Abstract
A major decline in pulmonary function is observed on the first day after upper abdominal surgery. This decline can reduce vital and inspiratory capacity and can culminate in restrictive lung diseases that cause atelectasis, reduced diaphragm movement, and respiratory insufficiency. The objective of this study was to evaluate the efficacy of preoperative ambulatory respiratory muscle training in patients undergoing esophagectomy. The sample consisted of 20 adult patients (14 men [70%] and 6 women [30%]) with a diagnosis of advanced chagasic megaesophagus. A significant increase in maximum inspiratory pressure was observed after inspiratory muscle training when compared with baseline values (from −55.059 ± 18.359 to −76.286 ± 16.786). Preoperative ambulatory inspiratory muscle training was effective in increasing respiratory muscle strength in patients undergoing esophagectomy and contributed to the prevention of postoperative complications.
Keywords: Physiotherapy, Inspiratory muscle training, Esophagectomy
Megaesophagus is an esophagopathy characterized by anatomic and functional alterations in the esophagus, which can be of idiopathic or chagasic etiology.1 In Brazil, Chagas disease affects about 6 million people, reducing the life expectancy and limiting the working capacity of affected individuals.2 It is estimated that 5% to 8% of patients with Chagas disease will develop megaesophagus.3
Idiopathic or chagasic megaesophagus is characterized by the loss or marked reduction of neurons in the myenteric plexus of the esophagus, causing uncoordinated contractions of the esophageal body (aperistalsis) and absent, uncoordinated, or incomplete opening of the lower esophageal sphincter (achalasia). These events, in turn, lead to esophageal stasis and progressive dilatation of the organ and reduce its contraction capacity.4
Among the various therapeutic procedures recommended for the treatment of megaesophagus, surgical intervention represents the best choice by relieving symptoms and improving the patient's nutritional status.5,6 However, a major decline in pulmonary function is observed on the first day after upper abdominal surgery. This decline can reduce vital and inspiratory capacity and can culminate in restrictive lung diseases that cause atelectasis, reduced diaphragm movement, and respiratory insufficiency.7,8 According to Paisani et al,9 inspiratory muscle weakness might be related to diaphragmatic dysfunction secondary to reflex inhibition of phrenic nerve activity caused by the anesthesia, with consequent impairment of respiratory mechanics.
Preoperative prophylactic physiotherapy has been shown to be an important and effective approach to prevent or reduce postoperative complications, in addition to optimizing treatment by familiarizing the patient with the physiotherapeutic procedures.10 The effects of preoperative respiratory physiotherapy have been documented in the literature, but most of these studies involved hospitalized patients.7,11 The objective of the present investigation was to evaluate the efficacy of preoperative ambulatory inspiratory muscle training (IMT) in patients with chagasic megaesophagus.
Materials and Methods
Patients
A qualitative–quantitative prospective cohort study was conducted at the Maria da Glória Outpatient Clinic of the Federal University of Triângulo Mineiro by the Department of Digestive Tract Surgery. The study was approved by the Ethics Committee of the Federal University of Triângulo Mineiro (process No. 660) and each patient signed a written informed consent form (Resolution 196/96 of the National Health Council).
A purposeful sample of 20 adult patients, including 14 (70%) men and 6 (30%) women, was studied. No patients were lost or excluded. All patients had megaesophagus and were submitted to laparoscopic transhiatal subtotal esophagectomy with esophagogastroplasty through a left cervicotomy. The procedure was started by creation of a pneumoperitoneum at 12 mmHg, where five punctures were performed for dissection of the abdominal and thoracic esophagus. A single-layer esophagogastric anastomosis was constructed with continuous sutures of polypropylene monofilament.
The criteria for inclusion in the study were a diagnosis of megaesophagus, aged more than 18 years, and an indication for laparoscopic transhiatal subtotal esophagectomy for the treatment of megaesophagus. Criteria for exclusion were the inability to perform respiratory muscle training, the presence of acute pulmonary disease, and refusal to undergo the treatment proposed.
All patients with a diagnosis of megaesophagus and a surgical indication were sent to the outpatient clinic for preoperative follow-up. During the first outpatient visit, the patients were submitted to physiotherapeutic assessment, which consisted of anamnesis and evaluation of respiratory muscle strength.
