Abstract
Fatty acid ethanolamides (FAEs), which include palmitoylethanolamide (PEA) and oleoylethanolamide (OEA), are endogenous agonists of peroxisome proliferator-activated receptor-α (PPAR-α) and important regulators of the inflammatory response. They are degraded in macrophages by the lysosomal cysteine amidase, N-acylethanolamine acid amidase (NAAA). Previous studies have shown that pharmacological inhibition of NAAA activity suppresses macrophage activation in vitro and causes marked anti-inflammatory effects in vivo, which is suggestive of a role for NAAA in the control of inflammation. It is still unknown, however, whether NAAA-mediated FAE deactivation might regulate pain signaling. In the present study, we examined the effects of ARN077, a potent and selective NAAA inhibitor recently disclosed by our group, in rodent models of hyperalgesia and allodynia caused by inflammation or nerve damage. Topical administration of ARN077 attenuated, in a dose-dependent manner, heat hyperalgesia and mechanical allodynia elicited in mice by carrageenan injection or sciatic nerve ligation. The anti-nociceptive effects of ARN077 were prevented by the selective PPAR-α antagonist GW6471 and did not occur in PPAR-α-deficient mice. Furthermore, topical ARN077 reversed the allodynia caused by ultraviolet B-radiation in rats, and this effect was blocked by pretreatment with GW6471. Sciatic nerve ligation or application of the pro-inflammatory phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) decreased FAE levels in sciatic nerve and skin tissue, respectively. ARN077 reversed these biochemical effects. The results identify ARN077 as a potent inhibitor of intracellular NAAA activity, which is active in vivo by topical administration. The findings further suggest that NAAA regulates peripheral pain initiation by interrupting endogenous FAE signaling at PPAR-α.
1. Introduction
The amides of fatty acids with ethanolamine (fatty acid ethanolamides, FAEs) are a family of lipid-derived messengers that participate in the control of multiple physiological functions, including pain and inflammation [18, 19, 22]. Saturated or mono-unsaturated members of this family, such as palmitoylethanolamide (PEA) and oleoylethanolamide (OEA), are produced in innate immune and neural cells by the action of a selective phospholipase, N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD), and exert anti-nociceptive and antiinflammatory effects in experimental animals and humans [6, 19, 24, 29]. Such effects are primarily, albeit not exclusively [1, 19], due to the ability of PEA and OEA to engage the ligand-activated transcription factor, peroxisome proliferator-activated receptor- (PPAR- ) [18, 19], to which they bind with high affinity [11]. The actions of these lipid messengers are terminated by enzyme-mediated hydrolysis, which is catalyzed by two intracellular lipid amidases: N-acylethanolamine acid amidase (NAAA) [31] and fatty acid amide hydrolase (FAAH) [8, 9].
Even though NAAA and FAAH share the ability to cleave lipid amide bonds, they differ markedly in primary structure, substrate selectivity and cellular localization. NAAA is an N-terminal nucleophile cysteine amidase, which displays a strong preference for saturated FAEs such as PEA [31]. FAAH, on the other hand, is a member of the ‘amidase signature’ family of serine hydrolases and displays broader substrate selectivity, but preferentially hydrolyzes mono-unsaturated and poly-unsaturated FAEs such as OEA and the endocannabinoid anandamide [9]. Moreover, NAAA appears to be primarily localized to the lysosomal compartment of macrophages [32], whereas FAAH is found on the outer face of mitochondria and endoplasmic reticulum of most mammalian cells [12].
Pro-inflammatory stimuli suppress NAPE-PLD expression in rat and mouse macrophages, stopping FAE production and causing a reduction in the cellular levels of these anti-inflammatory lipid mediators [27, 34]. The latter response is reversed by pharmacological blockade of NAAA-mediated FAE hydrolysis, suggesting that inhibition of intracellular NAAA activity might represent a novel mechanistic approach to control inflammation [27, 28]. In support of this view, we have shown that the compound (S)-N-(2-oxo-3-oxetanyl)-3-phenylpropanamide [(S)-OOPP], which inhibits rat NAAA with a median effective concentration (IC50) of ~0.4 M, prevents PEA and OEA hydrolysis in activated inflammatory cells and reduces tissue reactions to various pro-inflammatory triggers [27, 28].
While there is a growing appreciation for the role of NAAA in the control of inflammation, it is still unknown whether the ability of this enzyme to terminate FAE signaling might also contribute to pain regulation. To address this question, in the present study we utilized the compound ARN077 (5-phenylpentyl N-[(2S,3R)-2-methyl-4-oxo-oxetan-3-yl] carbamate), which we recently identified as a highly potent and selective inhibitor of human NAAA (IC50 ~7 nM) [2, 21]. Our results show that local administration of ARN077 normalizes FAE levels in inflamed mouse skin and blunts the hyperalgesia and allodynia evoked in mice by carrageenan and sciatic nerve injury and in rats by ultraviolet B (UVB) radiation.
2. Materials and Methods
2.1. Animals
We used male CD1 mice, Swiss Webster mice (25–30 g; Charles River, Wilmington, MA, USA), Sprague-Dawley rats (150–175 g; Charles River, Calco, Italy), male C57BL/6J (20–22 g; Charles River, Calco, Italy) and C57BL/6J mice homozygous for the PpartniJGonz-targeted mutation (20–22 g; Charles River, Calco, Italy). All procedures were performed in accordance with the Ethical Guidelines of the International Association for the Study of Pain, Italian regulations on the protection of animals used for experimental and other scientific purposes (D.M. 116192) and European Economic Community regulations (O.J. of E.C. L 358/1 12/18/1986). When appropriate, procedures were also approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Animals were group-housed in ventilated cages and had free access to food and water. They were maintained under a 12 h light/dark cycle (lights on at 8:00 a.m.) at controlled temperature (21±1°C) and relative humidity (55±10%). Behavioral testing was performed during the light cycle (between 9:00 a.m. and 5:00 p.m.). Animals were killed by cervical dislocation under anesthesia. Experimenters were unaware of the treatment protocol at the time of the test (blind procedure).
2.2. Chemicals
Glutamine, Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were from Euroclone (Pero, Italy); penicillin/streptomycin was from Invitrogen-Life Technologies (Monza, Italy), G418 from PAA (Pasching, Austria), 10-cis-heptadecenoylethanolamide from Avanti Polar Lipids (Alabaster, Alabama, USA), heptadecanoic acid; heptadecenoic acid, N-lauroyl ceramide from NuChek Prep (Elysian, Minnesota, USA); AM251, AM630, λ-carrageenan, gabapentin, GW6471 and 12-O-tetradecanoylphorbol 13-acetate (TPA) were from Sigma Aldrich. [3H]-anandamide (arachidonoyl-[1-3H] ethanolamine; 0.04 µCi/ml, specific activity 1.7 Ci/mmol; concentration 0.15 mCi/m) was from American Radiolabeled Chemicals (Saint Louis, Missouri, USA). ARN077 was synthesized in our laboratories, as described [21].
2.3. Recombinant rat NAAA
We amplified the full-length coding sequence of rat NAAA by polymerase chain reaction (PCR) using HotStar HiFidelity kit (Qiagen) and rat brain cDNA libraries as a templates, using primers designed on the NCBI database sequence NM_001010967 (forward, 5′-ATGGGGACCCCAGCCATCCGG-3′; reverse, 5′-TCAGCTTGGGTTTCTGATCATGGT-3′). The rat NAAA PCR product was subcloned into a pCMV-Flag vector (Stratagene, La Jolla, CA) by HindIII and XhoI (Roche, Indianapolis, IN) sites to construct a mammalian expression vector encoding Flag-tagged rat NAAA. We transfected the constructs in HEK 293 cells using JetPEI transfection reagent (Polyplus, Illkirch, France) and derived stable cell lines by selection with G418 (1mg/ml). Clones were selected based on expression levels assessed by Western blot analyses (mouse monoclonal MAB4494, R&D Systems, MN, USA). HEK 293 cells overexpressing rat NAAA (HEK-rNAAA cells) were grown in DMEM containing 10% FBS, 1% penicillin/streptomycin, 1% glutamine and 0.5 mg/ml G418.
