Abstract
Cripto is a small, GPI-anchored signaling protein that regulates cellular survival, proliferation, differentiation and migration during normal developmental processes and tumorigenesis. Cripto functions as an obligatory co-receptor for the TGF-β ligands Nodal, GDF1 and GDF3 but attenuates signaling of others such as activin-A, activin-B and TGF-β1. Soluble, secreted forms of Cripto also activate Src, ras/raf/MAPK and PI3K/Akt pathways via a mechanism that remains largely obscure. This review describes the biological roles and signaling mechanisms of Cripto, highlighting our identification of Glucose Regulated Protein 78 (GRP78) as a cell surface receptor/co-factor required for Cripto signaling via both TGF-β and Src/MAPK/PI3K pathways. We discuss emerging evidence indicating that Cripto/GRP78 signaling regulates normal somatic stem cells and their tumorigenic counterparts.
Keywords: TGF-β, Cripto, GRP78, stem cell, plasticity, cancer
1. Introduction
The TGF-β pathway is a ubiquitous regulator of myriad cellular functions including proliferation, differentiation, apoptosis, migration and secretion. TGF-β signaling can have tumor suppressive or oncogenic effects and despite a large body of work, the molecular determinants of which outcome will prevail remain poorly understood. Although simple at its core, consisting of TGF-β ligands, their cognate transmembrane serine kinase receptors and intracellular Smad proteins, the TGF-β pathway is finely tuned by a bewildering array of modulators including ligand traps, co-receptors, inhibitory Smads and Smad-associated transcriptional regulators. Given the importance of the TGF-β pathway under normal physiological conditions and in disease, it is critical to understand the roles and molecular mechanisms of its modulators and to consider them as therapeutic targets.
Cripto (Cripto-1, TDGF1) (Figure 1A) is a small, GPI-anchored protein that modulates the signaling of several TGF-β ligands that signal via the Smad2/3 pathway. Cripto functions as an obligatory cell surface co-receptor for a subset of ligands including Nodal [1,2], GDF1 [3] and GDF3 [4]. This co-receptor function plays essential roles in regulating stem cell differentiation [2,5,6] and vertebrate embryogenesis [1,2,7] and likely regulates normal tissue growth and remodeling in adult tissues [8-11]. Cripto co-receptor function has also been linked to tumor growth since Nodal signaling plays a key role in promoting plasticity and tumorigenicity [12-15]. In contrast to its co-receptor role, Cripto reduces the level of Smad activation by activin-A [16-18], activin-B [18,19] and TGF-β1 [18,20-22], thereby inhibiting the cytostatic effects of these ligands [18,20-22]. Cripto also has signaling activities that are thought to be independent of the TGF-β pathway [7,23]. The best characterized of these is its growth factor-like activity in which soluble/secreted forms of Cripto can lead to Src, ras/raf/MAPK and PI3K/Akt pathway activation [7,23]. Cripto has also been reported to promote signaling by Wnt [24] and Notch [25].
Figure 1. Structural features and interacting partners of Cripto and GRP78.
(A) Diagram of mouse Cripto indicating the locations of the signal peptide (SP), EGF-like domain, CFC domain and GPI-anchor attachment site (GPI). Several TGF-β ligands including Nodal, activin-A and TGF-β1 have been shown to bind to the EGF-like domain of Cripto. The CFC domain independently binds ALK4 and ALK7 and we have shown that it also binds GRP78. Monoclonal antibodies targeting either the EGF-like domain or the CFC domain have been shown to have anti-tumor activity in vivo. (B) Diagram highlighting features shared by GRP78 other Hsp70 family members including the ATP binding and peptide binding domains. Binding sites on GRP78 for Cripto and other extracellular proteins are indicated. N-20, C-20; polyclonal GRP78 antibodies (Santa Cruz Biotechnology, Inc.); Ab39, mAb targeting cell surface GRP78 [130]
In an effort to further elucidate the molecular basis for Cripto signaling, we conducted a screen aimed at identifying novel Cripto interacting proteins [21]. This led to the identification of GRP78 (Figure 1B), an HSP70 family member and ER chaperone best known for its coordination of the unfolded protein response (UPR) [26]. Although GRP78 is primarily targeted to the ER, a fraction of the protein is localized to the plasma membrane where it has been shown to have receptor-like function associated with increased cellular proliferation and survival [27-29]. Cell surface Cripto/GRP78 interaction is required for Cripto modulation of activin/Nodal/TGF-β signaling and activation of Src/MAPK/PI3K pathways [18,21]. This review provides an overview of Cripto function during development and oncogenesis, focusing on its roles as a regulator of the TGF-β pathway and its requirement for GRP78 as a cell surface signaling partner. We propose that targeting the Cripto/GRP78 complex represents a promising therapeutic strategy for the treatment of human tumors.
2. TGF-β pathway overview
The TGF-β superfamily contains over 30 secreted ligands in humans [30] and includes the activin [31], TGF-β [32], bone morphogenetic protein (BMP) [33] and Nodal-related families [1,2]. These ligands control a wide array of cellular processes including proliferation, homeostasis, differentiation, tissue morphogenesis, immune responses, angiogenesis, wound repair and endocrine function [31-35]. Disruption or dysregulation of TGF-β ligand signaling is associated with multiple pathological states including tumor growth and metastasis [36].
TGF-β superfamily members exert their biological effects by interacting with two classes of transmembrane receptors possessing serine/threonine kinase activities that are referred to as type I and type II [37]. Following the characterization of the first vertebrate receptor serine/threonine kinase by our group, the activin type II receptor (ActRII) [38], a dozen of these receptors are now known to exist in human [37]. Type I receptors are referred to as Activin receptor-like kinases (ALK1 to 7) [39-44]. The receptor activation mechanism was first established for TGF-β and consists of TGF-β binding to its type II receptor (TβRII) leading to the recruitment, phosphorylation and activation of its type I receptor TβRI (ALK5) [45]. A similar receptor activation mechanism has been demonstrated for activin receptors in which activin binding to ActRII or ActRIIB is followed by recruitment, phosphorylation and activation of the activin type I receptor ALK4 [46,47]. Notably, activin receptors are highly promiscuous and form signaling complexes with activins as well as several other superfamily members including Nodal, GDF1, GDF3 and myostatin (GDF8) [48]. TGF-β ligand/receptor assembly triggers activation of the type I receptor kinase which phosphorylates cytoplasmic Smad proteins on C-terminal serine residues [49-52]. Activins, Nodal and TGF-βs signal via Smad2 and Smad3 whereas BMPs signal via Smads 1, 5 and 8 [53]. Following phosphorylation by the type I receptor kinase, receptor-regulated Smads form hetero-oligomeric complexes with the common mediator Smad, Smad4, and then translocate to the nucleus and interact directly with DNA and/or cell-type specific co-activator or co-repressor proteins leading to the activation or repression of target genes [54,55].