Respiratory muscle strength testing
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were measured, which permit the simple, rapid, and reproducible evaluation of respiratory muscle strength. MIP and MEP were measured with a vacuum manometer (Comercial Médica, São Paulo, Brazil; −120 and +120 cm H2O).12 For the measurement, the patient was sitting comfortably and used a nasal clip and rigid plastic mouthpiece. The mouthpiece had a small, approximately 2 mm, orifice to neutralize the pressure of the oral cavity during contraction of the facial muscles. The patient received instructions regarding the maneuver and the importance to prevent the escape of air.13 For the measurement of MIP, the patient was asked to sustain a maximal inspiratory effort for at least 1 second from a maximum expiration (residual volume). MEP was obtained at the time when the patient performed a maximal expiratory effort, sustained for at least 1 second, after deep inspiration to total lung capacity.14
Three consecutive measurements of MIP and MEP were obtained at intervals of 1 minute and the highest value was recorded. The difference between values should not exceed 10% for each repetition and the last value should not be the highest.13 These parameters were collected weekly during the preoperative return visits to the outpatient clinic of the Department of Digestive Tract Surgery.
Peak expiratory flow
Peak expiratory flow (PEF) was measured with an Assess Peak Flow Meter and the highest value obtained was considered for analysis. PEF is an indirect measure of airway resistance and is an effort-dependent parameter.
Inspiratory muscle training
After baseline assessment, the patients underwent IMT for 4 weeks using a pressure Threshold device (Respironics). A load corresponding to 50% MIP was used for IMT and MIP was reevaluated weekly for load adjustment.15 Patients underwent IMT once a week (3 series of 10 repetitions) at the outpatient clinic under the supervision of the physiotherapist. In addition, they were instructed and encouraged to exercise at home on the other days of the week (3 series of 10 repetitions). For this purpose, patients received a Threshold device for use throughout the preoperative period and were asked to record the times when IMT was performed at home.
Statistical analysis
The normality and homogeneity of the numerical variables were evaluated by the Kolmogorov-Smirnov and Bartlett tests, respectively. The data were normally distributed and are expressed as mean ± SD. MIP, MEP, and PEF were compared by the paired t-test. Differences were considered to be statistically significant when P < 0.05.
Results
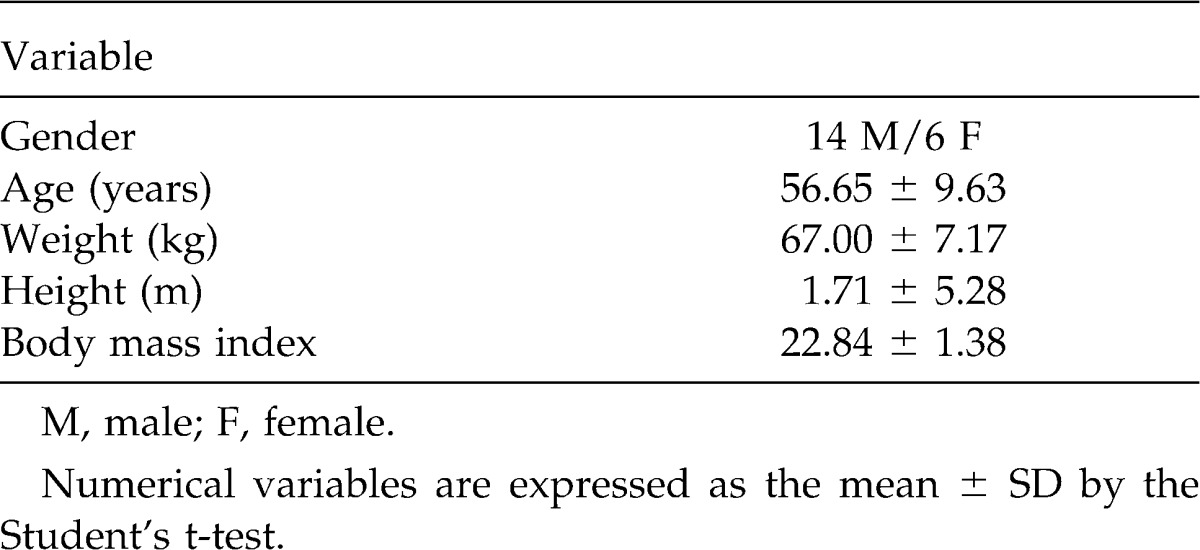
Twenty patients with a diagnosis of advanced chagasic megaesophagus were studied. The mean age of the patients was 51.29 years (range, 31–74 years). Table 1 shows the characteristics of the patients undergoing IMT.