2.4. NAAA Assay
Sample preparation
HEK-rNAAA cells were rinsed with phosphate-buffered saline (PBS, 10 mM, pH 7.4), scraped from the flasks, collected in 50 ml Falcon tubes on ice, and centrifuged 10 min at 4°C at 800×g. The pellets were suspended and sonicated in Tris-HCl buffer (20 mM, pH 7.4) containing 0.32 M sucrose. Rat native NAAA was obtained from lungs of male Sprague-Dawley rats. Rats were killed by decapitation and the lungs were dissected, minced over ice and transferred into ice-cold Tris-HCl buffer (20 mM, pH 7.4) containing 0.32 M sucrose (final volume-to-weight ratio, 9:1). Samples were homogenized, centrifuged at 800×g for 30 min at 4°C, and the supernatants were ultra-centrifuged at 12,000 × g for 30 min at 4°C. The pellets were suspended in 10 mM PBS (pH 7.4) on ice and subjected to a freeze/thaw cycle at −80°C. Suspensions were centrifuged at 105,000×g for 1 h at 4°C. Protein concentration was measured and samples stored at −80°C until used NAAA preparations (0.1 mg from rat lung or 10 µg from HEK-rNAAA cells) were pre-incubated with various concentrations of ARN077 (dissolved in dimethylsulphoxide, DMSO, final concentration 1%) in NAAA assay buffer (0.1 M NaH2PO4, 0.1 M sodium citrate, 0.1% Triton-X 100, 3 mM dithiothreitol, DTT, pH 4.5) for 30 min at 37°C before the addition of the enzyme substrate (10-cis-heptadecenoylethanolamide, 50 µM) at 37°C for 30 min. Reactions (in duplicate) were incubated for additional 30 min at 37°C and stopped by the addition of 0.2 ml ice-cold methanol containing 1 nmol heptadecanoic acid (NuChek Prep) as internal standard. Analyses were conducted by liquid chromatography/mass spectrometry (LC/MS).
LC/MS analyses
Samples were eluted isocratically on an Acquity UPLC BEH C18 column (50mm length, 2.1 mm i.d., 1.7 µm pore size, Waters) at 0.5 ml/min for 1.5 min with a solvent mixture of 95% methanol and 5% water, both containing 0.25% acetic acid and 5 mM ammonium acetate. Column temperature was set at 40 °C. Electrospray ionization was in the negative mode, capillary voltage was 2.7 kV, cone voltage was 45 kV, source temperature was 150°C with a desolvation temperature of 450°C. Nitrogen was used as drying gas at a flow rate of 800 L/hour and a temperature of 500°C. The [M-H]-ion was monitored in the selected-ion monitoring mode (m/z values: heptadecenoic acid 267.37, heptadecanoic acid 269.37). Calibration curves were generated using authentic heptadecenoic acid (NuCheck Prep). Inhibition of NAAA activity was calculated as reduction in heptadecenoic acid levels compared to vehicle controls. IC50 values were calculated by non-linear regression analysis of log[concentration]/inhibition curves using GraphPad Prism 5 (GraphPad Software Inc., CA – USA) applying a standard slope curve fitting.
2.5. Time-course and kinetics experiments
Time-course experiments were run under standard assay conditions, using ARN077 at a final concentration of 0.1 M. Kinetics experiments were run using ARN077 at a final concentration of 50 nM or 0.3 M, with substrate (10-cis-heptadecenoylethanolamide) at 10, 50, 100, 500, 1000, and 1500 µM.
2.6. Dialysis experiments
Recombinant rat NAAA was incubated in NAAA assay buffer containing either vehicle (DMSO, 1%) or ARN077 (0.3 M in DMSO 1%) at 37oC for 30 min. An aliquot was taken to determine NAAA activity (t = 0), and the remainder was injected into dialysis cassettes (10 kDa molecular weight cut-off; Thermo scientific, Massachusetts, USA) and dialyzed overnight in assay buffer under moderate stirring. DTT (3 mM) was added 1 h prior to the end of dialysis. After 12 h of dialysis, the samples were retrieved and assayed for NAAA activity.
2.7. Additional in vitro assays
Acid ceramidase (AC) activity
HEK 293 cells overexpressing rat AC were generated as described [27] and cell homogenates (25 µg protein) were incubated with 0.1 mM N-lauroyl ceramide (Nu-Chek Prep) as substrate in assay buffer (0.1 M sodium phosphate buffer, 0.1% Nonidet P-40, 150 mM NaCl and 3 mM DTT, pH 4.5) for 30 min at 37 °C in the presence of vehicle or various concentrations of ARN077. Reactions were stopped by addition of a mixture of chloroform/methanol (2:1, vol/vol) containing 1 nmol heptadecanoic acid (HDA; NuChek Prep). The organic phases were collected, dried under nitrogen, and analyzed by LC/MS in the negative-ion mode using HDA as internal standard (m/z=199 for lauric acid, m/z=269 for HDA). HDA was eluted on an XDB Eclipse C18 column isocratically at 2.2 ml/min for 1 min with a solvent mixture of 95% methanol and 5% water, both containing 0.25% acetic acid and 5 mM ammonium acetate. The column temperature was 50°C. Electrospray ionization (ESI) was in the negative mode, capillary voltage as 4 kV, and fragmentor voltage was 100 V. Nitrogen was used as drying gas at a flow rate of 13 L/min. Nebulizer pressure was set at 60 psi and 350°C. We monitored [M-H]-in the selected-ion monitoring (SIM) mode using HDA as internal standard.
FAAH activity
Rat brain homogenates (50 g protein) were incubated at 37°C in assay buffer (50 mM Tris pH 7.4, 0.05% fatty acid-free bovine serum albumin) containing either vehicle (DMSO, 1%) or ARN077. After 10 min, substrate (1 M anandamide and 0.6 nM [3H]- anandamide) was added and samples were incubated for additional 30 min. Reactions were stopped with ice-cold chloroform/methanol (1:1, vol:vol) and radioactivity in the aqueous phase was measured by liquid scintillation counting.
2.8. Animal Models
TPA-induced inflammation
20 adult male Swiss Webster mice were divided into 2 groups, each receiving either acetone or ARN077 (10% in acetone) topically on both ear pinnae (10 l on each surface). After 15 min, a solution of TPA (3% in acetone, 20 l) was applied to the left ear pinna while 20 l of acetone were applied to the right ear pinna. Animals were killed 6 h after treatment and the ears were excised. Four 2-mm punches from each ear were weighed to measure edema. Tissue FAE levels were quantified as previously described [3].
Carrageenan-induced inflammation
We induced paw edema by injecting λ-carrageenan (1% weight/vol in sterile water, 50 µl) into the left hind paw of lightly restrained adult male CD1 mice. Edema was measured with a plethysmometer (Ugo Basile, Comerio, Italy). Fresh drug suspensions were prepared daily and applied either topically on the plantar surface of the paw (in petrolatum containing 5% lauric acid, 20 l per animal) or given by intraplantar injection (in 80% sterile saline solution/10% PEG-400/10% Tween 80, 10 l per animal), 90 min after carrageenan injection.
Chronic constriction injury (CCI)
Sciatic nerve ligations were performed according to Bennett and Xie [4]. Adult male CD1 mice were anesthetized with 2–3% isoflurane, and the left sciatic nerve was exposed at mid-thigh level through a small incision and tied at two distinct sites (spaced at a 2-mm interval) with a silk thread. The wound was closed with a single muscle suture and skin clips, and dusted with streptomycin. In sham-operated animals, the nerve was exposed but not tied. Fresh drug suspensions were prepared in petrolatum/5% lauric acid and applied topically on the plantar surface of the paw (20 l per animal) 1 h before testing. Tissue FAE levels were quantified as previously described [3]. Briefly, frozen sciatic nerves were weighed (~7 mg) and homogenized in methanol (1 mL) containing [2H4]-anandamide, [2H4]-PEA and [2H4]-OEA as internal standards. Lipids were extracted with chloroform (2 vol) and washed with water (1 vol). Organic phases were collected and dried under nitrogen. The organic extract was fractionated by open-bed silica gel column chromatography. Anandamide, PEA and OEA were eluted with chloroform/methanol (9:1, vol/vol). Organic phases were evaporated under nitrogen and reconstituted in 60 µL of methanol. Levels of anandamide, PEA and OEA were measured using a Xevo TQ UPLC-MS/MS system (Waters, Milford, CT).