3. Cellular effects of TGF-β ligands are context-dependent
Ligands that activate the Smad2/3 pathway can have variable and even opposing effects on cellular proliferation, apoptosis and differentiation depending on the cell type and the cellular context [56]. The tumor suppressor function of the Smad2/3 pathway has been well characterized and derives from its ability to inhibit the cellular proliferation of multiple cell types and, in some cases, to cause terminal differentiation or apoptosis [36]. Maintenance of the cytostatic transcriptional program downstream of Smad2/3 pathway activation is critical for normal tissue homeostasis and tumor suppression and disruptions or alterations in this pathway have been observed in several types of human cancer [36]. Furthermore, following loss or attenuation of the cytostatic program, activation of the Smad2/3 pathway frequently has pro-tumorigenic effects that are exacerbated in the context of activated stem cell/growth factor pathways such as ras, Wnt and Notch [12,36,56-61]. For example, tumor cells that have become refractory to the cytostatic effects of TGF-β signaling generally secrete high levels of TGF-β ligands that act in an autocrine/paracrine manner both on tumor cells themselves and other cell types within the tumor microenvironment including stromal fibroblasts, endothelial cells and immune cells. Activation of the Smad2/3 pathway in this context can cause increased proliferation, motility, invasion and epithelial to mesenchymal transition (EMT) of tumor cells as well as increased angiogenesis and decreased immune surveillance [12,36,56-61]. Since these effects can lead to increased tumor growth and metastasis, intensive research efforts are currently aimed at understanding the molecular mechanisms that cause this cytostatic to oncogenic switch in Smad2/3 signaling.
4. Cripto modulates the TGF-β pathway during development
Nodal [35], GDF1 [3] and GDF3 [4] utilize activin signaling receptors but differ from activins since they require co-receptors from the Epidermal Growth Factor-Cripto, FRL-1, Cryptic (EGF-CFC) protein family [3,35]. The EGF-CFC family consists of small, glycosylated, extracellular signaling proteins including human and mouse Cripto and Cryptic, Xenopus FRL-1 and zebrafish one-eyed pinhead (oep) [62,63]. Members of the EGF-CFC family have an N-terminal signal peptide, an EGF-like domain, a cysteine-rich CFC domain unique to the family and a C-terminal site for GPI attachment [62,63] (Figure 1A). The EGF-like domain binds Nodal and the CFC domain binds the activin/Nodal type I receptor ALK4 and both of these interactions are required for Nodal signaling [64,65]. Substantial biochemical evidence indicates that Nodal, GDF1 and GDF3 bind Cripto and that these ligands require Cripto or a related EGF-CFC co-receptor to form active signaling complexes with activin receptors [3,4,8,64-66].
EGF-CFC proteins are known to act cell autonomously as anchored cell surface co-receptors but they also have activity when expressed as soluble proteins lacking a GPI attachment site [7,8,67,68] or when they are released from the cell surface following enzymatic cleavage of their GPI anchors [65,69-71]. In this regard, the GPI-cleaved form of Cripto was shown to be much more active as a paracrine Nodal co-receptor than mutant forms of soluble Cripto lacking the GPI attachment site [70]. In addition to its cell surface roles, Cripto has also been reported to regulate intracellular trafficking and processing of Nodal [72] and Notch proteins [25]. Genetic studies in zebrafish and mice have shown that EGF-CFC proteins are required for mesoderm and endoderm formation, cardiogenesis, and the establishment of left/right asymmetry during embryonic development [2,7,35,62,71,73]. Cripto knockout mouse embryos lack a primitive streak and fail to form embryonic mesoderm [74]. This phenotype is similar to that observed in ActRIIA-/-;ActRIIB-/- mice [75], ALK4-/- mice [76] and Nodal-/- mice [77,78], consistent with a requirement for coordinated Nodal signaling via activin receptors and Cripto to initiate primitive streak elongation and mesoderm formation [1,2]. Of note, Nodal activity was observed in Cripto knockout mice during embryogenesis, suggesting it can act independently of EGF-CFC co-receptors [79], However, a subsequent study showed that the phenotype of Cripto; Cryptic double mutant mice is virtually indistinguishable from that of Nodal knockout mice, supporting the requirement of EGF-CFC proteins for Nodal signaling. This work further provided evidence that Cryptic can compensate for the absence of Cripto during early embryogenesis by acting as a Nodal co-receptor in a non-cell autonomous manner [71]. Thus, these data and other available evidence strongly support a necessary role for EGF-CFC co-receptors as mediators of Nodal signaling in most, if not all, circumstances.
Cripto has also been recognized as a cell surface marker selectively expressed in embryonic stem cells [80-82] and iPS cells [83-85] and both Nodal and Cripto have been shown to play important roles as regulators of stem cell pluripotency maintenance and differentiation [5-7,82,86,87]. Although it is predominantly expressed during embryogenesis, Cripto has recently been shown to regulate developmental processes in adult tissues. Cripto was shown to function as a key regulator of hematopoetic stem cells (HSCs) within the hypoxic niche and to maintain the stem cell potential of HSCs ex vivo [88]. Cripto was also recently reported to regulate myostatin signaling in myoblasts derived from adult mouse muscle tissue [11]. Cripto expression has been reported in several other adult tissues including mammary gland [8], adipose tissue [9], pancreas [89] and endometrium [10,90], suggesting it may have a broad role in regulating adult tissue stem cells.
5. Cripto regulation of Activin/Nodal signaling
As mentioned above, Cripto has the interesting property of acting as a co-receptor for certain TGF-β ligands while inhibiting the signaling of others. Careful analysis demonstrated dose-dependent attenuation of activin-A signaling and activation of Nodal signaling by Cripto [17] despite the fact that these ligands are closely related structurally and utilize the same signaling receptors. Incremental increases in Cripto expression progressively inhibited maximal activin-A signaling to ∼50% of its original levels at which point higher levels of Cripto expression had no further effect [17]. These observations suggest that Cripto functions as a non-competitive activin antagonist rather than as a competitive antagonist as had been previously proposed [16,19,91]. Interestingly, maximal Nodal signaling was indistinguishable from that of activin in the presence of high levels of Cripto, i.e., ∼50% of maximal activin-A signaling in the absence of Cripto [17]. This discovery that activin is capable of signaling at higher levels than Nodal is consistent with the finding that activin regulates nearly twice as many genes as Nodal during Xenopus development including cell cycle genes associated with its antiproliferative role during gastrulation [92]. Since activin-A and Nodal each elicited similar maximal signaling responses in the presence of Cripto, we hypothesized that both ligands form structurally similar signaling complexes containing Cripto and activin receptors. In support of this, covalent crosslinking experiments demonstrated that activin-A assembles complexes containing Cripto, ActRII and ALK4 when these proteins were overexpressed in 293T cells or when they were expressed at endogenous levels in P19 cells [17]. Furthermore, Nodal could compete with activin-A for assembly of Cripto/ActRII/ALK4 complexes and bound the same site as activin-A on ActRII [17]. Together, these results indicate that activin and Nodal assemble structurally and functionally similar signaling complexes containing ActRII, ALK4 and Cripto. Our signaling data also indicate that these Cripto-containing complexes are less active than receptor complexes assembled by activin in the absence of Cripto. We have proposed a model in which the opposing effects of Cripto on activin and Nodal signaling stem from the ability of Cripto to act as an obligatory Nodal co-receptor on the one hand and non-competitive activin antagonist on the other despite the fact that it forms similar receptor complexes with both ligands [17] (Figure 2). Thus, by either promoting or attenuating the signaling of TGF-β ligands that utilize the Smad2/3 pathway, Cripto can regulate expression patterns and cell fate decisions that are highly sensitive to the magnitude and duration of Smad2/3 signaling [93-95] (Figure 2).
Figure 2. Model of Cripto modulation of activin and Nodal signaling.

(A) In the absence of Cripto, Nodal cannot signal and activin can signal at high levels leading to activation of genes responsive to both low and high levels of Smad2/3 signaling. (B) Cripto enables Nodal signaling and attenuates activin signaling and forms receptor complexes with these ligands that are structurally and functionally similar. The weaker signaling of Cripto-containing complexes precludes the regulation of genes that require high, sustained levels of Smad2/3 signaling.