Table 1.
Gender, age, and anthropometric characteristics of the patients undergoing inspiratory muscle training
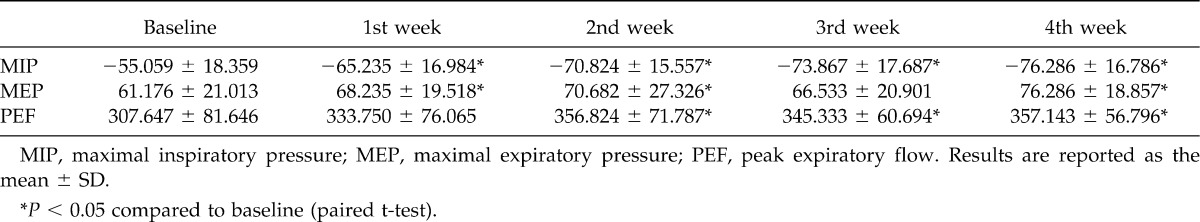
MIP increased gradually during the 4 weeks of IMT treatment, with a significant difference in the first week (P = 0.0017), second week (P < 0.0001), third week (P = 0.0001), and fourth week (P < 0.0001) when compared to baseline.
The results showed an increase in MEP after 4 weeks of IMT, although no expiratory muscle strength training was performed. This increase was significant in the first week (P = 0.0347), second week (P = 0.0233), and fourth week (P = 0.0004) when compared with baseline.
PEF also increased after IMT treatment, with the observation of a significant difference between baseline and the second week (P = 0.0172), third week (P = 0.0484), and fourth week (P = 0.0064). Table 2 shows the mean and SD of MIP, MEP, and PEF at baseline and during the 4 weeks of IMT treatment.
Table 2.
Maximal inspiratory pressure, maximal expiratory pressure, and peak expiratory flow at baseline and during the 4 weeks of inspiratory muscle training
Discussion
Megaesophagus is one of the clinical manifestations of Chagas disease. Although benign, the course of this condition is chronic and progressive, with relevant repercussions on the nutritional and psychologic status of affected patients. Megaesophagus is more common among men,16 in agreement with the present study in which 70.58% of the patients were men.
Preoperative IMT programs are used to increase respiratory muscle strength and endurance and several studies have demonstrated their benefits.7,17,18 Riganas et al19 reported a 28% increase of inspiratory muscle strength after 6 weeks of IMT, and Stein et al20 demonstrated a 37% increase of MIP after 4 weeks of ambulatory IMT. Other studies found a significant increase of inspiratory muscle strength between the second and fourth week of preoperative IMT.21,22 High-risk surgical patients who underwent preoperative IMT presented a reduction of 50% in postoperative pulmonary complications when compared with those did not submitted to this training.23 Hulzebos et al,24 Weiner et al,25 and Nomori et al12 reported significant increases in inspiratory muscle strength and endurance after short-term preoperative IMT. Other studies demonstrated improvement of blood oxygenation, dyspnea, and MIP in patients undergoing IMT for a period of 6 to 8 weeks.26,27
Ysayama et al28 showed that IMT for 4 weeks before esophagectomy resulted in a significant increase of respiratory muscle strength and endurance. In the present study, a significant increase in inspiratory muscle strength was demonstrated by the increase in MIP values. These results suggest that the gain in inspiratory muscle strength led to an increase of ventilatory capacity and pulmonary volume, with the consequent improvement of respiratory function and performance.
Although the patients only underwent inspiratory muscle strength training, MEP values were also increased, a finding confirming the hypothesis that IMT increases the amplitude of thoracoabdominal movements. Other studies also demonstrated an increase in expiratory muscle strength and consequent clinical improvement after IMT.29,30
Satisfactory results were also obtained for PEF values, with the observation of a significant increase after 2 weeks of IMT. In this respect, studies in the literature have shown that IMT effectively promotes biomechanical reorganization and improves muscle function, which, in turn, leads to significant improvement of PEF and variables of severity.31,32
The present study had the following limitations: lack of a control group for comparison of the results obtained, and IMT was not supervised daily by a physiotherapist and may therefore have not been performed correctly. However, we believe that daily training under the supervision of a professional might have yielded even more satisfactory results.
