Abstract
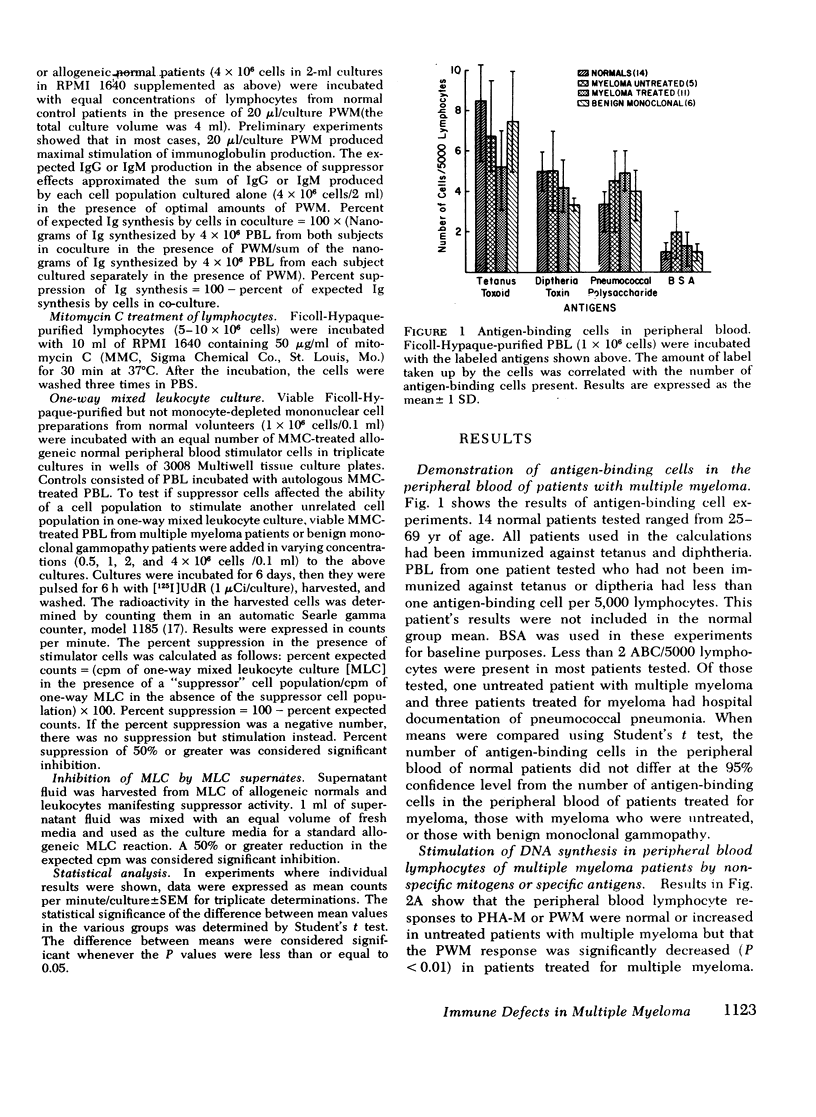
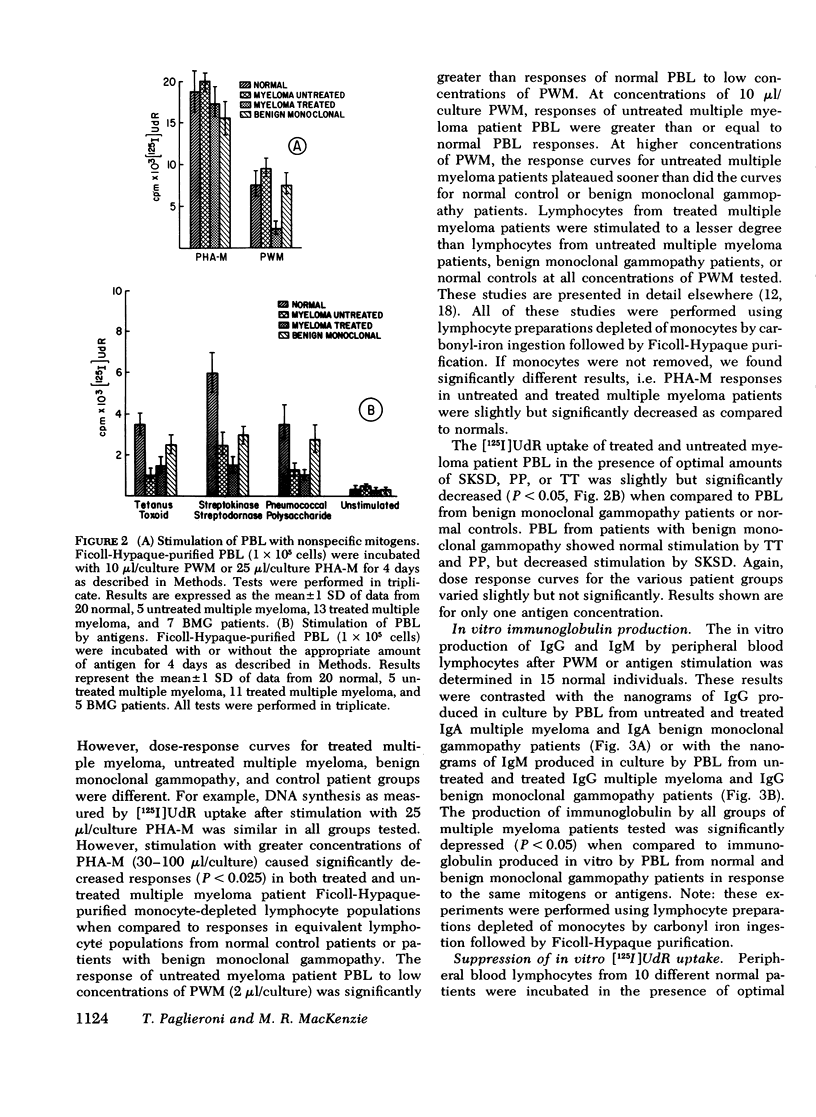
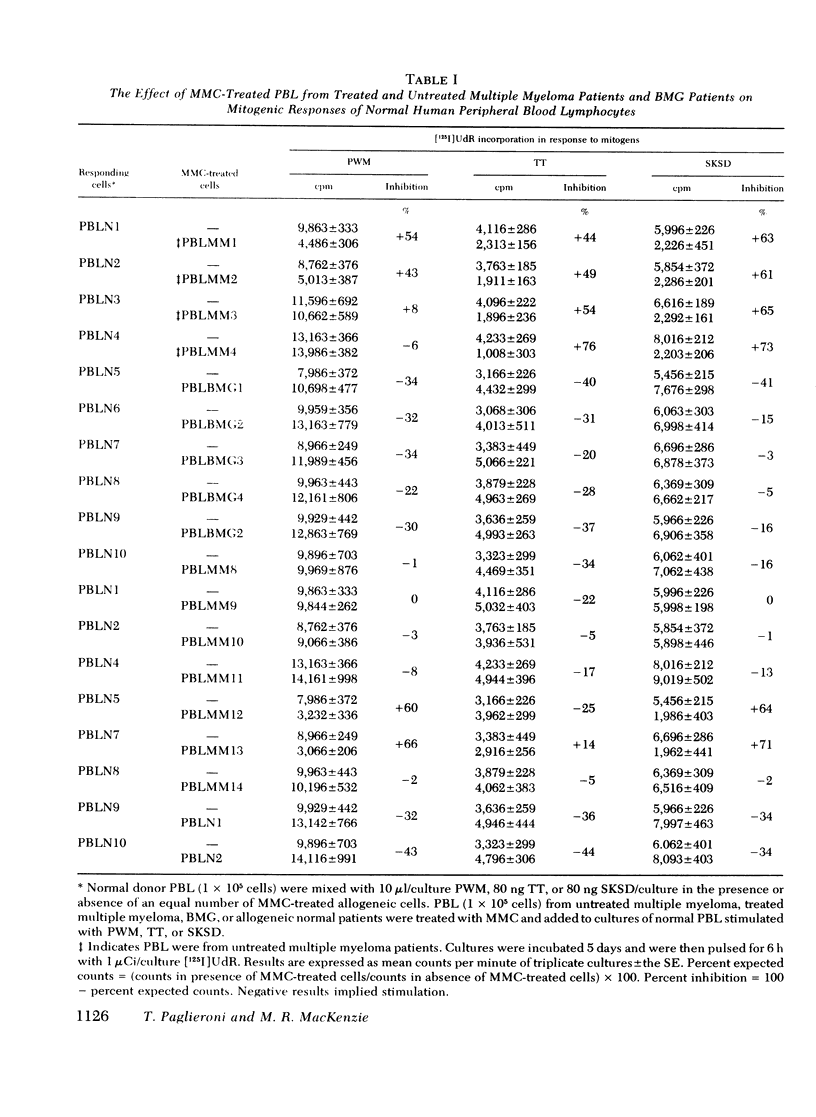
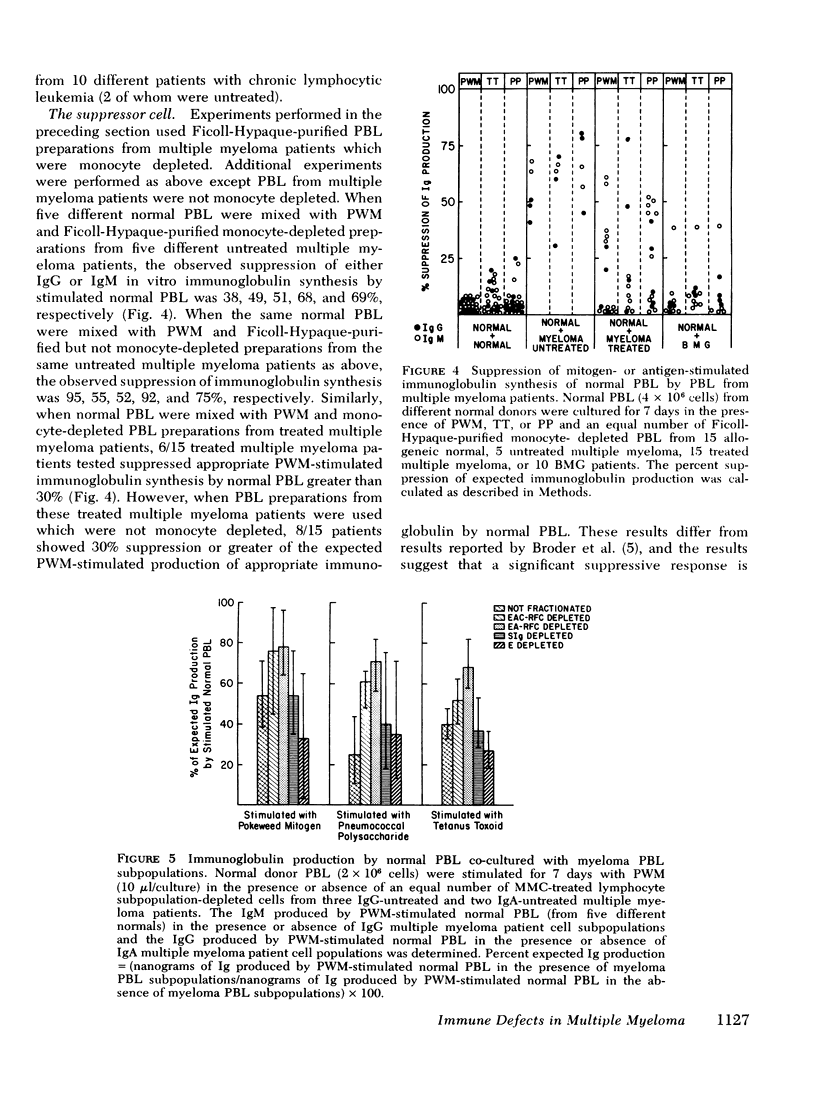
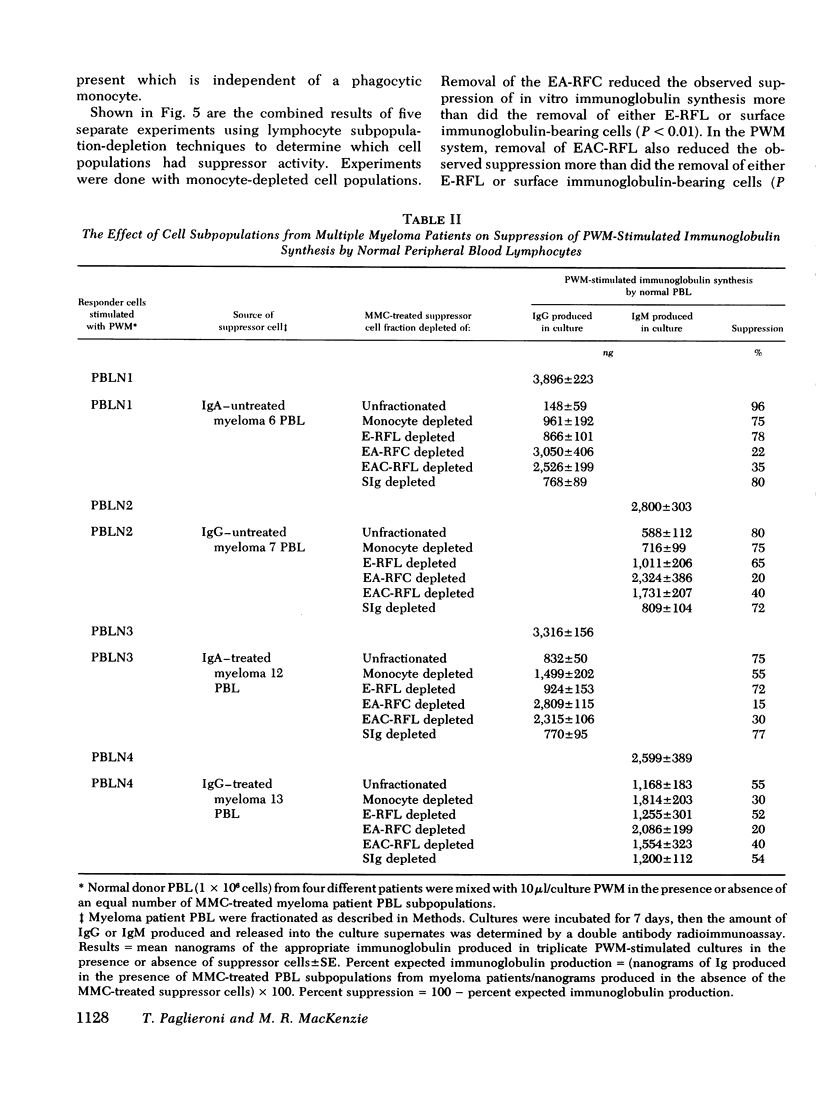
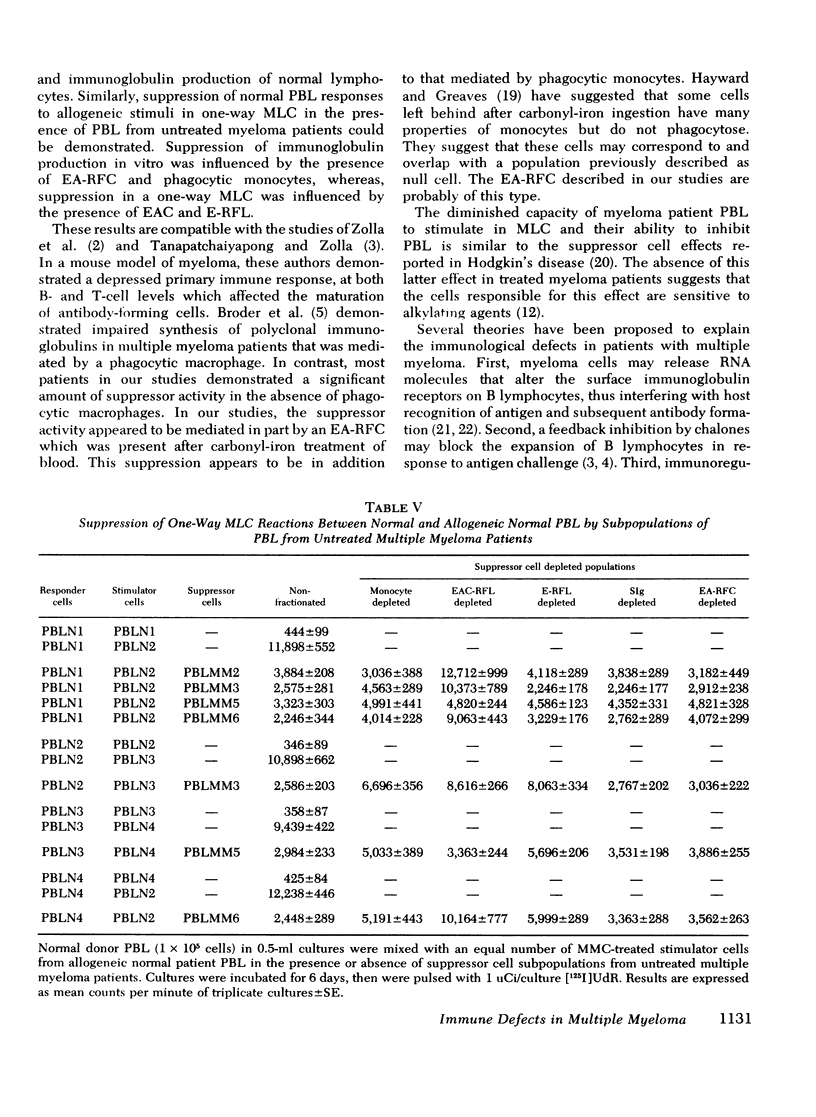
The reduced capacity of patients with multiple myeloma to respond to antigen challenge is well recognized. Response to antigen involves antigen recognition, cell proliferation, and synthesis and secretion of antibody. This study examines this sequence of events in peripheral blood lymphocytes from untreated and treated patients with myeloma, from individuals with benign monoclonal gammopathy, and from normal healthy donors. Antigen-binding capacity was assessed by testing the ability of lymphocytes to bind radio-labeled pneumococcal polysaccharide, tetanus toxoid, or diptheria toxin. The in vitro proliferative response to these antigens as