6. Cripto modulation of the TGF-β pathway during oncogenesis
In addition to its physiological roles during development, Cripto has also been implicated in tumorgenesis. Cripto was originally isolated as a putative oncogene from a human teratocarcinoma cell line [96] and is expressed at high levels in human breast, colon, stomach, pancreas, lung, ovary, endometrial, testis, bladder and prostate tumors though negligibly expressed in the normal tissue counterparts of these tumors [63]. Soluble Cripto is also detected in plasma from patients with breast or colon carcinoma at levels that were significantly elevated relative to those of healthy volunteers, indicating Cripto may represent a novel biomarker for detection of these cancers [97]. Cripto was expressed at high levels in tumors from neu (ErbB2), TGF-α, int-3, polyoma middle T (PyMT) and simian virus 40 large T antigen [98] transgenic mice and also from Smad7/v-rasHa mice where Cripto was proposed to promote the malignant phenotype [99]. Early studies demonstrated that Cripto has a transforming ability since its overexpression could confer anchorage independent growth to mammary epithelial cells [100]. Cripto has subsequently been shown to have multiple oncogenic functions including promotion of cellular proliferation, survival, migration, invasion, angiogenesis and EMT [7]. Cripto also functions as an oncogene in vivo since transgenic MMTV-Cripto and WAP-Cripto mice develop mammary hyperplasias and tumors [7,101-103] and since monoclonal antibodies targeting Cripto reduce the growth of tumor xenografts in nude mice [19,104]. Therefore, in addition to its developmental roles, Cripto can promote tumorigenesis and is selectively expressed in human tumors.
Cripto is thought to promote oncogenesis via modulation of TGF-β ligand signaling and through mechanisms that are independent of TGF-β ligands and their signaling receptors [7,23,48]. As a modulator of TGF-β ligand signaling, Cripto can promote the tumorigenic phenotype either as a co-receptor for receptor activation or through attenuation of cytostatic pathway activation [12-16,18-22,91,105-107]. The oncogencity of Cripto in its role as an obligatory co-receptor is indicated by several studies showing that Nodal promotes tumor cell growth, aggressiveness and plasticity [12-15,106,107]. Pioneering studies in human melanoma showed that Nodal is secreted from aggressive melanoma cells and that blockade of Nodal signaling inhibited melanoma cell invasiveness, colony forming ability and tumorigenicity [12]. Nodal expression and tumorigenicity were also reduced in melanoma and breast cancer cell lines when they were grown in an embryonic microenvironment containing the Nodal antagonist Lefty [13]. Nodal was subsesquently found to be expressed in cancerous but not normal prostate specimens and its overexpression in prostate cancer cell lines enhanced anchorage independent growth while inhibiting AR signaling and down regulating the expression of androgen-responsive genes [106]. In glioma cell lines, Nodal was shown to cause increased cell invasiveness, proliferation and tumor growth, while Nodal knockdown caused differentiation of these cells toward an astrocytic phenotype [14]. Nodal knockdown was also shown to inhibit angiogenesis and VEGF expression in tumors derived from glioma cell lines [107]. Finally, Nodal and activin signaling were recently reported to promote self-renewal and tumorigenicity of pancreatic cancer stem cells [15]. In each of these studies, Cripto expression was detected in the Nodal-responsive cells [12-15,106,107] and, importantly, Cripto and Nodal were each shown to be selectively expressed in the tumorigenic stem cell compartment of pancreatic cancer [15]. Together, these studies clearly indicate the important role of Cripto-dependent Nodal signaling in promoting cellular plasticity and tumorigenesis.
Cripto can also attenuate the cytostatic, tumor suppressive effects of activin and TGF-β signaling. We originally showed that Cripto binds activin-A and attenuates activin-A signaling and, based on these findings, hypothesized that Cripto exerts its oncogenic effects at least in part by inhibiting the tumor suppressor function of the Smad2/3 pathway [16]. In support of this, it was reported that Cripto also binds activin-B and, importantly, that it inhibits cytostatic Smad2/3 signaling downstream of both activin-A and activin-B [18,19]. Similar to what was shown with activins, we showed that Cripto binds TGF-β1 and reduces TGF-β1 crosslinking to its type I receptor, TβRI [20]. This suggested a role for Cripto in altering or disrupting TGF-β receptor assembly and, consistent with this, Cripto overexpression inhibited TGF-β-depedent Smad2 phosphorylation and antiproliferative effects in human mammary epithelial MCF10A cells. Cripto knockdown in HeLa cells enhances TGF-β1 signaling indicating a role for endogenous Cripto as a TGF-β antagonist [20]. Together, these results demonstrate that Cripto binds TGF-β1 in a complex with TβRII, inhibits TGF-β receptor assembly and reduces downstream Smad2/3 signaling and cytostatic effects. Cripto antagonism of TGF-β signaling has subsequently been observed in embryonal carcinoma NCCIT cells, [18] hematopoetic stem cells [88] and in keratinocytes, where its antagonism of TGF-β signaling was cited as a potential mechanism underlying skin carcinogenesis [22].
Thus, Cripto can have oncogenic effects both as a co-receptor and as an antagonist of TGF-β signaling. We propose that overall, Cripto modulation of the TGF-β pathway promotes tumorigenesis by establishing and enforcing low to moderate levels of Smad2/3 signaling while preventing high, sustained Smad2/3 signaling. As described above, in the presence of Cripto, Nodal and activin achieved signaling maxima that were indistinguishable from each other but only ∼50% of maximal activin signaling in the absence of Cripto [17]. Similarly, TGF-β signaling appeared to be only partially inhibited even at high levels of Cripto expression [20]. These observations are noteworthy since high, sustained levels of Smad2/3 signaling are required to initiate and maintain the cytostatic program while lower levels of Smad2/3 signaling elicit transcription of genes associated with tumor progression [56,108]. This is consistent with available data that have shown that in the presence of Cripto, TGF-β and activin lose their cytostatic effects but acquire oncogenic signaling properties similar to those of Nodal deriving, at least in part, from their similar ability to activate the Smad2/3 pathway at low levels [17,18].
7. Cripto has growth factor activity independent of the TGF-β pathway
In addition to its effects on TGF-β superfamily ligands, Cripto has multiple signaling activities that are thought to be independent of TGF-β ligands and receptors. These include its function as a soluble growth factor that can be negatively regulated by caveolin-1 [109], its role as a co-receptor for Wnt signaling [24] and its ability to promote Notch processing [25]. Pioneering studies demonstrated that soluble Cripto activates both the mitogen activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) pathways [7,110,111]. Treatment of HC-11 mammary epithelial cells with soluble Cripto resulted in tyrosine phosphorylation of the SH2-adaptor protein Shc, association of Shc with Grb2 and activation of the p42/44 Erk/MAPK pathway [112]. Treatment of cells with soluble Cripto also caused phosphorylation of the p85 regulatory subunit of PI3K leading to phosphorylation and activation of Akt in SiHa cervical carcinoma cells [111]. The receptor mechanism mediating these Cripto effects has remained elusive. Although Cripto treatment induced tyrosine phosphorylation of ErbB4, it was unable to directly bind ErbB4 or other members of the EGF receptor family [112]. In addition, while soluble forms of Cripto have been reported to cause Smad2 phosphorylation in an ALK4- and Nodal-dependent manner, activation of MAPK/PI3K pathways by soluble Cripto was reported to be ALK4- and Nodal-independent [113]. Subsequent studies showed that Src is activated following treatment of cells with soluble Cripto and that Src activation is necessary for Cripto-dependent activation of MAPK and PI3K pathways [114]. The GPI-anchored proteoglycan glypican-1 was also reported to bind Cripto and facilitate Cripto-dependent Src activation [114]. However, the transmembrane signaling mechanism coupling Cripto to activation of Src and MAPK/PI3K pathways has not yet been elucidated.