UVB-induced inflammation
Rats were anaesthetized with a mixture of tiletamine and zolazepam (each at 15 mg/kg, intraperitoneal), placed laying on their backs and shrouded in a UV opaque material with only the relevant surface of the plantar right hind paw exposed, perpendicular to the narrowband UVB light source situated above the level of the limb. The UVB source consisted in a bank of 4 TL01 fluorescent tubes (Philips, UK, λmax=312 nm) spaced 2.5 cm apart producing an even field of irradiation. The amount of UVB irradiation to which animals were exposed was calculated by using a calibrated meter (IL1400A with SEL240/UVB-1/TD filter, ABLE Instruments & Controls Ltd, UK). The 24 h time point represents the peak of thermal sensory changes after 250 mJ/cm2 UVB irradiation. ARN077 was suspended in petrolatum/5% lauric acid (50 µl) and administered topically to the right hind paw, 4 h before the tests. GW6471, AM251 and AM630 were dissolved in saline containing 10% PEG-400 and 10% Tween 80 to a final concentration of 1µg/µl and 60 µl of this solution were injected into the right paw of the rats.
2.9. Behavioral tests
All experiments were performed in a quiet room, and experimenters were blinded to the treatment protocol at the time of the test. Heat hyperalgesia was assessed by the method of Hargreaves et al. [14] measuring the latency to withdraw the hind paw from a focused beam of radiant heat (thermal intensity: infrared 3.0) applied to the plantar surface in a plantar test apparatus (Ugo Basile). The cutoff time was set at 30 s. Withdrawal latency was measured on the injured ipsilateral paw in either carrageenan-treated mice or UVB-irradiated rats. Tactile allodynia (dynamic plantar esthesiometer; Ugo Basile) was assessed as described by La Rana et al. [17] measuring the latency to withdraw the hind paw from a steel rod of diameter 0.5mm applied to the hind paw with ascending force of 0 to 5 g over a period of 20 s at a rate of 0.25 g/s.
2.10. Statistical Analyses
IC50 values were calculated by non-linear regression analysis of log[concentration]/inhibition curves using GraphPad Prism 5 (GraphPad Software, CA, USA) applying a standard slope curve fitting. In the reversibility study the significance of differences between groups was determined using two-way analysis of variance (ANOVA) followed by Bonferroni’s test for multiple comparisons. The significance of differences between groups in the TPA-induced inflammation was evaluated using Student’s t test for within-group analysis. The significance of differences for PEA, OEA and anandamide levels in the CCI model was determined using oneway ANOVA followed by Bonferroni’s test for multiple comparisons. Data obtained in the carrageenan and CCI models were compared using two-way analysis of variance (ANOVA) followed by Bonferroni’s test for multiple comparisons. The significance of differences between groups in the UVB model was determined using one-way ANOVA followed by Bonferroni’s test for multiple comparisons.
3. Results
3.1. ARN077 inhibits rat NAAA activity in vitro
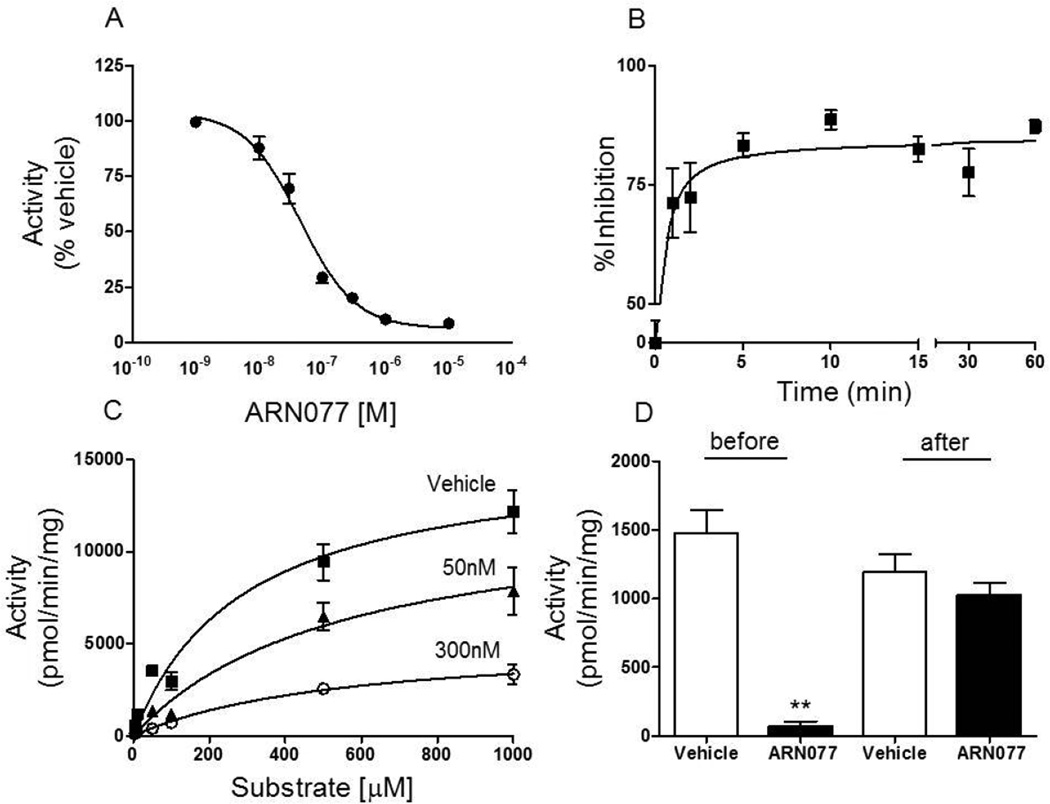
As previously shown for human NAAA [21], ARN077 displayed a potent inhibitory activity towards native rat lung NAAA (IC50 = 45 ± 3 nM; n = 3) and recombinant rat NAAA (IC50 = 11 nM). Kinetic analyses showed that ARN077 inhibits recombinant rat NAAA through a rapid (t1/2 = 0.4 min), non-competitive and reversible mechanism (Fig. 1 B–D; Table 1). By contrast, the compound did not show significant inhibitory activity toward FAAH (control, 198.9 ± 18.8 pmol/mg/min; 10 µM ARN077, 211.6 ± 21.0 pmol/mg/min; n = 4) or acid ceramidase, a cysteine amidase that is structurally related to NAAA [32] (control, 296 ± 4.6 pmol/mg/min; 10 µM ARN077, 308 ± 11 pmol/mg/min; 100 µM ARN077, 270 ± 4.7 pmol/mg/min; n = 3).
Figure 1.
Effects of ARN077 on rat NAAA activity in vitro. (A) Concentration-dependent inhibition of recombinant rat NAAA activity by ARN077. (B) Time-course of the NAAA reaction in the presence of ARN077 (0.1 µM); (C) Michaelis-Menten analysis of the NAAA reaction in the presence of vehicle (squares; DMSO, 1%), 50 nM ARN077 (triangles) or 300 nM ARN077 (circles). (D) Effects of dialysis (24 h, 0–4°C) on NAAA activity by ARN077 (0.3 µM). Results are expressed as mean ± SEM (n = 6). ** p<0.01.
