Abstract
Objective
To investigate the effect of a resin infiltrant on the surface microhardness and roughness of healthy enamel and, as a subsidiary aim, to compare it with a fissure sealant.
Materials and methods
Twenty freshly extracted premolars were used. Sound enamel surfaces were treated with a resin infiltrant (Icon) or fissure sealant (Seal-Rite). The average roughness (Ra, μm) of the specimens was measured with a profilometer (Surtronic 10 Tylor Hobson). Surface hardness was determined by utilizing Vicker’s surface hardness (VHN) with a Micromet II Microhardness tester. Each specimen acted as its own control. Data were analyzed with 2-way analysis of variance (ANOVA), and mean values were compared with independent t-test. All analyses were performed with the SPSS program version 16 (USA). Differences with a P-value of ⩽0.05 were considered statistically significant.
Results
Comparison of enamel surfaces before and after application of resin infiltrant revealed no significant differences in surface hardness; however, enamel surfaces treated by infiltrant showed significantly higher VHN (244.0 ± 79.8) values than those treated with fissure sealant (37.5 ± 14.2). Surface roughness did not differ before and after application of either material to sound enamel. Enamel surfaces treated with fissure sealant (5.3 ± 1.4) were significantly smoother than those treated with infiltrant (6.9 ± 2.0).
Conclusion
Within the limitations of the study, the results showed that enamels treated with the resin infiltrant showed approximately the same microhardness and surface roughness as sound enamel, indicating that this material might be suitable for the treatment of enamel subsurface lesions.
Keywords: Icon, Surface roughness, Surface hardness, Resin infiltration, Fissure sealant, Seal-Rite
1. Introduction
Dental caries is one of the most common chronic diseases worldwide. Different approaches to caries removal have been attempted through the years, starting from the use of a hand drill, which was surpassed in 1871 by James Morison’s treadle instrument (Siegel and Von Fraunhofer, 1998). Other procedures for caries removal include air abrasion, atraumatic restorative therapy, chemo-mechanical caries removal (CMCR), and laser. Today, conventional caries treatment usually involves use of a high-speed handpiece to access the lesion and a low-speed handpiece to remove the caries.
CMCR is a noninvasive alternative for the removal of carious dentin. The technique involves application of a chemical solution to the decayed dentinal tissue. The decayed dentine is softened and then scraped off with blunt hand instruments (Beeley et al., 2000; ElKholany et al., 2004; Maragakis et al., 2001; Yazici et al., 2003). Dentin surface formed in this manner is highly irregular and well suited for bonding with composite resin or glass ionomer (Beeley et al., 2000). This system is thought to be useful for the treatment of deciduous teeth, patients with dental phobias, and medically compromised patients (Beeley et al., 2000). The system was originally marketed in the USA in the 1980s as Cavidex (Beeley et al., 2000; ElKholany et al., 2004). However, its efficacy in caries removal had to be improved and its hand instruments were suboptimal: application of the system required a large reservoir with a pump and large quantities of solution (Yazici et al., 2003).
Carisolv, which was developed by Swedish Medi Team, recently was introduced in European markets (Yazici et al., 2003). This system works through the same mode as Cavidex, but overcomes the major shortcomings of its predecessor (Maragakis et al., 2001). Carisolv consists of a 2-component gel and numerous special hand instruments. The gel is a red, highly viscous fluid that utilizes three naturally occurring amino acids (glutamic acid, leucin acid, and lycine) (Elkholany et al., 2004; Yazici et al., 2003). It also contains sodium chloride, erythrocin, water, sodium hydroxide, and a transparent fluid consisting of a low concentration of sodium hypochlorite (Yazici et al., 2003). When the gel and fluid are mixed in the syringe and applied to the decayed dentinal tissue, the mixture softens the decayed dentine, allowing it to be scraped off with blunt hand instruments. The partially degraded collagen in carious dentin is chlorinated by the chemo-mechanical caries removal solution (Maragakis et al., 2001). This technique has the advantages of adhesive bonding and compatibility with both soft tissues and restorative materials (Elkholany et al., 2004). However, some disadvantages have been associated with this method. In particular, Yazici et al. (2003) suggested that a conventional rotary instrument (bur) is more effective than Carisolv in the removal of carious tissue. The lengthy procedure of CMCR is another drawback (Elkholany et al., 2004).