8. GRP78, a putative Cripto signaling partner
The mechanisms of Cripto's many signaling functions are only partially understood at the molecular level. Therefore, in order to better understand Cripto signaling function, we sought to identify and characterize additional Cripto binding partners. We conducted a protein-protein interaction screen using Cripto as bait and these efforts led to the identification of the HSP70 family member GRP78 as a novel Cripto binding protein [21]. Figure 1B illustrates the structural features of GRP78 including the ATP binding (ATPase) and peptide-binding domains that are highly conserved among HSP70 family members and that mediate classical chaperone functions [115,116] (Figure 1B).
GRP78 is best known for its roles in the ER where it promotes the folding, maturation and assembly of nascent proteins and also coordinates the unfolded protein response (UPR) that alleviates ER stress [26,117]. Although it is constitutively expressed, GRP78 levels increase greatly in response to demanding conditions such as hypoxia and nutrient deprivation and it has been heavily implicated in cytoprotection and chemoresistance in the tumor microenvironment where these conditions prevail [118,119]. Similar to Cripto knockout mice, GRP78 knockout mice have an early embryonic lethal phenotype and GRP78 was found to be required for proliferation and survival of embryonic inner cell mass cells that are the precursors of pluripotent stem cells [120]. Cripto is a critical regulator of cardiogenesis [5,121,122] and, interestingly, both GRP78 [123] and Cripto [124] are expressed in the embryonic heart. This raises the possibility that GRP78 may be required for Cripto function during cardiac development. Like Cripto, GRP78 is highly elevated in multiple types of solid tumors relative to their normal tissue counterparts [26,118,119] and this may reflect the aforementioned stress responsive and protective properties. The first direct evidence for the involvement of GRP78 in cancer progression came from the demonstration that GRP78 knockdown in fibrosarcoma cells using antisense prevented tumor formation in nude mice [125]. It was further shown that a suicide transgene driven by the GRP78 promoter in breast cancer cells completely blocked tumor growth in mice [126]. More recently, it was demonstrated that GRP78 heterozygous mice develop normally but are resistant to transgene-induced mammary tumor growth [127]. In this study, it was demonstrated that reduced GRP78 levels in heterozygous mice resulted in decreased tumor cell proliferation, increased tumor cell apoptosis and reduced tumor angiogenesis [127]. Therefore, it is reasonable to infer that the conditions found within solid tumors cause induction of GRP78 and that its expression enhances tumor growth.
Since Cripto is a GPI-anchored extracellular protein, it was surprising for us to discover that it interacts with a protein localized to the ER. However, it has now been widely reported that a fraction of GRP78 localizes to the plasma membrane [27-29]. Cell surface GRP78 was first detected in human rhabdomyosarcoma cells following treatment with thapsigargin, a potent inducer of GRP78 expression [128]. Global profiling of the cell surface proteome of cancer cells also identified GRP78 [129]. Furthermore, phage display-derived human monoclonal antibodies isolated based on their ability to bind to the surface of live primary human breast cancer cells were shown to specifically recognize GRP78 [130]. GRP78 can exist as a transmembrane protein [131] but it was also isolated as a soluble factor secreted by tumor cells and exerting pro-survival effects on endothelial cells and myeloma cells [132]. Regardless of its topology, GRP78 actively accumulates at the cell surface following induction by ER stress or ectopic expression [133] and several extracellular proteins have been reported to bind cell surface GRP78 near its N- or C-terminus [21,130,134-137] (Figure 1C). Importantly, cell surface GRP78 signaling functions appear to be diversified and include acting as a co-receptor for viruses and MHC Class I antigen presentation [138] and as a receptor for Kringle 5 of plasminogen [134] and activated α2-macroglobulin (α2M) [139]. Interestingly, α2M binding to GRP78 on the surface of prostate carcinoma 1-LN cells triggered pro-proliferative and anti-apoptotic behavior via activation of MAPK and PI3K pathways [139,140]. Cell surface GRP78 was similarly shown to have an essential role in mediating T-cadherin signaling via Akt and resulting pro-survival effects in endothelial cells [135]. Consistent with these studies, tumorigenesis and Akt activation resulting from Pten loss was blocked by conditional knockout of GRP78 expression both in prostate epithelium [141] and the hematopoetic system [142]. Significantly, GRP78 autoantibodies were isolated from the serum of prostate cancer patients and the reactivity of these antibodies to cell surface GRP78 correlated strongly with androgen insensitivity, increased cancer aggressiveness and decreased patient survival [143]. Also, cytotoxic peptide conjugates specifically targeting cell surface GRP78 prevented the growth of breast and prostate tumors in mice [144,145]. Finally, targeting cell surface GRP78 inhibited stemness, radioresistance and tumorigenicity of head and neck cancer initiating cells [146].
9. Cripto/GRP78 signaling via TGF-β and Src/MAPK/PI3K pathways
Since Cripto is a cell surface protein and GRP78 is primarily localized to the ER, we first sought to determine whether GRP78 interacts with Cripto at the cell surface. We overexpressed Cripto and GRP78 in 293T cells, labeled cell surface proteins with cell impermeable biotin and subjected cell lysates to anti-Cripto immunoprecipitation followed by blotting with avidin-HRP. GRP78 co-immunoprecipitated with Cripto in this experiment and was biotinylated indicating Cripto and GRP78 associate at the cell surface. Endogenously expressed Cripto and GRP78 also interact at the cell surface as indicated by their co-immunoprecipitation from biotin-treated P19 cells [21]. Consistent with these data, immunofluorescence studies showed that Cripto and GRP78 co-localize at the cell surface when overexpressed in 293T cells and when expressed at endogenous levels in P19 cells [21].
Based on available evidence indicating cell surface GRP78 and Cripto may have similar functions, we hypothesized that GRP78 and Cripto interact as signaling partners. In support of this, GRP78 knockdown in HeLa cells enhanced TGF-β1-induced Smad2 phosphorylation [21] in a manner that was similar to the effect of Cripto knockdown in the same cells [20]. GRP78 did not directly interact with TGF-β type I and type II receptors suggesting it exerts its effects on TGF-β signaling via its interaction with Cripto. Importantly, Cripto and GRP78 functioned collaboratively to inhibit cytostatic TGF-β signaling since overexpression of both proteins reduced TGF-β1-dependent Smad2 phosphorylation and growth inhibition to a much greater extent than overexpression of either protein alone [21]. Cripto and GRP78 also acted together to increase colony growth and attenuate the growth inhibitory effects of TGF-β1 on PC3 cells in soft agar. Together, these data indicate that Cripto and GRP78 form a complex at the cell surface that promotes cell growth and inhibits cytostatic TGF-β signaling [21].