Table 1.
| Vehicle | ARN077 (50nM) |
ARN077 (300nM) |
|
|---|---|---|---|
| Vmax (pmol/min/mg) | 15,359 | 12,489 | 5,245 |
| Km (µM) | 288 | 545 | 551 |
3.2. ARN077 protects OEA and PEA from degradation in vivo
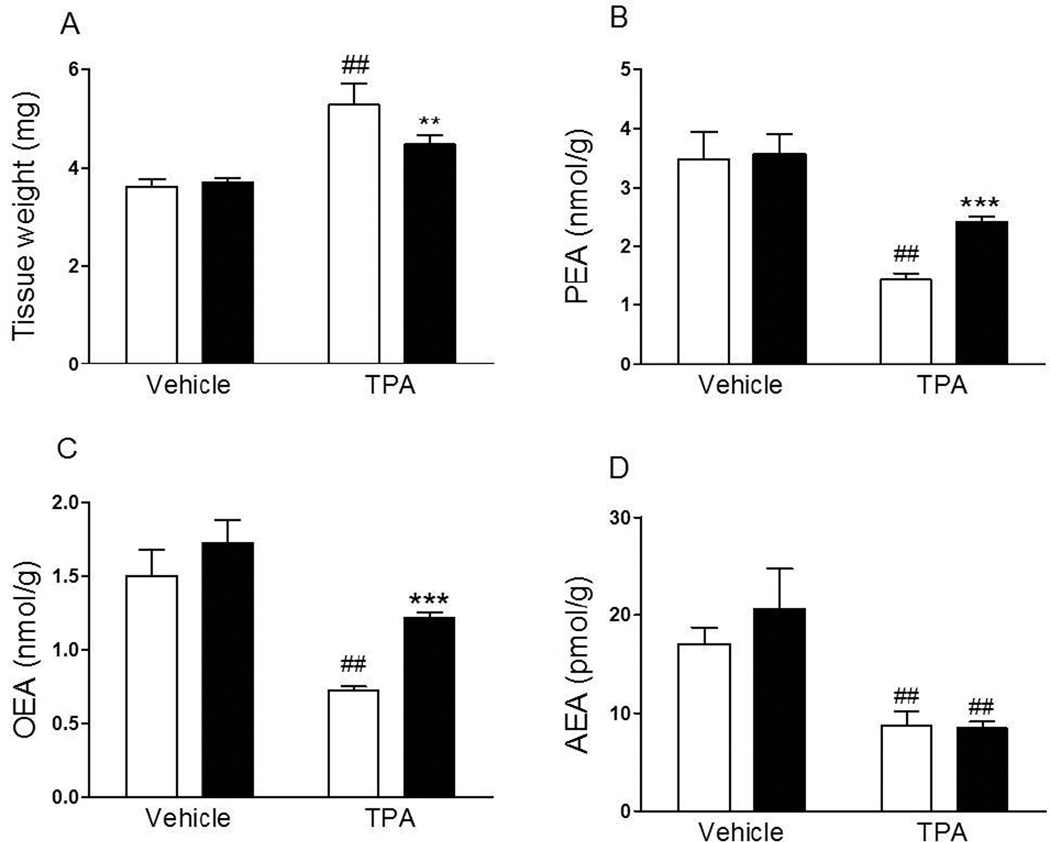
Application of the pro-inflammatory phorbol ester TPA (3% in acetone) to the ear skin of Swiss Webster mice produced local edema (Fig. 2 A), which was accompanied by a reduction in tissue PEA, OEA and anandamide levels (Fig. 2 B, C and D). Pre-treatment with ARN077 (10% in acetone, 15 min before TPA) suppressed TPA-induced edema (Fig. 2 A) and partially normalized tissue PEA and OEA content (Fig. 2 B, C), but not anandamide content (Fig. 2 D).
Figure 2.
Effects of topical application of ARN077 on TPA-induced edema and skin FAE levels. ARN077 (10% in acetone) reduced tissue weight (A) normalized PEA and OEA, but not AEA, content in mouse ear skin (B, C and D) 6 h after co-application with TPA (3% in 10 l). Results are expressed as mean ± SEM (n = 5 each group). ** p<0.01 and *** p<0.001 vs. TPA-ARN077-treated group; ## p<0.01 vs. TPA vehicle-treated group.
3.4. Effects of ARN077 in the mouse carrageenan model
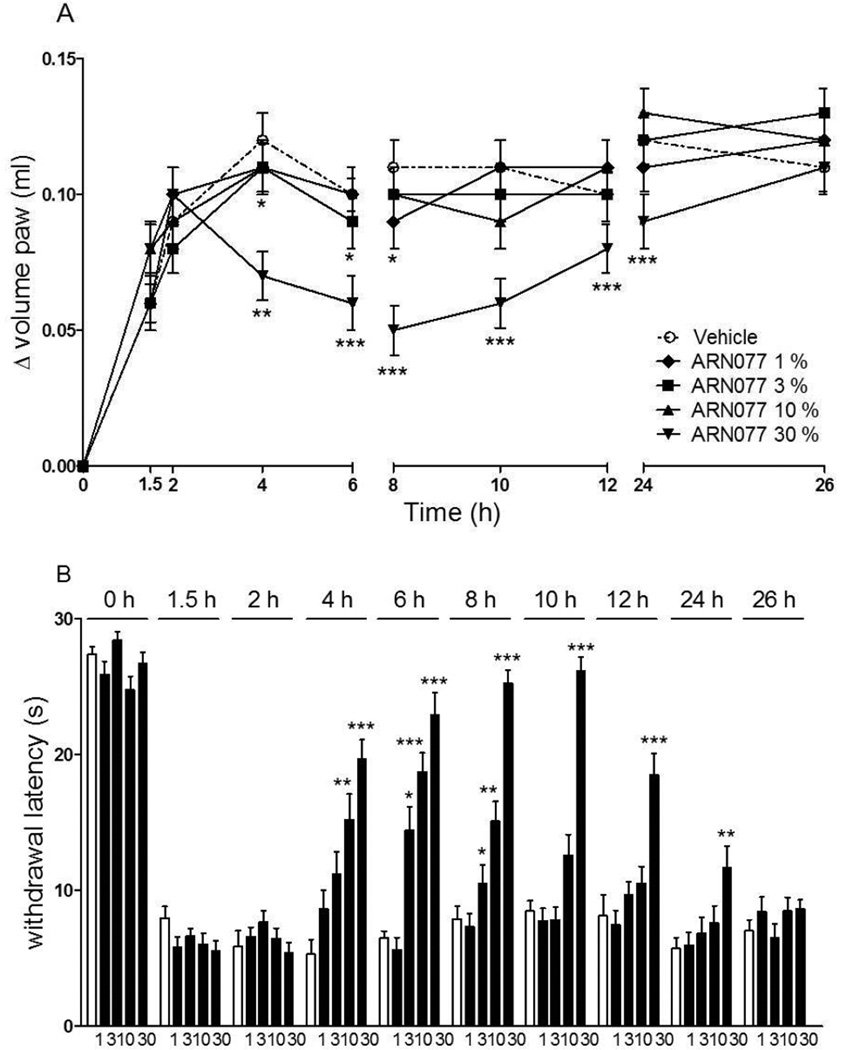
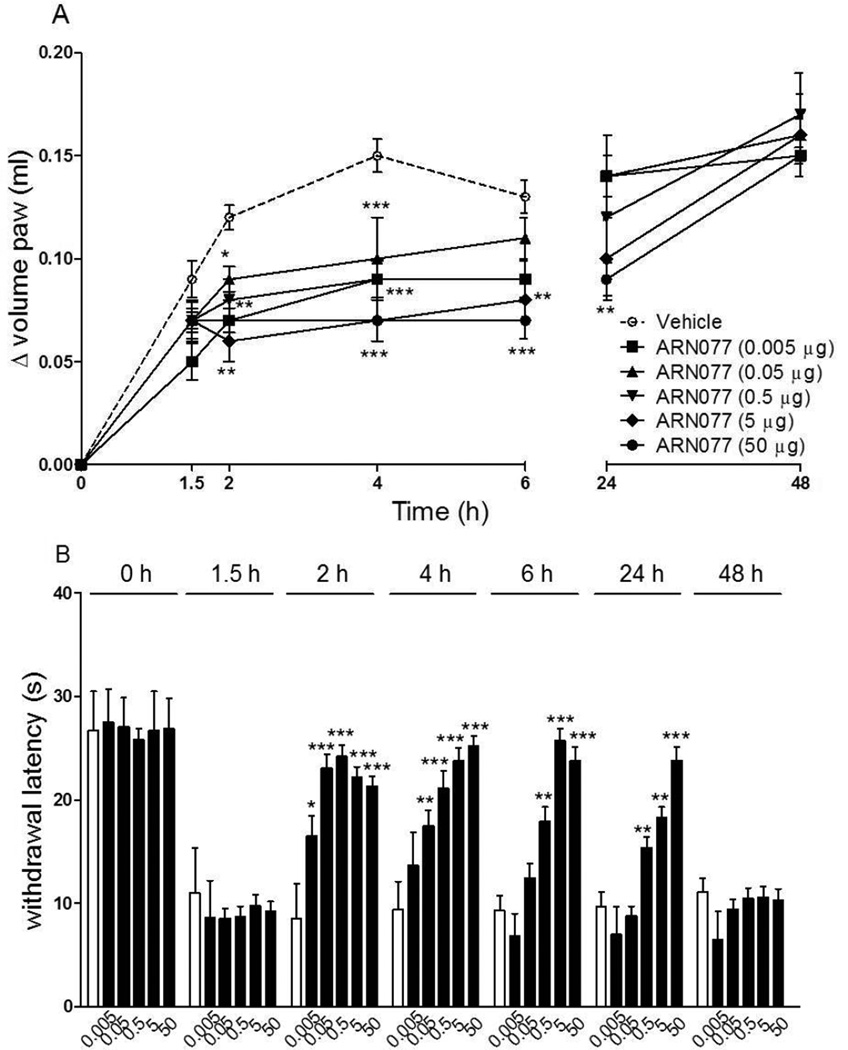
Intraplantar administration of carrageenan in CD1 mice resulted in the development of paw edema and heat hyperalgesia (Fig. 3 A, B). Both responses were markedly attenuated, in a dose- and time-dependent manner, by a single topical dosing with ARN077 (1–30% weight/volume, in petrolatum/5% lauric acid) applied 90 min after carrageenan (Fig. 3 A, B). When ARN077 was administered at its highest dosage (30%), the effect was still statistically detectable 24 h following application (Fig. 3 B, P<0.001). An anti-nociceptive response of similar magnitude and duration was seen when ARN077 was administered by intraplantar injection (50 µg/paw, P<0.01) (Fig. 4 A, B).