well as to pokeweed mitogen and streptokinase-streptodornase was evaluated. The secretion of immunoglobulin in response to pneumococcal polysaccharide, tetanus toxoid, and pokeweed mitogen by 2-4 × 106 lymphocytes in 7-day cultures was determined. The effects of coculture of myeloma peripheral blood lymphocytes and normal peripheral blood lymphocytes on immunoglobulin production and mixed leukocyte reactions were explored. All myeloma patients had normal numbers (3-8/5,000 cells) of antigen-binding cells. However, most showed a diminished antigen-induced blast transformation as measured by uptake of [125I]5-iodo-2′-deoxyuridine in culture. Immunoglobulin production in response to specific antigen in myeloma lymphocytes was 30-80% less than in normal lymphocytes. Immunoglobulin synthesis and mixed leukocyte responses by normal peripheral blood lymphocytes could be suppressed by myeloma lymphocytes. Multiple suppressor populations were present. Thus, the immune defect in myeloma is beyond the antigen recognition step and involves both the proliferation of antigen-sensitive cells and immunoglobulin production. Further suppressive effects are imposed on normal cells, implying defects in immunoregulation in this disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Voynow N. K. One-way stimulation in mixed leukocyte cultures. Science. 1966 Jul 29;153(3735):545–547. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- CONE L., UHR J. W. IMMUNOLOGICAL DEFICIENCY DISORDERS ASSOCIATED WITH CHRONIC LYMPHOCYTIC LEUKEMIA AND MULTIPLE MYELOMA. J Clin Invest. 1964 Dec;43:2241–2248. doi: 10.1172/JCI105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bhoopalam N., Yakulis V., Heller P. Changes in lymphocyte surface immunoglobulins in myeloma and the effect of an RNA-containing plasma factor. Ann Intern Med. 1975 Nov;83(5):625–631. doi: 10.7326/0003-4819-83-5-625. [DOI] [PubMed] [Google Scholar]