The present results demonstrate that preoperative ambulatory IMT has beneficial effects, as it was found to improve respiratory muscle strength in patients undergoing esophagectomy. Further studies are needed to better understand the gain in preoperative inspiratory muscle strength and of how much this gain is sustained during the postoperative period, permitting to identify factors that further improve the response of these patients to training.
References
- 1.Ramos R. I., Varrica L. M., Dantas R. O. Differences in response of the proximal esophagus to wet swallows in patients with Chagas' disease and idiopathic achalasia. Dis Esophagus. 2006;19:401–405. doi: 10.1111/j.1442-2050.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 2.Moncayo A., Silveira C. A. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz. 2009;104(I):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 3.Crema E., Ribeiro L. B., Terra J. A., Jr, Silva A. A. Laparoscopic transhiatal subtotal esophagectomy for the treatment of advanced megaesophagus. Ann Thorac Surg. 2005;80:1196–1201. doi: 10.1016/j.athoracsur.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 4.Valezi A. C., Mali Júnior J., Marson A. C., De Brito E. M., De Souza J. C. L. Laparoscopic management of esophageal achalasia: experience in 12 cases. Rev Col Bras Cir. 2004;31:148–153. [Google Scholar]
- 5.Penhavel F. A., Waitzberg D. L., Trevenzol H. P., Alves L., Zilberstein B., Gama-Rodrigues J. Pre-and postoperative nutritional evaluation in patients with chagasic megaesophagus. Nutr Hosp. 2004;19:89–94. [PubMed] [Google Scholar]
- 6.Crema E., Cruvinel L. A., Werneck A. M., de Oliveira R. M., Silva A. A. Manometric and radiologic aspects of Chagas' megaesophagus: the importance to its surgical treatment. Rev Soc Bras Med Trop. 2003;36:665–669. doi: 10.1590/s0037-86822003000600004. [DOI] [PubMed] [Google Scholar]
- 7.Dronkers J., Veldman A., Hoberg E., van der Waal C., van Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training a randomized controlled pilot study. Clin Rehabil. 2008;22:134–142. doi: 10.1177/0269215507081574. [DOI] [PubMed] [Google Scholar]
- 8.Ramos G. C., Pereira E., Gabriel Neto S., Oliveira E. C. Pulmonary performance test after conventional and laparoscopic cholecystectomy. Rev Col Bras Cir. 2007;34(5):326–330. [Google Scholar]
- 9.Paisani D. M., Benassule E., Chiavegato L. D. Cirurgia Abdominal in Sarmento GVJ Fisioterapia respiratória no paciente critic. São Paulo: Editora Manole; 2007. pp. 315–322. [Google Scholar]
- 10.Crema E., Benelli A. G., Silva A. V., Martins A. J., Pastore R., Kujavao G. H., et al. Assessment of pulmonary function in patients before and after laparoscopic and open esophagogastric surgery. Surg Endosc. 2005;19:133–136. doi: 10.1007/s00464-004-8102-z. [DOI] [PubMed] [Google Scholar]
- 11.Nomori H., Kobayashi R., Fuyuno G., Morinaga S., Yashima H. Preoperative respiratory muscle training. Assessment in thoracic surgery patients with special reference to postoperative pulmonary complications. Chest. 1994;105(6):1782–1788. doi: 10.1378/chest.105.6.1782. [DOI] [PubMed] [Google Scholar]
- 12.Neder J. A., Andreoni S., Lerario M. C., Nery L. E. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719–727. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 13.Parreira V. F., França D. C., Zampa C. C., Fonseca M. M., Tomich G. M., Brito R. R. Maximal respiratory pressures: actual and predicted values in healthy subjects. Revista Brasileira de Fisioterapia. 2007;11(5):361–368. [Google Scholar]
- 14.Souza R. B. Pressões respiratórias estáticas máximas. J Bras Pneumol. 2002;28(3):155–165. [Google Scholar]
- 15.Lotters F., van Tol B., Kwakkel G., Gosselink R. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. 2002;20:570–576. doi: 10.1183/09031936.02.00237402. [DOI] [PubMed] [Google Scholar]
- 16.Hulzebos E. H., van Meeteren N. L., van den Buijs B. J., de Bie R. A., Brutel de la Rivière A., Helders P. J. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin Rehabil. 2006;20(11):949–959. doi: 10.1177/0269215506070691. [DOI] [PubMed] [Google Scholar]