Figure 3.
Effects of topical application of ARN077 on carrageenan-induced hyperalgesia and edema. ARN077 (1–30% in petrolatum/5% lauric acid) reduced paw edema (A) and heat hyperalgesia (B) measured immediately before (0 h) and 1.5 h after carrageenan injection, and at various times after ARN077 application. Results are expressed as mean ± SEM (n = 6, each group). ** p<0.01 and *** p<0.001 vs. vehicle.
Figure 4.
Effects of intraplantar injection of ARN077 on carrageenan-induced hyperalgesia and edema. ARN077 (0.005–50 g/mouse, dissolved in 80% sterile saline/10% PEG-400/10% TWEEN 80) reduced paw edema (A) and heat hyperalgesia (B), measured immediately before (0 h) and 1.5 h after carrageenan injection, and at various times after ARN077 application. Results are expressed as mean ± SEM (n = 6, each group). * p<0.05, ** p< 0.01 and *** p<0.001 vs. vehicle.
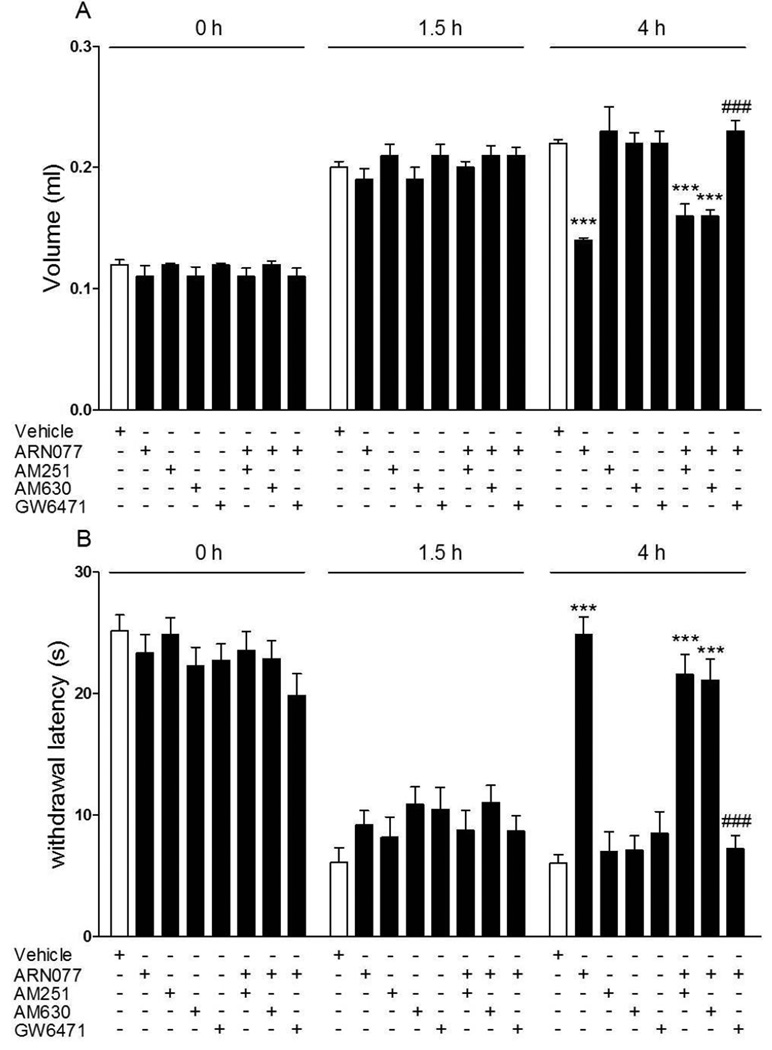
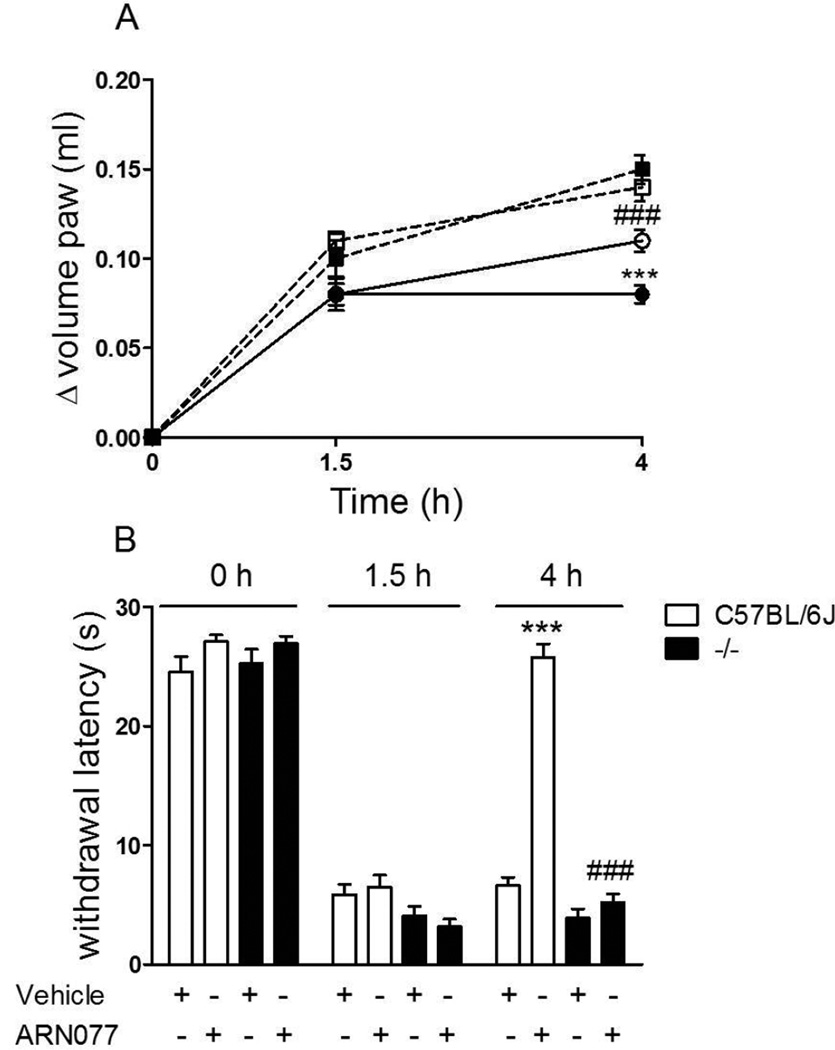
The anti-inflammatory and anti-hyperalgesic effects of topical ARN077 (30%) were prevented by the selective PPAR- antagonist GW6471 [33] (administered by intraplantar injection 30 min before ARN077; 1 g per mouse), but not by the CB1 inverse agonist AM251 or the CB2 antagonist AM630 (administered by intraplantar injection 30 min before ARN077; 1 g per mouse) (Fig. 5 A, B). Initial tests determined that the 1 g per mouse dose of GW6471, used in these studies, was maximally active at reducing the anti-hyperalgesic effect of ARN077 (Supplementary Fig. 1). Furthermore, in C57BL/6J mice lacking PPAR- [13] topical ARN077 (30%) failed to inhibit carrageenan-induced edema (Fig. 6 A) and heat hyperalgesia (Fig. 6 B), whereas it was fully active in wild-type C57BL/6J mice (Fig. 6 A, B).