Treatment of caries requires the understanding that caries development is a dynamic process involving periods of demineralization and remineralization (Silverstone, 1977). Enamel remineralization has been studied for about 100 years. Noninvasive treatment of early caries lesions by remineralization may represent a major advance in clinical management of the disease (Reynolds, 2008). During the development of subsurface caries lesions, minerals are dissolved out of the enamel, resulting in increased porosities that appear clinically as so-called “white-spot” lesions (Ten Cate et al., 2003). Such lesions result from the dissolution of calcium hydroxyl apatite from the enamel and the production of microporosities within the remaining calcified tissue (Robinson et al., 1995). These lesions are commonly treated by enhancing remineralization, e.g., through improved oral hygiene, fluoridation (Paris et al., 2007b), or other processes (Gray and Shellis, 2002).
Different products have been launched in the market since 2000 that rely on the calcium phosphate remineralization system. Their effect is mainly based on enhancement of the natural remineralization capacity of saliva. This technology could help some patients, but is not generally recommended (Cury and Tenuta, 2009), nor is it a solution to the problem of controlling caries disease (Reynolds, 2008). A promising alternative therapy for the arrest of caries lesion might be the infiltration of low-viscosity light-curing resins into the subsurface lesion. Because the porosities of enamel caries act as diffusion pathways for acids and dissolved minerals, infiltration of these lesions with resin might occlude the pathways, leading to the arrest of caries progression (Gray and Shellis, 2002; Paris et al., 2007b). However, the restoration of enamel and initial dentinal lesions is associated with an unfavorable benefit/damage ratio. Moreover, restorations possess a limited life span.
Sealing of initial enamel lesions with resins might be a promising approach, as suggested by results with the fissure sealing technique (Simonsen, 1991), in which a barrier between the lesion and source of acid production is established. The fissure sealing concept has been extended to smooth enamel surfaces (Schmidlin and Besek, 2003). A recently designed adhesive patch to seal smooth enamel surfaces claims to protect enamel from chemo-mechanical challenge significantly better than a double layer of unfilled resin (Shmidlin et al., 2005). This adhesive patch is reported to be associated with a clinically acceptable surface roughness and, therefore, merits further clinical investigation (Shmidlin et al., 2006).
By means of a new virtually painless method, the caries infiltration product Icon was introduced in Germany in 2009. This product utilizes a special resin to fill and seal diseased enamel, with no unnecessary loss of healthy hard tissue (Drilling no thanks, 2009). Icon is an innovative product for the microinvasive treatment of early cariogenic lesions in the approximal and vestibular regions. It can be used to treat caries in a timely manner without drilling. The approximal version of the product is specially developed for hard tissues, preserving treatment of incipient proximal caries; the vestibular version is particularly suited for orthodontic patients after braces removal. To our knowledge, only a few studies have been conducted regarding Icon, and those have shown promising results (Paris et al., 2007a). Therefore, the aim of the present study was to investigate the effect of Icon on the surface microhardness and roughness of healthy enamel and, as a subsidiary aim, to compare it with that of a fissure sealant.
2. Materials and methods
2.1. Materials
The materials tested in this study were Icon – Smooth Surface (resin infiltrant) (DMG, Germany) and Seal-Rite (pit and fissure sealant) (Pulpdent, USA). The compositions and instructions for these materials are shown in Table 1.
Table 1.
Composition and manufacturer’s instructions of tested materials.
| Material | Icon® caries infiltrant | Seal-RiteTM fissure sealant |
|---|---|---|
| Manufacturer | DMG – Hamburg, Germany | Ultradent Products Inc., Pulpdent, USA |
| Composition |
|
|
| Directions of usage |
|
|
| Batch no. | 626382 | 063 |
| 090403 |
2.2. Sample preparation
A total of 20 caries-free human premolar teeth that had been extracted for orthodontic purposes were thoroughly cleaned using slurry pumice and a prophylaxis brush in a contra-angled handpiece. Teeth used in this study were collected and stored in a thymol solution (0.025%) until the day of measurement. Only teeth with no cracks, restorations, or developmental lesions were selected.
Roots of teeth were removed and the crowns were sectioned longitudinally in a mesio-distal direction by using a Buehler Isomet 2000 Precision Saw with profuse water irrigation. A total of 40 specimens were embedded in ortho resin, such that the crown was projected and ensuring that the convex smooth tooth surfaces were as parallel to the scanning stage as possible. All specimens were stored in distilled water after the measurements were made.