In subsequent studies, we tested the requirement of cell surface GRP78 for Cripto signaling and biological activity. Like mouse P19 cells, human germ cell tumor-derived NCCIT cells express high levels of Cripto endogenously and we analyzed Cripto signaling in NCCIT lines stably transduced with empty vector, Cripto shRNA, GRP78 shRNA or both shRNAs [18]. Cripto knockdown or GRP78 knockdown similarly enhanced activin-A, activin-B and TGF-β1 signaling while inhibiting Nodal signaling and knockdown of both proteins had a more pronounced effect than knockdown of either alone [18]. Furthermore, an antibody (N-20, Santa Cruz Biotechnology, Inc.) previously shown to block cell surface GRP78 signaling functions [134,135] blocked Cripto effects on activin-A, activin-B, TGF-β1 and Nodal signaling. Thus, treatment of NCCIT control cells with the N-20 antibody enhanced activin-A, activin-B and TGF-β1 signaling and decreased Nodal signaling in a manner similar to that of Cripto knockdown or GRP78 knockdown. Also, this GRP78 antibody had no effect on signaling by activin/TGF-β/Nodal in Cripto knockdown cells indicating it exerts its effects by blocking Cripto. We explored the mechanistic basis for these effects and showed that deletion of the N-terminal N-20 epitope on GRP78 disrupts Cripto/GRP78 binding and also that Cripto and the N-20 antibody compete for binding to GRP78 [18]. Significantly, the N-20 antibody also increased activin-A signaling and decreased Nodal signaling in hES cells as measured by Smad2 phosphorylation [18]. Together, these findings indicate that GRP78 is required for Cripto signaling via the TGF-β pathway and provide the first evidence for Cripto/GRP78 signaling in ES cells.
As discussed above, soluble forms of Cripto activate Src/MAPK/PI3K pathways and we tested if cell surface GRP78 is also required for this Cripto growth factor-like activity. In support of this possibility, we showed that treatment with soluble Cripto protein caused Akt, Erk and Src phosphorylation in Cripto knockdown NCCIT cells, but not in cells in which both Cripto and GRP78 were knocked down [18]. Furthermore, and similar to what was observed with Cripto regulation of TGF-β ligands, the N-20 antibody blocked Cripto-induced Akt phosphorylation and cellular proliferation. The N-20 antibody also blocked Cripto-dependent Akt and Src phosphorylation and inhibited Cripto-induced pro-proliferative and EMT-like effects in MCF10A human mammary epithelial cells [18]. Interestingly, Cripto/GRP78 signaling inhibited cytostatic effects of activin and TGF-β and promoted pro-proliferative responses to activin, TGF-β and Nodal in both MCF10A cells and in NCCIT cells [18,21]. The molecular basis for this switch in the proliferative effects of activin/Nodal/TGF-β signaling remains to be elucidated, but likely derives from low/moderate Smad2/3 signaling coupled with activated Src/MAPK/PI3K signaling. In summary, our results indicate that cell surface GRP78 functions as a critical mediator of Cripto signaling via both TGF-β and Src/MAPK/PI3K pathways (Figure 3) and they suggest that GRP78 is likely required for Cripto function generally.
Figure 3. Cripto binding to cell surface GRP78 promotes stemness and the tumorigenic phenotype.
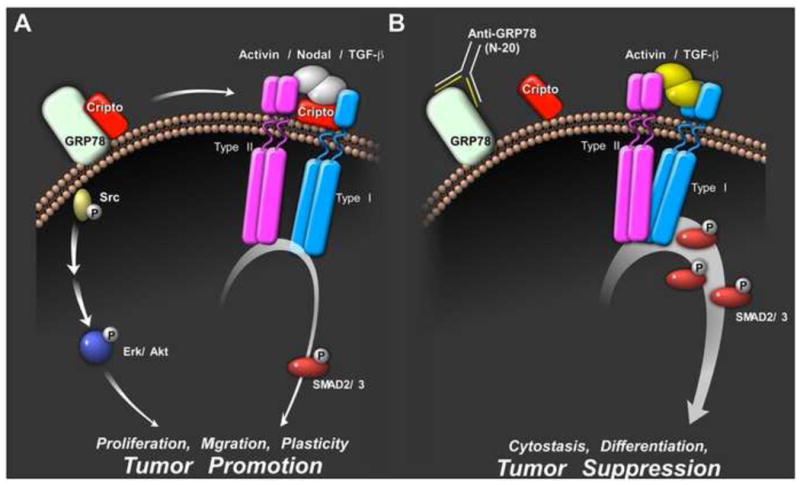
(A) The cell surface Cripto/GRP78 complex interaction required for Cripto regulation of the Smad2/3 pathway both as a Nodal co-receptor and antagonist of activin and TGF-β signaling. Soluble Cripto also binds cell surface GRP78 to cause activation of PI3K and MAPK signaling via activation of Src. The effects of Cripto/GRP78 signaling on Src/MAPK/PI3K and Smad2/3 pathways are likely to function synergistically to promote stemness, plasticity and tumorigenesis. (B) The N-20 antibody blocks Cripto binding to cell surface GRP78 and inhibits Cripto-dependent signaling via MAPK, PI3K and Smad2/3 pathways. By blocking Cripto, this GRP78 antibody blocks Src/MAPK/PI3K pathway activation and enhances activin and TGF-β signaling thereby promoting cytostasis and tumor suppression.
Subsequent to our studies, other groups further implicated Cripto signaling via cell surface GRP78 both in the regulation of head and neck cancer tumor initiating cells [146] and in normal adult hematopoetic stem cells [88]. Wu et al. [146] showed that cell surface GRP78 is co-expressed with Cripto in head and neck cancer initiating cells (HN-CIC), a tumorigenic subpopulation of head and neck squamous cell carcinoma. Knockdown of cell surface GRP78 reduced the self-renewal properties and tumorigenicity of HN-CIC consistent with a role for Cripto signaling via GRP78 to promote tumor initiation and stemness in these cells [146]. However, the importance of Cripto/GRP78 interaction in promoting stemness and tumorigenicity of HN-CIC remains to be directly demonstrated. More recently, a critical and direct role for Cripto/GRP78 signaling in adult hematopoetic stem cells (HSC) was identified. Miharada et al. [88] demonstrated that HSCs contain GRP78+ and GRP78-subpopulations and that only the GRP78+ population is responsive to Cripto treatment. Cripto treatment activated the PI3K/Akt pathway in these cells and maintained the stem cell potential of the GRP78+ population ex vivo as evidenced by its ability to promote multilineage colony growth in vitro and reconstitution of the hematopoietic system upon transplantation [88]. Significantly, the Cripto effects on HSCs were completely blocked if cells were treated with the N-20 GRP78 antibody [88]. Overall, the authors showed that Cripto/GRP78 signaling represents a critical downstream mediator of HIF1α required for maintenance of undifferentiated HSC in hypoxic niche in bone [88]. This study substantiates the requirement of cell surface GRP78 as a Cripto signaling partner and with an increasing literature on both Cripto and cell surface GRP78 suggests the potential for similar Cripto/GRP78 signaling in stem cell maintenance in other adult tissue types.
Overall, available evidence supports a model in which the cell surface Cripto/GRP78 complex acts as a signaling node that promotes cellular plasticity and the tumorigenic phenotype (Figure 3A). According to this model, Cripto and GRP78 cooperatively attenuate cytostatic Smad2/3 signaling in response to activin and TGF-β and cause activin, Nodal and TGF-β to promote stemness and oncogenesis through coordination of restricted Smad2/3 signaling and activation of Src, MAPK and PI3K pathways. The N-20 GRP78 antibody blocks Cripto binding to cell surface GRP78 and thereby inhibits Cripto signaling via both Smad2/3 and Src/MAPK/PI3K pathways [18] (Figure 3B).
10. Conclusions
Collectively, the evidence outlined above supports a role for Cripto signaling via cell surface GRP78 to promote stemness and plasticity during normal development and tumor progression. At the molecular level, Cripto acts via GRP78 to alter TGF-β signaling either directly, by forming complexes with TGF-β ligands and their signaling receptors, or indirectly by activating Src/MAPK/PI3K and possibly Notch and Wnt pathways which can then engage in crosstalk with the Smad2/3 pathway. We propose that the cell surface Cripto/GRP78 complex acts via these direct and indirect mechanisms to cause a switch in Smad2/3 signaling from cytostatic to oncogenic.