Figure 5.
The PPAR- antagonist GW6471 blocks the anti-hyperalgesic and anti-inflammatory effects of topical ARN077 in the carrageenan model. Intraplantar administration of GW6471 (1 g, 1 h after carrageenan injection) prevented the effects of topical ARN077 (1–30% in petrolatum/5% lauric acid) on paw edema (A) and heat hyperalgesia (B). Intraplantar administration of the CB1 inverse agonist AM251 or the CB2 antagonist AM630 had no such effect. Edema and hyperalgesia were measured immediately before (0 h) and 1.5 h after carrageenan injection, and at the time of maximal effect of ARN077 (4 h). Results are expressed as mean ± SEM (n = 6 each group). *** p<0.001 vs. vehicle and ### p<0.001 vs. ARN077.
Figure 6.
Effects of ARN077 in wild-type and PPAR- -deficient mice. ARN077 (30%) reduced paw edema (A) and heat hyperalgesia (B) in wild-type C57BL/6J mice, but not in PPAR- -null mice. Edema and hyperalgesia were measured immediately before (0 h) and 1.5 h after carrageenan injection, and at the peak time of 4 h after ARN077 application. Open symbols, wild-type C57BL/6J mice; Closed symbols, PPAR- -deficient mice. Results are expressed as mean ± SEM (n = 6 each group). *** p<0.001 vs. wild-type controls.
3.4. Effects of ARN077 in the mouse CCI model
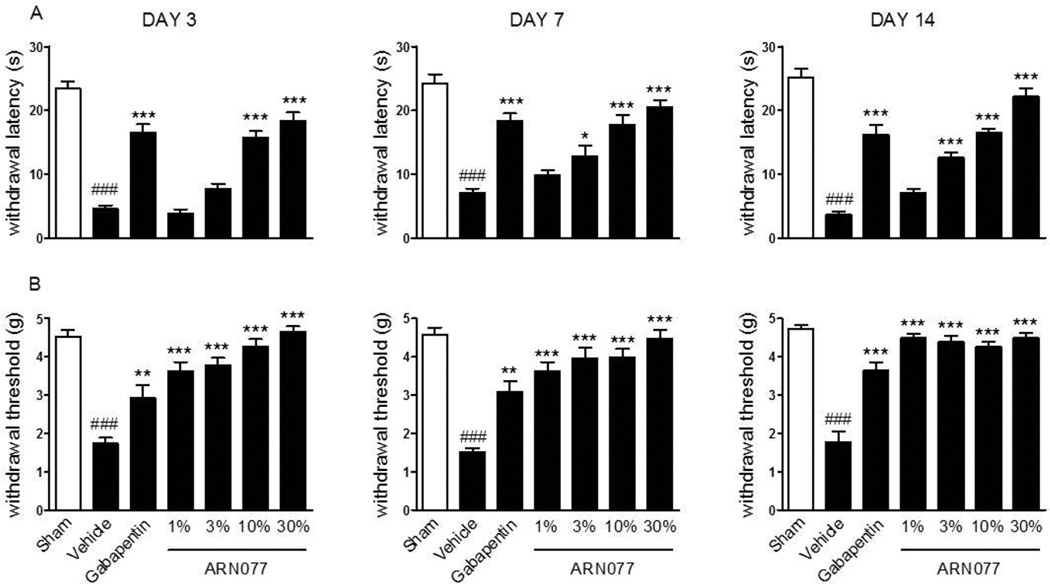
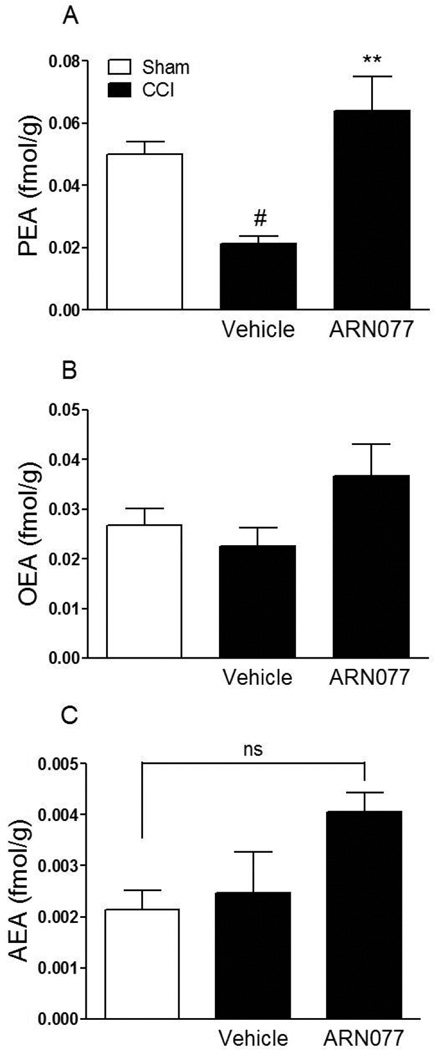
To test the ability of ARN077 to alleviate established chronic pain, we determined whether the compound might influence the persistent hyperalgesia and allodynia associated with injury of the sciatic nerve in CD1 mice. We applied ARN077 (1–30%) on day 3, 7 and 14 following left sciatic nerve ligation, and measured nocifensive behaviors 2 h after each dosing. As shown in Fig. 7, a single application of ARN077 was sufficient to reduce significantly both hyperalgesia and allodynia (P<0.001). Surgical ligation was accompanied by a substantial decrease in PEA content in sciatic nerve tissue (Fig. 8 A). This effect was prevented by treatment with ARN077 (10%), which normalized the levels of PEA in ligated, but not sham-operated mice (Fig. 8 A). Nerve ligation and ARN077 treatment had no statistically detectable effect on OEA or anandamide content (Fig. 8 B, C).
Figure 7.
Antihyperalgesic effects of ARN077 in the chronic nerve constriction model. Topical ARN077 (1–30%) reduced both heat hyperalgesia (A) and mechanical allodynia (B). The effect of gabapentin (50 mg-kg−1, oral gavage) is shown for comparison. ARN077 was administered once daily 3, 7, and 14 days following the ligation. Hyperalgesia and allodynia were measured 1 h after treatment. Results are expressed as mean ± SEM (n = 6, each group). ** p< 0.01 and *** p<0.001 vs. vehicle; ### p<0.001 vs. sham-operated mice.