Two groups of 20 specimens were measured for their Vicker’s surface hardness (VHN) with a Micromet II Microhardness tester (Item code 80355, Buehler, Lakebluff, Illinois, USA) and for their surface roughness with a surface profilometer (Surtronic 10, Ra, Rank Tylor Hobson Ltd., Leicester, England in Denmark, serial #112/1540-1243513). Each specimen acted as its own control.
The 20 specimens in each of the two main groups were subdivided into two subgroups: subgroup 1 (n = 10) treated with Icon caries infiltrant and subgroup 2 (n = 10) treated with Seal-Rite fissure sealant. Measurements were taken before and after application of the materials. The resin infiltrant and fissure sealant were applied to the teeth according to manufacturer’s instructions (Table 1). Both materials were polymerized with an Elipar Highlight (ESPE, Germany) with 400–500 nm, and the intensity was measured throughout the experiment with an Optilux Radiometer (Kerr Corp., Danbury, CT).
2.3. Measurement of enamel surface hardness
Surface hardness measurements were made by using a microscope with 200× magnification. The test was performed by applying a load of 300 g to the specimens for 15 s. The load and time were constant for all samples throughout the study. Measurements after surface treatments of both materials were delayed for 24 h while the teeth were kept in an incubator in distilled water at 37 °C. Three indentations were made in each specimen before and after surface treatment. To ensure accuracy of the measurements, indentations were done on the flattest points of the enamel surface.
2.4. Measurement of enamel surface roughness
Surface roughness was characterized by the average roughness (Ra), which represents the arithmetical average value of all absolute distances of the roughness profile from the center line within the measuring length (Whitehead et al., 1995). Three readings were recorded on each surface. The cut-off value (distance transversed by the stylus over which the data were collected) for surface roughness was 0.8 mm, and the traversing distance of the stylus was 5.0 mm. The radius of the tracing diamond tip was 0.5 μm, measuring force was 10 mN, and speed was 2 mm/s. The machine was calibrated after every five samples to ensure reliable readings. After evaluation, specimens were sputtered with gold (Polaron E-5200 Energy Beam Sciences, Agawan, MA) and examined by scanning electron microscopy (SEM; JSM, 6360LV, JEOL, Tokyo, Japan).
2.5. Statistical analysis
For each measured parameter, mean values were calculated before and after either treatment (infiltrant or fissure sealant) and were compared with an independent t-test. All analyses were performed with the SPSS program version 16 (USA). Differences with a P-value of ⩽0.05 were considered statistically significant.
3. Results
The numerical analyses of the hardness and roughness of the enamel surfaces are presented in Table 2. No significant differences in the enamel surfaces were found before and after application of the resin infiltrant (Fig. 1). Application of the fissure sealant significantly decreased the VHN of the enamel surface (37.5 ± 14.2) (382.6 ± 71.5). Enamel surfaces treated with the resin infiltrant showed significantly higher VHN (246.4 ± 123.9) than those treated with fissure sealant (37.5 ± 14.2) (Fig. 1).
Table 2.
Mean, SD and P value of tested materials for both measurements.
| Materials tested | Surface hardness (kg/mm2) |
Surface roughness (μm) |
||
|---|---|---|---|---|
| VHN ± SD | P value | Ra ± SD | P value | |
| Control Icon | 290.6 ± 74.7 | .100 | 7.2 ± 2.8 | .662 |
| Icon® | 246.4 ± 123.9 | 6.9 ± 2.0 | ||
| Control Seal-Rite™ | 382.6 ± 71.5 | .000 | 6.3 ± 2.6 | .070 |
| Seal-Rite™ | 37.5 ± 14.1 | 5.3 ± 1.4 | ||
| Icon® | 246.4 ± 123.9 | .000 | 6.9 ± 2.0 | .001 |
| Seal-Rite™ | 37.5 ± 14.1 | 5.3 ± 1.4 | ||
Figure 1.
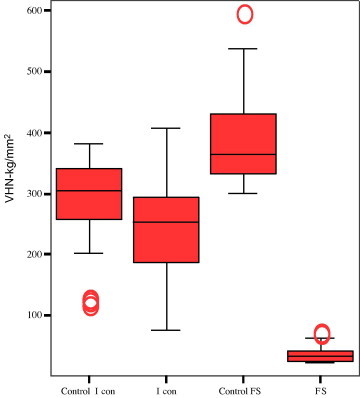
The mean surface hardness (VHN) of tested materials (box and whisker plots with quartiles).