Available data clearly indicate that Cripto binding to cell surface GRP78 is required for Cripto signaling. Therefore, molecules that interfere with Cripto signaling, such as caveolin-1, may do so by interfering with Cripto/GRP78 binding. However, the mechanistic details of how GRP78 enables Cripto signaling remain to be elucidated. One possibility is that GRP78 serves as an adaptor protein that physically couples Cripto to signaling targets such as TGF-β ligands/receptors and tyrosine kinase receptors such as erbB4. In such a role, GRP78 could act either as an extracellular protein or as a transmembrane protein. However, as a transmembrane protein, GRP78 may possess a cytoplasmic domain capable of activating downstream signaling independently of coupling to other receptors. Perhaps a more likely scenario is that extracellular GRP78 acts in its capacity as a chaperone and that Cripto is one of its client proteins. This is supported by the fact that GRP78 binds a large variety of extracellular proteins and regulates multiple diverse signaling processes. Furthermore, secreted GRP78 can regulate cell signaling in a non-cell autonomous manner. Future studies will be required to clarify the precise mechanism through which cell surface GRP78 facilitates Cripto signaling.
In summary, Cripto regulates the signaling of several TGF-β superfamily members that activate the Smad2/3 pathway and activates Src/MAPK/PI3K and Wnt/Notch pathways. GRP78 forms a complex with Cripto at the cell surface and this interaction appears to be essential for all aspects of Cripto signaling. Cripto/GRP78 signaling is emerging as a key regulator of stem cell function and tumorigenesis and targeting the cell surface Cripto/GRP78 complex represents an attractive therapeutic strategy for the treatment of human cancer.
Acknowledgments
Benjamin T. Spike and Debbie Doan carefully read the manuscript and provided helpful comments and discussions. Jonathan A. Kelber and Jamie Simon contributed to the design and artwork of the figures. This work was supported in part by the Clayton Medical Research Foundation, Inc., (WWV is a senior CMRF investigator), DoD Award Number W81XWH-10-1-0891 and the Cancer Center Core Grant (P30 CA014195-38). This manuscript is dedicated to Wylie Vale in remembrance of his warmth, his wisdom and his irrepressible enthusiasm for science.
Abbreviations
- GPI
glcyosylphosphatidylinositol
- GDF
growth and differentiation factor
- BMP
Bone Morphogenetic Protein
- GRP78
Glucose Regulated Protein 78 kDa
- TDGF1
Teratocarcinoma-Derived Growth Factor 1
- FRL-1
Fibroblast Growth Factor (FGF) Receptor Ligand-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–34. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 3.Cheng SK, Olale F, Bennett JT, Brivanlou AH, Schier AF. EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1. Genes Dev. 2003;17:31–6. doi: 10.1101/gad.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–29. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- 5.Parisi S, D'Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–14. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minchiotti G. Nodal-dependant Cripto signaling in ES cells: from stem cells to tumor biology. Oncogene. 2005;24:5668–75. doi: 10.1038/sj.onc.1208917. [DOI] [PubMed] [Google Scholar]
- 7.Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731–41. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- 8.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and - independent signaling pathways in mammary epithelial cells. Mol Cell Biol. 2002;22:2586–97. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci U S A. 2008;105:7252–6. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorgiou I, Nicholls PK, Wang F, Lackmann M, Makanji Y, Salamonsen LA, Robertson DM, Harrison CA. Expression of nodal signalling components in cycling human endometrium and in endometrial cancer. Reprod Biol Endocrinol. 2009;7:122. doi: 10.1186/1477-7827-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemaladewi DU, de Gorter DJ, Aartsma-Rus A, van Ommen GJ, Ten Dijke P, t Hoen PA, Hoogaars WM. Cell-type specific regulation of myostatin signaling. FASEB J. 2011 doi: 10.1096/fj.11-191189. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 13.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105:4329–34. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CC, Jan HJ, Lai JH, Ma HI, Hueng DY, Lee YC, Cheng YY, Liu LW, Wei HW, Lee HM. Nodal promotes growth and invasion in human gliomas. Oncogene. 2010;29:3110–23. doi: 10.1038/onc.2010.55. [DOI] [PubMed] [Google Scholar]
- 15.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, Torres-Ruiz R, Garcia E, Hidalgo M, Cebrian DA, Heuchel R, Lohr M, Berger F, Bartenstein P, Aicher A, Heeschen C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci U S A. 2003;100:5193–8. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelber JA, Shani G, Booker EC, Vale WW, Gray PC. Cripto is a noncompetitive activin antagonist that forms analogous signaling complexes with activin and nodal. J Biol Chem. 2008;283:4490–500. doi: 10.1074/jbc.M704960200. [DOI] [PubMed] [Google Scholar]
- 18.Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, Gray PC. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–36. doi: 10.1038/onc.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–87. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray PC, Shani G, Aung K, Kelber J, Vale W. Cripto binds transforming growth factor beta (TGF-beta) and inhibits TGF-beta signaling. Mol Cell Biol. 2006;26:9268–78. doi: 10.1128/MCB.01168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–77. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla A, Ho Y, Liu X, Ryscavage A, Glick AB. Cripto-1 alters keratinocyte differentiation via blockade of transforming growth factor-beta1 signaling: role in skin carcinogenesis. Mol Cancer Res. 2008;6:509–16. doi: 10.1158/1541-7786.MCR-07-0396. [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka T, Karasawa H, Castro NP, Rangel MC, Salomon DS, Bianco C. An evolving web of signaling networks regulated by Cripto-1. Growth Factors. 2011 doi: 10.3109/08977194.2011.641962. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–71. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, Rangel MC, Sakamoto K, Sun Y, Callahan R, Salomon DS. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. J Cell Biol. 2009;187:343–53. doi: 10.1083/jcb.200905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–6. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 28.Sato M, Yao VJ, Arap W, Pasqualini R. GRP78 signaling hub a receptor for targeted tumor therapy. Adv Genet. 2010;69:97–114. doi: 10.1016/S0065-2660(10)69006-2. [DOI] [PubMed] [Google Scholar]
- 29.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–8. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15:681–94. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- 31.Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P, Petraglia F, Yu J, Plotsky P, Spiess J, Rivier J. Chemical and biological characterization of the inhibin family of protein hormones In Laurentian Hormone Conference. In: Clark JH, editor. Recent Progress in Hormone Research. 1988. pp. 1–34. [DOI] [PubMed] [Google Scholar]
- 32.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 33.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 34.Roberts AB, Sporn MB. The transforming growth factor-βs. In: Sporn MB, Roberts AB, editors. Handbook Exp Pharmacol. Springer-Verlag; Heidelberg: 1990. pp. 419–472. [Google Scholar]
- 35.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–9. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 36.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- 39.ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993;8:2879–2887. [PubMed] [Google Scholar]
- 40.Ebner R, Chen RH, Shum L, Lawler S, Zioncheck TF, Lee A, Lopez AR, Derynck R. Cloning of type I TGF-β receptor and its effect on TGF-β binding to the type II receptor. Science. 1993;260:1344–1348. doi: 10.1126/science.8388127. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida K, Mathews LS, Vale WW. Cloning and characterization of a transmembrane serine kinase that acts as an activin type I receptor. Proc Natl Acad Sci USA. 1993;90:11242–11246. doi: 10.1073/pnas.90.23.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassing CH, Yingling JM, Howe DJ, Wang T, He WW, Gustafson ML, Shah P, Donahoe PK, Wang XF. A transforming growth factor-β type I receptor that signals to activate gene expression. Science. 1994;263:87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- 43.ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchida K, Sawchenko PE, Nishikawa SI, Vale WW. Molecular cloning of a novel type I receptor serine/threonine kinase for the TGFβ superfamily from rat brain. Mol Cell Neurosci. 1996;7:467–478. doi: 10.1006/mcne.1996.0034. [DOI] [PubMed] [Google Scholar]