- 17.Reeve J. C., Nicol K., Stiller K., McPherson K. M., Birch P., Gordon I. R., et al. Does physiotherapy reduce the incidence of postoperative pulmonary complications following pulmonary resection via open thoracotomy? A preliminary randomised single-blind clinical trial. Eur J Cardiothorac Surg. 2010;37(5):1158–1166. doi: 10.1016/j.ejcts.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Forti E., Ike D., Barbalho-Moulim M., Rasera I., Jr, Costa D. Effects of chest physiotherapy on the respiratory function of postoperative gastroplasty patients. Clinics (Sao Paulo) 2009;64(7):683–689. doi: 10.1590/S1807-59322009000700013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riganas C. S., Vrabas I. S., Christoulas K., Mandroukas K. Specific inspiratory muscle training does not improve performance or VO2max levels in well trained rowers. J Sports Med Phys Fitness. 2008;48:285–292. [PubMed] [Google Scholar]
- 20.Stein R., Maia C. P., Silveira A. D., Chiappa G. R., Myers J., Ribeiro J. P. Inspiratory muscle strength as a determinant of functional capacity early after coronary artery bypass graft surgery. Arch Phys Med Rehabil. 2009;90:1685–1691. doi: 10.1016/j.apmr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Garcia R. C. P., Costa D. Treinamento muscular respiratório em pós-operatório de cirurgia cardíaca eletiva. Rev Bras Fisioter. 2002;6:139–146. [Google Scholar]
- 22.Weiner P., Berar-Yanay N., Davidovich A., Magadle R., Weiner M. Specific inspiratory muscle training in patients with mild asthma with high consumption of inhaled beta(2)-agonists. Chest. 2000;117(3):722–727. doi: 10.1378/chest.117.3.722. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia E., Fulgenzi A., Ferrero M. E. Rationale of the combined use of inspiratory and expiratory devices in improving maximal inspiratory pressure and maximal expiratory pressure of patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2009;90:913–918. doi: 10.1016/j.apmr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Hulzebos E. H., Helders P. J., Favie N. J., De Bie R. A., Brutel de la R. A., van Meeteren N. L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851–1857. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- 25.Weiner P., Zeidan F., Zamir D., Pelled B., Waizman J., Beckerman M., et al. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J Surg. 1998;22:427–431. doi: 10.1007/s002689900410. [DOI] [PubMed] [Google Scholar]
- 26.Martinez A., Lisboa C., Jalil J., Munoz V., Dias O., Casanegra P., et al. Entre- namiento selectivo de los musculos respiratorios en pacientes com insuficiencia cadiaca cronica. Ver Méd Chil. 2001;29(2):133–139. [PubMed] [Google Scholar]
- 27.Vargas B. D. Evaluación de la fuerza de la musculatura respiratoria y su tolerancia a la fatiga luego de una actividad fisica. Kinesiologia. 1992;32:17–23. [Google Scholar]
- 28.Ysayama L., Lopes L. R., Silva A. M. O., Andreollo N. A. The influence of the respiratory muscular training in the recovery of esophagectomies. ABCD Arq Bras Cir Dig. 2008;21(2):61–64. [Google Scholar]
- 29.Pires V. A., Costa D., Jamami M., Oishi J., Baldissera V. Comparaçäo de duas técnicas de treinamento muscular respiratório em pacientes sob ventilaçäo mecânica com insucesso de desmame. Rev Bras Fisioter. 2000;4(2):93–104. [Google Scholar]
- 30.Ribeiro K. P., Toledo A., Whitaker D. B., Reyes L. C. V., Costa D. Inspiratory muscle training in rehabilitation of COPD patients. Saúde Rev. 2007;9(22):39–46. [Google Scholar]
- 31.Lima E. V., Lima W. L., Nobre A., dos Santos A. M., Brito L. M., Costa Mdo R. Inspiratory muscle training and respiratory exercises in children with asthma. J Bras Pneumol. 2008;34(8):552–558. doi: 10.1590/s1806-37132008000800003. [DOI] [PubMed] [Google Scholar]
- 32.Enright S., Chatham K., Ionescu A. A., Unnithan V. B., Shale D. J. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126(2):405–411. doi: 10.1378/chest.126.2.405. [DOI] [PubMed] [Google Scholar]