No significant difference in surface roughness was found between before and after application of either material to sound enamel (Fig. 2). Significantly rougher surface values were recorded for surfaces treated with infiltrant (6.9 ± 2.0) compared to those treated with fissure sealant (5.3 ± 1.4) (Fig. 2). The SEM evaluation revealed clearly visible enamel surface alterations after application of either material (Fig. 3A–C).
Figure 2.
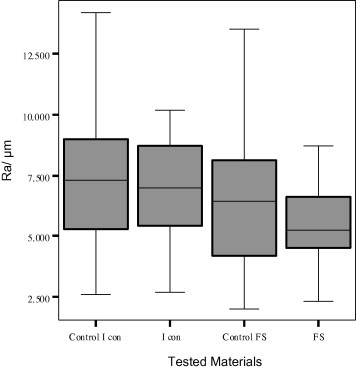
The mean surface roughness (Ra) of tested materials (box and whisker plots with quartiles).
Figure 3.
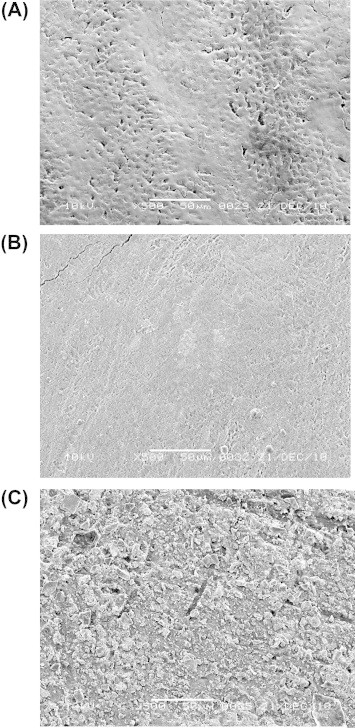
SEM pictures at 500× magnification. Representative photographs of the enamel surface, (A) pre-operative view, (B) following the application of Icon®, (C) following the application of Seal-Rite™.
4. Discussion
Arresting of enamel lesions by infiltration with composite resins and penetration of adhesives into previously demineralized enamel seem to be promising approaches for the nonoperative treatment of carious lesions (Mueller et al., 2006). Treatment with fissure sealant relies upon maintenance of an intact margin between the sealant and the tooth (Mueller et al., 2006). A resin layer on top of the lesion is not required to accomplish this goal, if the lesion body is homogenously infiltrated with resin (Mueller et al., 2006). Paris et al. (2006), who wiped away the overlying resin before curing the sealants in their study, concluded that leaving excessive material could actually be disadvantageous clinically, because sealant margins and even a thin excess of resin material could provide retention sites for plaque and caries (Paris et al., 2006).
In the current study, the fissure sealant was applied with a brush and was evenly distributed on the surface without wiping it away. The recorded VHN in the fissure sealant group was significantly lower than those of the control and resin infiltrant groups (Table 2). This finding could be attributed to the resin layer remaining on the top of the enamel surface. Insufficient material penetration and material viscosity reportedly have an adverse effect on the success of fissure sealing (Francescut and Lussi, 2006; Irinoda et al., 2000).
In contrast to fissure sealing, in which a diffusion barrier is placed on top of the lesion surface, the infiltration technique aims to create a diffusion barrier inside the lesion, by replacing lost minerals with resin (Paris et al., 2007b). In the present study, VHN values of the infiltrant group were significantly higher than those of the fissure sealant group. Icon infiltrant is a methacrylate-based resin matrix containing BISGMA and TEGDMA, whereas Seal-Rite contains 34.4% UDMA. This difference in composition might explain the different surface hardness readings between the materials. Hydrochloric acid gel erodes the surface layer more effectively than 37% phosphoric acid. Use of longer acid conditioning with Icon (2 min with hydrochloric acid) could have led to deeper resin penetration than etching with phosphoric acid gel (Paris et al., 2007b).
Icon-dry (which contains 99% ethanol) was applied for 30 s prior to application of the infiltrant. Addition of ethanol increases the penetration coefficient by decreasing the viscosity and contact angle (Paris et al., 2007a). Mixtures containing large amounts of HEMA, TEGDMA, and ethanol are associated with high penetration coefficients and satisfactory hardening; therefore, they might be promising tools for rapid caries penetration (Paris et al., 2007a). A previous study found optimal results when using a short (5 s) etch with 36% phosphoric acid, dehydration with absolute ethanol for 2 min, and application of multiple layers of bounding resin (Gray and Shellis, 2002).