- 45.Wrana J, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1992;370:341–347. [Google Scholar]
- 46.Attisano L, Wrana JL, Montalvo E, Massague J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebrun JJ, Vale WW. Activin and inhibin have antagonistic effects on ligand-dependent heterodimerization of the type I and type II activin receptors and human erythroid differentiation. Mol Cell Biol. 1997;17:1682–1691. doi: 10.1128/mcb.17.3.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab. 2005;16:73–8. doi: 10.1016/j.tem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 50.Macias SM, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–24. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 52.Kretzschmar M, Liu F, Hata A, Doody J, Massague J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–95. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- 53.Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev. 2000;11:5–13. doi: 10.1016/s1359-6101(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 54.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 55.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 56.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 58.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 59.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 60.Uttamsingh S, Bao X, Nguyen KT, Bhanot M, Gong J, Chan JL, Liu F, Chu TT, Wang LH. Synergistic effect between EGF and TGF-beta1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27:2626–34. doi: 10.1038/sj.onc.1210915. [DOI] [PubMed] [Google Scholar]
- 61.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–74. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 62.Shen MM, Schier AF. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000;16:303–9. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- 63.Saloman DS, Bianco C, Ebert AD, Khan NI, De Santis M, Normanno N, Wechselberger C, Seno M, Williams K, Sanicola M, Foley S, Gullick WJ, Persico G. The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer. Endocr Relat Cancer. 2000;7:199–226. doi: 10.1677/erc.0.0070199. [DOI] [PubMed] [Google Scholar]
- 64.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–57. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 65.Yan YT, Liu JJ, Luo Y, E C, Haltiwanger RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22:4439–49. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–22. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–51. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 68.Minchiotti G, Manco G, Parisi S, Lago CT, Rosa F, Persico MG. Structure-function analysis of the EGF-CFC family member Cripto identifies residues essential for nodal signalling. Development. 2001;128:4501–10. doi: 10.1242/dev.128.22.4501. [DOI] [PubMed] [Google Scholar]
- 69.Chu J, Ding J, Jeays-Ward K, Price SM, Placzek M, Shen MM. Non-cell-autonomous role for Cripto in axial midline formation during vertebrate embryogenesis. Development. 2005;132:5539–51. doi: 10.1242/dev.02157. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe K, Hamada S, Bianco C, Mancino M, Nagaoka T, Gonzales M, Bailly V, Strizzi L, Salomon DS. Requirement of glycosylphosphatidylinositol anchor of Cripto-1 for trans activity as a Nodal co-receptor. J Biol Chem. 2007;282:35772–86. doi: 10.1074/jbc.M707351200. [DOI] [PubMed] [Google Scholar]
- 71.Chu J, Shen MM. Functional redundancy of EGF-CFC genes in epiblast and extraembryonic patterning during early mouse embryogenesis. Dev Biol. 2010;342:63–73. doi: 10.1016/j.ydbio.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constam DB. Riding shotgun: a dual role for the epidermal growth factor-Cripto/FRL-1/Cryptic protein Cripto in Nodal trafficking. Traffic. 2009;10:783–91. doi: 10.1111/j.1600-0854.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 73.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 74.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–7. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 75.Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213:157–69. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- 76.Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev. 1998;12:844–57. doi: 10.1101/gad.12.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–7. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- 78.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–28. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 79.Liguori GL, Borges AC, D'Andrea D, Liguoro A, Goncalves L, Salgueiro AM, Persico MG, Belo JA. Cripto-independent Nodal signaling promotes positioning of the A-P axis in the early mouse embryo. Dev Biol. 2008;315:280–9. doi: 10.1016/j.ydbio.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 80.Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo AB, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RD, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O'Brien CM, Oh SK, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RA, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–16. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 81.Hough SR, Laslett AL, Grimmond SB, Kolle G, Pera MF. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS One. 2009;4:e7708. doi: 10.1371/journal.pone.0007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, Salomon DS. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol. 2010;177:532–40. doi: 10.2353/ajpath.2010.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–19. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez-Piza I, Richaud-Patin Y, Vassena R, Gonzalez F, Barrero MJ, Veiga A, Raya A, Belmonte JC. Reprogramming of human fibroblasts to induced pluripotent stem cells under xeno-free conditions. Stem Cells. 2010;28:36–44. doi: 10.1002/stem.248. [DOI] [PubMed] [Google Scholar]
- 86.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 88.Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, Cammenga J, Karlsson S. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–44. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 89.Zhang YQ, Cleary MM, Si Y, Liu G, Eto Y, Kritzik M, Dabernat S, Kayali AG, Sarvetnick N. Inhibition of sctivin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic {beta}-cells. Diabetes. 2004;53:2024–2033. doi: 10.2337/diabetes.53.8.2024. [DOI] [PubMed] [Google Scholar]
- 90.Torres PB, Florio P, Galleri L, Reis FM, Borges LE, Petraglia F. Activin A, activin receptor type II, nodal, and cripto mRNA are expressed by eutopic and ectopic endometrium in women with ovarian endometriosis. Reprod Sci. 2009;16:727–33. doi: 10.1177/1933719109334967. [DOI] [PubMed] [Google Scholar]
- 91.Shen MM. Decrypting the role of Cripto in tumorigenesis. J Clin Invest. 2003;112:500–2. doi: 10.1172/JCI19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramis JM, Collart C, Smith JC. Xnrs and Activin regulate distinct genes during Xenopus development: Activin regulates cell division. PLoS ONE. 2007;2:e213. doi: 10.1371/journal.pone.0000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guzman-Ayala M, Lee KL, Mavrakis KJ, Goggolidou P, Norris DP, Episkopou V. Graded Smad2/3 activation is converted directly into levels of target gene expression in embryonic stem cells. PLoS One. 2009;4:e4268. doi: 10.1371/journal.pone.0004268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee KL, Lim SK, Orlov YL, Yit le Y, Yang H, Ang LT, Poellinger L, Lim B. Graded Nodal/Activin signaling titrates conversion of quantitative phospho-Smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genet. 2011;7:e1002130. doi: 10.1371/journal.pgen.1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown S, Teo A, Pauklin S, Hannan N, Cho CH, Lim B, Vardy L, Dunn NR, Trotter M, Pedersen R, Vallier L. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells. 2011;29:1176–85. doi: 10.1002/stem.666. [DOI] [PubMed] [Google Scholar]
- 96.Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ‘EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. Embo J. 1989;8:1987–91. doi: 10.1002/j.1460-2075.1989.tb03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, De Luca A, Jones B, Balogh G, Russo J, Mailo D, Palaia R, D'Aiuto G, Botti G, Perrone F, Salomon DS, Normanno N. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res. 2006;12:5158–64. doi: 10.1158/1078-0432.CCR-06-0274. [DOI] [PubMed] [Google Scholar]