- Eggers A. E., Wunderlich J. R. Suppressor cells in tumor-bearing mice capable of nonspecific blocking of in vitro immunization against transplant antigens. J Immunol. 1975 May;114(5):1554–1556. [PubMed] [Google Scholar]
- Froland S. S., Wisloff F., Michaelsen T. E. Human lymphocytes with receptors for IgG. A population of cells distinct from T- and B-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):124–138. doi: 10.1159/000231207. [DOI] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Giacomoni D., Yakulis V., Wang S. R., Cooke A., Dray S., Heller P. In vitro conversion of normal mouse lymphocytes by plasmacytoma RNA to express idiotypic specificities on their surface characteristic of the plasmacytoma immunoglobulin. Cell Immunol. 1974 Mar 30;11(1-3):389–400. doi: 10.1016/0008-8749(74)90037-9. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunity to murine sarcoma virus-inducted tumors. II. Suppression of T cell-mediated immunity by cells from progressor animals. J Immunol. 1974 May;112(5):1826–1838. [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Hayward A. R., Greaves M. F. Identification of cells with monocyte markers in panhypogammaglobulinaemia. Scand J Immunol. 1975 Sep;4(5-6):563–570. doi: 10.1111/j.1365-3083.1975.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Some aspects of the immune defense against cancer. I. In vitro studies on animal tumors. Cancer. 1971 Nov;28(5):1266–1268. doi: 10.1002/1097-0142(1971)28:5<1266::aid-cncr2820280525>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Pellegrino A., Reisfeld R. A. A rapid microtechnique for in vitro stimulation of human lymphocytes by phytohemagglutinin. Clin Immunol Immunopathol. 1973 Nov;2(1):67–73. doi: 10.1016/0090-1229(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovics J. G. Letter: Suppressor cells and human malignant disease. Br Med J. 1976 May 1;1(6017):1072–1073. doi: 10.1136/bmj.1.6017.1072-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitler L. E., Spath P., Petz L., Cooper N., Fudenberg H. H. Phagocytes and C4 in paraproteinaemia. Br J Haematol. 1975 Feb;29(2):279–292. doi: 10.1111/j.1365-2141.1975.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Tanapatchaiyapong P., Zolla S. Humoral immunosuppressive substance in mice bearing plasmacytomas. Science. 1974 Nov 22;186(4165):748–750. doi: 10.1126/science.186.4165.748. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zolla S., Naor D., Tanapatchaiyapong P. Cellular basis of immunodepression in mice with plasmacytomas. J Immunol. 1974 Jun;112(6):2068–2076. [PubMed] [Google Scholar]