Although a fissure sealant releases fluoride, its surface should be sufficiently smooth so as not to accumulate substrates and microorganisms (Aranda and García-Godoy, 1995). Shmidlin et al. (2006) assessed the surface roughness of a newly devised adhesive patch as a smooth surface sealant. The patch was composed of methacrylic groups and contained elastic, cross-linked, urethane-based polymer material. The patch had a clinically acceptable surface roughness, and is of considerable interest in the ongoing search for a controllable application technique of sealants to smooth enamel surfaces. The fissure sealant used in the present study showed good roughness results, with a lower Ra than those of the control and infiltrant groups. This result might be related to the remaining resin layer on top of the enamel surface. The infiltrant group demonstrated no significant difference in Ra compared to the control group. Similar results were found by Burgess and Cakir (2009), who found that caries infiltration did not lead to an increased surface roughness of infiltrated lesions compared to sound enamel.
The SEM results demonstrated that infiltrant application to the enamel surface had sealed the enamel porosities and the resulting product appeared smooth, whereas the sealant appeared to be projecting from the sealed surface. Those observations might explain the recorded surface hardness and roughness values of enamel when treated with the tested materials. However, surface roughness in vitro may be quite different when compared to the dynamic complex biological system in the oral cavity in vivo. Thus, direct extrapolations to clinical conditions must be exercised with caution.
The current experimental conditions appeared suitable to test the initial behavior of the resin infiltration material for in vivo screening. However, the study conditions described in this report differ from the in vivo situation. In particular, we did not address the roles of saliva, erosive and abrasive challenges, or resin expansion and contraction by thermal cycling.
5. Conclusion
Within the limitations of the study, it can be concluded that the microhardness of the enamel surface treated with Icon was approximately the same as that of sound enamel. The treated enamel showed a clinically acceptable surface roughness, indicating that this infiltrant might be suitable for the treatment of enamel subsurface lesions. Enamel surfaces treated with fissure sealant showed significantly lower values than those treated with resin infiltrant.
Ethical Statement
There is no ethical issue regarding this study.
Conflict of interest
No conflict of interest declared.
Acknowledgments
This research was conducted at the CDRC lab as non-funded research no. 2218. The authors would like to express their thanks to Prof. Mohammad T. Bukhary for his cooperation, Mr. Nassr Almaflehi for carrying on the statistics of the study and Mr. Bong Tuazon for his help with the samples’ preparation and assistance.
Contributor Information
Nadia Malek Taher, Email: nmataher@hotmail.com.
Haifa Abdulrahman Alkhamis, Email: Alkhamis.haifa@gmail.com.
Sarah Mesha’l Dowaidi, Email: Sarah.dowaidi@gmail.com.
References
- Aranda M., García-Godoy F. Clinical evaluation of the retention and wear of a light-cured pit and fissure glass ionomer sealant. J. Clin. Pediatr. Dent. 1995;19(4):273–277. [PubMed] [Google Scholar]
- Beeley J.A., Yip H.K., Stevenson A.G. Chemomechanical caries removal: a review of the techniques and latest developments. Br. Dent. J. 2000;188:427–430. doi: 10.1038/sj.bdj.4800501. [DOI] [PubMed] [Google Scholar]
- Burgess J.O., Cakir D. DMG; Hamburg, Germany: 2009. Surface Roughness Determination of a Caries Infiltrant Resin. Data on file. [Google Scholar]
- Cury J.A., Tenuta L.M. Enamel remineralization: controlling the caries disease or treating early caries lesions? Braz. Oral Res. 2009;23:23–30. doi: 10.1590/s1806-83242009000500005. [DOI] [PubMed] [Google Scholar]
- Elkholany N.R., Abdelaziz K.M., Zaghloul N.M., Aboulenine N. Chemo-mechanical method: a valuable alternative for caries removal. Dental News. 2004;11(3):16–22. [Google Scholar]