Figure 8.
Effects of topical application of ARN077 (10%) on sciatic nerve FAE levels in the chronic nerve constriction model. ARN077 (10%) normalized PEA content in mouse ligated nerve (A) and did not change OEA and anandamide content (B, C) 7 days after sciatic nerve ligation. Sciatic nerves are collected 1 hour after topical application of ARN077 on plantar surface of paw. Results are expressed as mean ± SEM (n = 5 each group). ** p<0.01 vs. vehicle-CCI; # p<0.05 vs. sham.
3.5. Effects of ARN077 in the rat UVB model
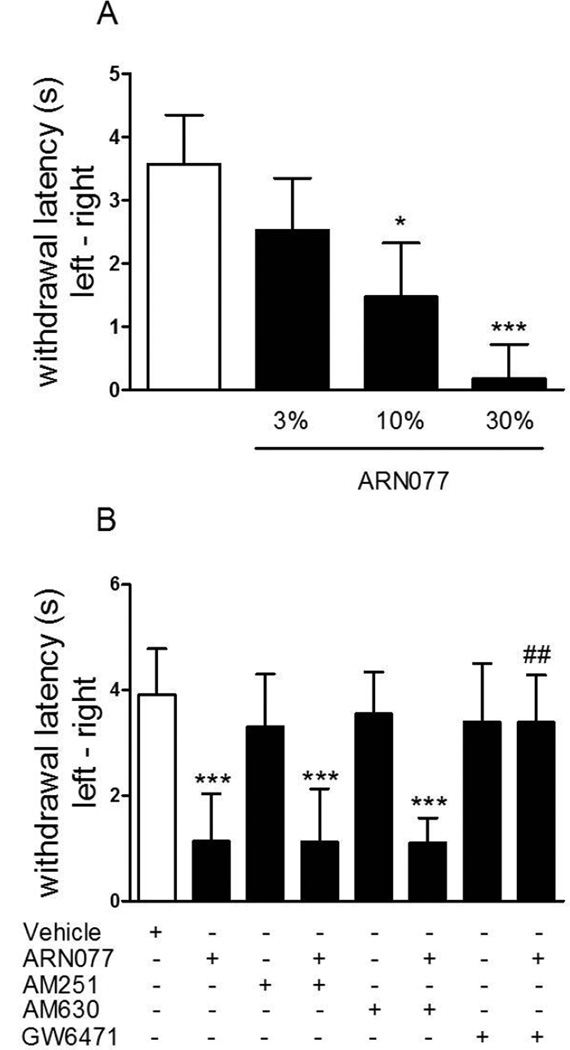
Significant hyperalgesia was achieved in rats by exposing the glabrous skin of the right hind paw to UVB radiation (30 min, 250 mJ/cm2). Topical application of ARN077 (3–30%, 20 h after irradiation) reduced UVB-induced heat hyperalgesia in a dose-dependent manner (Fig. 9 A, P<0.05 for 10% and P<0.001 for 30%). Pre-treatment with the CB1 inverse agonist AM251 and the CB2 antagonist AM630 (each at 60 µg/60 µl/paw failed to reverse the anti-hyperalgesic effect of ARN077, which was completely prevented by the pharmacological blockade of PPAR-(Fig. 9 B).
Figure 9.
Anti-hyperalgesic effects of ARN077 in the UVB model. (A) Topical application of ARN077 (3, 10 and 30%) reduced heat hyperalgesia in a dose-dependent manner. (B) The anti-hyperalgesic effects of ARN077 were blocked by intraplantar administration of the selective PPAR- antagonist GW6471, but not by a selective CB1 (AM251) or CB2 (AM630) antagonist (60 µg/60 µl). Results are expressed as mean ± SEM (n = 6, each group). ** p<0.01 and *** p<0.001 vs. vehicle; ### p<0.001 vs ARN077.
4. Discussion
The present results show that the compound ARN077, a potent and selective NAAA inhibitor recently identified by our group [2, 21], inhibits rat NAAA activity in vitro and protects endogenous FAEs from NAAA-catalyzed degradation in inflamed tissues of live mice. The results further show that ARN077 attenuates hyperalgesic and allodynic states elicited, in mice and rats, by local inflammation or nerve damage. The anti-nociceptive effects of ARN077 are prevented by the selective PPAR- antagonist GW6471 and are absent in PPAR- -deficient mice. We interpret these findings to indicate that ARN077 modulates nociceptive responses in mice and rats by interrupting NAAA-mediated FAE degradation and restoring intrinsic FAE signaling at PPAR-.
ARN077 inhibits rat NAAA with high potency through a mechanism that is rapid, noncompetitive and fully reversed after overnight dialysis. These results are consistent with the possibility that the compound forms a hydrolysable thioester bond with the catalytic cysteine of rat NAAA (Cys131), as recently demonstrated for human NAAA [2]. This interaction appears to be selective, in that ARN077 does not inhibit two lipid amidases that are functionally or structurally related to NAAA: namely FAAH, a serine hydrolase that cleaves various FAEs, including OEA and PEA [9], and acid ceramidase, a cysteine hydrolase acid that shares 33.9–35.2% amino-acid identity with NAAA [32]. Like other -lactone-based NAAA inhibitors, ARN077 is rapidly hydrolyzed in plasma [10, 27]. This instability precludes systemic administration, but not topical application to body surfaces. Indeed, we found that, when applied on mouse skin, ARN077 protects endogenous FAEs from the NAAA-mediated degradation of these compounds elicited by pro-inflammatory stimuli (TPA) or tissue damage (sciatic nerve ligation).
Previous work has shown that local administration of PEA or OEA produces marked antnociceptive effects in mice, which are mimicked by synthetic PPAR- agonists and are absent in mice lacking PPAR- [6, 19, 24, 29]. Moreover prior studies have suggested that PEA and/or OEA levels are reduced in various models of inflammation, and pharmacological blockade of NAAA activity prevents this reduction [27, 34]. We report now that topical ARN077 partially normalizes FAE levels in skin and sciatic nerve tissue, which are reduced by inflammation and surgical ligation, respectively, and attenuates nociception in mice and rats through a mechanism that requires PPAR- activation. A plausible interpretation of these data is that ARN077 regulates nociceptive processing by blocking intracellular NAAA activity in macrophages and, consequently, potentiating FAE-mediated modulation of sensory neuron activity [19]. Consistent with this view, it has been shown that (a) macrophages produce FAEs [5] and display high levels of NAAA expression [32]; (b) primary sensory neurons contain PPAR- [19]; and (c) genetic ablation of this nuclear receptor renders mice more sensitive to visceral and neuropathic pain [23]. Our finding that the nociceptive responses to carrageenan and sciatic nerve ligation are similar between wild-type and PPAR- -null mice may be interpreted to indicate that PPAR-is not involved in regulating pain initiation. It is important to point out, however, that our experiments were not designed to identify differences in nociceptive thresholds. Addressing this point would require a different protocol, in which escalating doses of pain-inducing stimuli are administered in control and PPAR- -null mice. This is not easily accomplished with the models we selected, which were developed to test anti-hyperalgesic (rather than pro-hyperalgesic) properties of drugs. Finally, it is noteworthy that the FAE-PPAR- signaling system is not only present in macrophages and sensory neurons, but also in keratinocytes and other skin cell types [20]. This suggests a mode of action that might involve an intercellular cross-talk between different cells in skin and other tissues.