- Francescut P., Lussi A. Performance of a conventional sealant and a flowable composite on minimally invasive prepared fissures. Oper. Dent. 2006;31:543–550. doi: 10.2341/05-91. [DOI] [PubMed] [Google Scholar]
- Gray G.B., Shellis P. Infiltration of resin into white spot caries-like lesions of enamel: an in vitro study. Eur. J. Prosthodont. Restor. Dent. 2002;10:27–32. [PubMed] [Google Scholar]
- Irinoda Y., Matsumura Y., Kito H., Nakano T., Toyama T., Nakagaki H. Effect of sealant viscosity on the penetration of resin into etched human enamel. Oper. Dent. 2000;25:274–282. [PubMed] [Google Scholar]
- Maragakis G.M., Hahn P., Hellwig E. Chemomechanical caries removal: comprehensive review of the literature. Int. Dent. J. 2001;51:291–299. doi: 10.1002/j.1875-595x.2001.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Mueller J., Meyer-Lueckel H., Paris S., Kielbassa A.M. Inhibition of lesion progression by penetration of resins in vitro: influence of the application procedure. Oper. Dent. 2006;31:338–345. doi: 10.2341/05-39. [DOI] [PubMed] [Google Scholar]
- Paris S., Meyer-Lueckel H., Mueller J., Hummel M., Kielbassa A.M. Progression of sealed initial bovine enamel lesions under demineralising conditions in vitro. Caries Res. 2006;40:129–134. doi: 10.1159/000091058. [DOI] [PubMed] [Google Scholar]
- Paris S., Meyer-Lueckel H., Colfen H., Kielbassa A.M. Penetration coefficients of commercially available and experimental composites intended to infiltrate enamel carious lesions. Dent. Mater. 2007;23:742–748. doi: 10.1016/j.dental.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Paris S., Meyer-Lueckel H., Kielbassa A.M. Resin infiltration of natural caries lesions. J. Dent. Res. 2007;86(7):662–666. doi: 10.1177/154405910708600715. [DOI] [PubMed] [Google Scholar]
- Reynolds E.C. Calcium phosphate-based remineralization systems: scientific evidence? Aust. Dent. J. 2008;53(3):268–273. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Weatherell J.A., Kirkham J. The chemistry of dental caries. In: Robinson C., Kirkham J., Shore R.C., editors. Dental Enamel Formation to Destruction. CRC Press; New York: 1995. pp. 223–243. [Google Scholar]
- Schmidlin P.R., Besek M.J. Atraumatic tooth separation and proximal sealing: filling the gap between preventive and restorative dentistry. Pract. Proced. Aesthet. Dent. 2003;15(1):65–69. [PubMed] [Google Scholar]
- Shmidlin P.R., Zehnder M., Zimmermann M.A., Zimmermann J., Roos M., Roulet J.F. Sealing smooth enamel surfaces with a newly devised adhesive patch: a radiochemical in vitro analysis. Dent. Mat. J. 2005;21(6):545–550. doi: 10.1016/j.dental.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Schmidlin P.R., Gohring T.N., Roos M., Zehnder M. Wear resistance and surface roughness of a newly devised adhesive patch for sealing smooth enamel surfaces. J. Oper. Dent. 2006;31:115–121. doi: 10.2341/04-202. [DOI] [PubMed] [Google Scholar]
- Silverstone L.M. Remineralization phenomena. J. Caries Res. 1977;11(Suppl.):59–84. doi: 10.1159/000260296. [DOI] [PubMed] [Google Scholar]
- Siegel S.C., Von Fraunhofer J.A. Dental cutting: the historical development of diamond burs. J. Am. Dent. Assoc. 1998;129(6):740–745. doi: 10.14219/jada.archive.1998.0316. [DOI] [PubMed] [Google Scholar]
- Simonsen R.J. Retention and effectiveness of dental sealant after 15 years. J. Am. Dent. Assoc. 1991;122:34–42. doi: 10.14219/jada.archive.1991.0289. [DOI] [PubMed] [Google Scholar]
- Ten Cate J.M., Larsen M.J., Pearce E.I.F., Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O., Kidd E.A.M., editors. Dental Caries. Blackwell Munksgaard; Oxford: 2003. pp. 49–69. [Google Scholar]
- Whitehead S.A., Shearer A.C., Watts D.C., Wilson N.H. Comparison of methods for measuring surface roughness of ceramic. J. Oral Rehabil. 1995;22(6):421–427. doi: 10.1111/j.1365-2842.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Yazici A.R., Atila P., Ozgunaltay G., Muftuoglu S. In vitro comparison of the efficacy of CarisolvTM and conventional rotary instrument in caries removal. J. Oral. Rehabil. 2003;30:1177–1182. doi: 10.1111/j.1365-2842.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- <http://www.drilling-no-thanks.co.uk/upload/files/download//z_downloads_8_ur_icon_mendes_en_2009_08_lay.pdf>.