FAAH inhibitors such as URB597 [16, 30] and URB937 [7] produce significant anti-nociceptive effects in animal pain models [24, 26]. Many of these effects are reversed by antagonizing CB1-or CB2-type cannabinoid receptors, which is consistent with the key role played by FAAH in the intracellular degradation of the endocannabinoid anandamide [7, 24]. Indeed, only a subset of the anti-nociceptive actions of FAAH inhibitors are attenuated by pharmacological blockade of PPAR- [15, 25], which is suggestive that a high degree of spatial segregation exists between anandamide-mediated signaling at cannabinoid receptors, which can be potentiated by FAAH inhibitors, and OEA/PEA-mediated signaling at PPAR- , which is preferentially enhanced by NAAA inhibitors. Future experiments should investigate the respective functional domains of these distinct, but related signaling complexes. Another important question that remains to be addressed pertains to the relative roles played by PEA and OEA in PPAR- -mediated analgesic signaling. Our results show that the pro-inflammatory phorbol ester TPA decreases the levels of both PEA and OEA in skin tissue, whereas surgical ligation lowers only PEA levels in sciatic nerve tissue. The reasons for this discrepancy are unknown at present, but obvious candidates include differences in anatomical setting (skin vs sciatic nerve) and time-course (hours vs days). A comparison with other experimental models of inflammation – such as adjuvant-induced arthritis or antigen-induced dermatitis – may shed light on this important issue. The carrageenan inflammation model, which we used in the present study, does not appear to be suitable in this regard, because it does not allow for the isolation of inflamed paw tissue from the surrounding non-inflamed area, resulting in heterogeneous and unreliable measurements.
In conclusion, the ability of ARN077 to attenuate hyperalgesic and allodynic states that accompany local inflammation and peripheral nerve damage suggests that endogenous FAE-mediated signaling at PPAR- serve an important function in pain regulation. The lipid amidase, NAAA, disables this intrinsic anti-nociceptive mechanism and might thus provide a novel target for analgesic therapy.
Supplementary Material
Supplementary Figure 1. Dose-dependent effects of the PPAR- antagonist GW6471 in the carrageenan model. Intraplantar administration of GW6471 (0.1–1 g, 1 h after carrageenan injection) prevented the effects of topical ARN077 (30% in petrolatum/5% lauric acid) on heat hyperalgesia. Hyperalgesia was measured immediately before (0 h) and 1.5 h after carrageenan injection, and at the time of maximal effect of ARN077 (4 h). Results are expressed as mean ± SEM (n = 6 each group). *** p<0.001 vs. vehicle and ### p<0.001 vs. ARN077.
Summary.
The N-acylethanolamine acid amidase (NAAA) inhibitor, ARN077, exerts profound antinociceptive effects in animal pain models by enhancing endogenous lipid signaling at peroxisome proliferator-activated receptor-α. NAAA may be a new target for analgesic drugs.
Acknowledgements
We thank Clara Albani, Ernest Fung and Opeyemi Oluyemi for help with experiments. This study was partially supported by grants from the National Institutes on Drug Abuse (RO1-DA-012413, DP1 DA031387 to D.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have the following conflicts of interest to report: Daniele Piomelli, Andrea Duranti, Giorgio Tarzia and Marco Mor are inventors in patent applications protecting the structures and uses of NAAA inhibitors.
References
- 1.Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- 2.Armirotti A, Romeo E, Ponzano S, Mengatto L, Dionisi M, Karacsonyi C, Bertozzi F, Garau G, Tarozzo G, Reggiani A, Bandiera T, Tarzia G, Mor M, Piomelli D. β-Lactones inhibit N-1432 acylethanolamine acid amidase by S-acylation of the catalytic N-terminal cysteine. ACS. Med. Chem.Lett. 2012;3:422–426. doi: 10.1021/ml300056y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2755–2767. doi: 10.1016/j.jchromb.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- 6.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 7.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 9.Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 10.Duranti A, Tontini A, Antonietti F, Vacondio F, Fioni A, Silva C, Lodola A, Rivara S, Solorzano C, Piomelli D, Tarzia G, Mor M. N-(2-Oxo-3-oxetanyl)carbamic Acid Esters as N-Acylethanolamine Acid Amidase Inhibitors: Synthesis and Structure-Activity and Structure-Property Relationships. J Med Chem. 2012 doi: 10.1021/jm300349j. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 12.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 13.Guzmán M, Lo Verme J, Fu J, Oveisi F, Blázquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- 14.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 15.Jhaveri MD, Richardson D, Robinson I, Garle MJ, Patel A, Sun Y, Sagar DR, Bennett AJ, Alexander SP, Kendall DA, Barrett DA, Chapman V. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology. 2008;55:85–93. doi: 10.1016/j.neuropharm.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 17.La Rana G, Russo R, D'Agostino G, Sasso O, Raso GM, Iacono A, Meli R, Piomelli D, Calignano A. AM404, an anandamide transport inhibitor, reduces plasma extravasation in a model of neuropathic pain in rat: role for cannabinoid receptors. Neuropharmacology. 2008;54:521–529. doi: 10.1016/j.neuropharm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 19.Lo Verme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 20.Michalik L, Wahli W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochim Biophys Acta. 2007;1771:991–998. doi: 10.1016/j.bbalip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Ponzano S, Bertozzi F, Mengatto L, Dionisi M, Berteotti A, Fiorelli C, Tarozzo G, Reggiani A, Duranti A, Tarzia G, Mor M, Cavalli A, Piomelli D, Bandiera T. Synthesis and Structure–Activity Relationship (SAR) of 2-Methyl-4-2 oxo-3-oxetanyl Carbamic Acid Esters, A Potent Class of N-3 Acylethanolamine Acid Amidase (NAAA) Inhibitors. J Med Chem. 2012 doi: 10.1021/jm400739u. In the press. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J, Murillo-Rodríguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Medina J, Flores JA, Tasset I, Tunez I, Valverde O, Fernandez-Espejo E. Alteration of neuropathic and visceral pain in female C57BL/6J mice lacking the PPAR-α gene. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2662-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Russo R, LoVerme J, La Rana G, D'Agostino G, Sasso O, Calignano A, Piomelli D. Synergistic antinociception by the cannabinoid receptor agonist anandamide and the PPAR-alpha receptor agonist GW7647. Eur J Pharmacol. 2007;566:117–119. doi: 10.1016/j.ejphar.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagar DR, Kendall DA, Chapman V. Inhibition of fatty acid amide hydrolase produces PPAR-alpha-mediated analgesia in a rat model of inflammatory pain. Br J Pharmacol. 2008;155:1297–1306. doi: 10.1038/bjp.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, Moreno-Sanz G, Reggiani A, Piomelli D. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res. 2012;65:553–563. doi: 10.1016/j.phrs.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci U S A. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solorzano C, Antonietti F, Duranti A, Tontini A, Rivara S, Lodola A, Vacondio F, Tarzia G, Piomelli D, Mor M. Synthesis and structure-activity relationships of N-(2-oxo-3-oxetanyl)amides as N-acylethanolamine-hydrolyzing acid amidase inhibitors. J Med Chem. 2010;53:5770–5781. doi: 10.1021/jm100582w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suardíaz M, Estivill-Torrús G, Goicoechea C, Bilbao A, Rodríguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Tarzia G, Duranti A, Tontini A, Piersanti G, Mor M, Rivara S, Plazzi PV, Park C, Kathuria S, Piomelli D. Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J Med Chem. 2003;46:2352–2360. doi: 10.1021/jm021119g. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi K, Zhao LY, Okamoto Y, Araki N, Ueno M, Sakamoto H, Ueda N. Predominant expression of lysosomal N-acylethanolamine-hydrolyzing acid amidase in macrophages revealed by immunochemical studies. Biochim Biophys Acta. 2007;1771:623–632. doi: 10.1016/j.bbalip.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE, McKee DD, Galardi CM, Plunket KD, Nolte RT, Parks DJ, Moore JT, Kliewer SA, Willson TM, Stimmel JB. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPAR-alpha. Nature. 2002;415:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, Piomelli D. Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol Pharmacol. 2011;79:786–792. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dose-dependent effects of the PPAR- antagonist GW6471 in the carrageenan model. Intraplantar administration of GW6471 (0.1–1 g, 1 h after carrageenan injection) prevented the effects of topical ARN077 (30% in petrolatum/5% lauric acid) on heat hyperalgesia. Hyperalgesia was measured immediately before (0 h) and 1.5 h after carrageenan injection, and at the time of maximal effect of ARN077 (4 h). Results are expressed as mean ± SEM (n = 6 each group). *** p<0.001 vs. vehicle and ### p<0.001 vs. ARN077.