- 98.Kenney NJ, Smith GH, Maroulakou IG, Green JH, Muller WJ, Callahan R, Salomon DS, Dickson RB. Detection of amphiregulin and Cripto-1 in mammary tumors from transgenic mice. Mol Carcinog. 1996;15:44–56. doi: 10.1002/(SICI)1098-2744(199601)15:1<44::AID-MC7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Lee J, Cooley M, Bhogte E, Hartley S, Glick A. Smad7 but not Smad6 cooperates with oncogenic ras to cause malignant conversion in a mouse model for squamous cell carcinoma. Cancer Res. 2003;63:7760–8. [PubMed] [Google Scholar]
- 100.Ciardiello F, Dono R, Kim N, Persico MG, Salomon DS. Expression of cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 1991;51:1051–4. [PubMed] [Google Scholar]
- 101.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–76. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 102.Sun Y, Strizzi L, Raafat A, Hirota M, Bianco C, Feigenbaum L, Kenney N, Wechselberger C, Callahan R, Salomon DS. Overexpression of human Cripto-1 in transgenic mice delays mammary gland development and differentiation and induces mammary tumorigenesis. Am J Pathol. 2005;167:585–97. doi: 10.1016/S0002-9440(10)63000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon DS. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- 104.Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer Res. 2004;64:4018–23. doi: 10.1158/0008-5472.CAN-03-3888. [DOI] [PubMed] [Google Scholar]
- 105.Salomon DS. Melanoma pathogenesis and Nodal: a partial picture? Nat Med. 2006;12:1231. doi: 10.1038/nm1106-1231a. author reply 1231. [DOI] [PubMed] [Google Scholar]
- 106.Lawrence MG, Margaryan NV, Loessner D, Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR, Lai J, Postovit LM, Clements JA, Hendrix MJ. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate. 2011;71:1198–209. doi: 10.1002/pros.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hueng DY, Lin GJ, Huang SH, Liu LW, Ju DT, Chen YW, Sytwu HK, Chang C, Huang SM, Yeh YS, Lee HM, Ma HI. Inhibition of Nodal suppresses angiogenesis and growth of human gliomas. J Neurooncol. 2011;104:21–31. doi: 10.1007/s11060-010-0467-3. [DOI] [PubMed] [Google Scholar]
- 108.Nicolas FJ, Hill CS. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22:3698–711. doi: 10.1038/sj.onc.1206420. [DOI] [PubMed] [Google Scholar]
- 109.Bianco C, Strizzi L, Mancino M, Watanabe K, Gonzales M, Hamada S, Raafat A, Sahlah L, Chang C, Sotgia F, Normanno N, Lisanti M, Salomon DS. Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells. Am J Pathol. 2008;172:345–57. doi: 10.2353/ajpath.2008.070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Santis ML, Kannan S, Smith GH, Seno M, Bianco C, Kim N, Martinez-Lacaci I, Wallace-Jones B, Salomon DS. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21ras-and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–66. [PubMed] [Google Scholar]
- 111.Ebert AD, Wechselberger C, Frank S, Wallace-Jones B, Seno M, Martinez-Lacaci I, Bianco C, De Santis M, Weitzel HK, Salomon DS. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells. Cancer Res. 1999;59:4502–5. [PubMed] [Google Scholar]
- 112.Kannan S, De Santis M, Lohmeyer M, Riese DJ, 2nd, Smith GH, Hynes N, Seno M, Brandt R, Bianco C, Persico G, Kenney N, Normanno N, Martinez-Lacaci I, Ciardiello F, Stern DF, Gullick WJ, Salomon DS. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J Biol Chem. 1997;272:3330–5. doi: 10.1074/jbc.272.6.3330. [DOI] [PubMed] [Google Scholar]
- 113.Bianco C, Normanno N, De Luca A, Maiello MR, Wechselberger C, Sun Y, Khan N, Adkins H, Sanicola M, Vonderhaar B, Cohen B, Seno M, Salomon D. Detection and localization of Cripto-1 binding in mouse mammary epithelial cells and in the mouse mammary gland using an immunoglobulin-cripto-1 fusion protein. J Cell Physiol. 2002;190:74–82. doi: 10.1002/jcp.10037. [DOI] [PubMed] [Google Scholar]
- 114.Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–7. [PubMed] [Google Scholar]
- 115.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 116.Brocchieri L, Conway de Macario E, Macario AJ. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 118.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 119.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 120.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–97. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu C, Liguori G, Adamson ED, Persico MG. Specific arrest of cardiogenesis in cultured embryonic stem cells lacking Cripto-1. Dev Biol. 1998;196:237–47. doi: 10.1006/dbio.1998.8862. [DOI] [PubMed] [Google Scholar]
- 122.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–94. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 123.Mao C, Tai WC, Bai Y, Poizat C, Lee AS. In vivo regulation of Grp78/BiP transcription in the embryonic heart: role of the endoplasmic reticulum stress response element and GATA-4. J Biol Chem. 2006;281:8877–87. doi: 10.1074/jbc.M505784200. [DOI] [PubMed] [Google Scholar]
- 124.Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–68. doi: 10.1242/dev.118.4.1157. [DOI] [PubMed] [Google Scholar]
- 125.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–4. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong D, Dubeau L, Bading J, Nguyen K, Luna M, Yu H, Gazit-Bornstein G, Gordon EM, Gomer C, Hall FL, Gambhir SS, Lee AS. Spontaneous and controllable activation of suicide gene expression driven by the stress-inducible grp78 promoter resulting in eradication of sizable human tumors. Hum Gene Ther. 2004;15:553–61. doi: 10.1089/104303404323142006. [DOI] [PubMed] [Google Scholar]
- 127.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 128.Delpino A, Piselli P, Vismara D, Vendetti S, Colizzi V. Cell surface localization of the 78 kD glucose regulated protein (GRP 78) induced by thapsigargin. Mol Membr Biol. 1998;15:21–6. doi: 10.3109/09687689809027514. [DOI] [PubMed] [Google Scholar]
- 129.Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–16. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 130.Jakobsen CG, Rasmussen N, Laenkholm AV, Ditzel HJ. Phage display derived human monoclonal antibodies isolated by binding to the surface of live primary breast cancer cells recognize GRP78. Cancer Res. 2007;67:9507–17. doi: 10.1158/0008-5472.CAN-06-4686. [DOI] [PubMed] [Google Scholar]
- 131.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–24. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 132.Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood. 2009;114:3960–7. doi: 10.1182/blood-2009-03-209668. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–75. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davidson DJ, Haskell C, Majest S, Kherzai A, Egan DA, Walter KA, Schneider A, Gubbins EF, Solomon L, Chen Z, Lesniewski R, Henkin J. Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res. 2005;65:4663–72. doi: 10.1158/0008-5472.CAN-04-3426. [DOI] [PubMed] [Google Scholar]
- 135.Philippova M, Ivanov D, Joshi MB, Kyriakakis E, Rupp K, Afonyushkin T, Bochkov V, Erne P, Resink TJ. Identification of proteins associating with GPI-anchored T-cadherin on the surface of vascular endothelial cells: the role for Grp78/BiP in T-cadherin-dependent cell survival. Mol Cell Biol. 2008;28:4004–17. doi: 10.1128/MCB.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gonzalez-Gronow M, Cuchacovich M, Llanos C, Urzua C, Gawdi G, Pizzo SV. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–31. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- 137.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–88. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol. 2002;76:633–43. doi: 10.1128/JVI.76.2.633-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Misra UK, Gonzalez-Gronow M, Gawdi G, Wang F, Pizzo SV. A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates alpha2M*-induced signalling. Cell Signal. 2004;16:929–38. doi: 10.1016/j.cellsig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 141.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:19444–9. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu Y, Bhojwani D, Carroll WL, Lee AS. Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Blood. 2011;119:817–25. doi: 10.1182/blood-2011-06-357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mintz PJ, Kim J, Do KA, Wang X, Zinner RG, Cristofanilli M, Arap MA, Hong WK, Troncoso P, Logothetis CJ, Pasqualini R, Arap W. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- 144.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–84. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–47. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu MJ, Jan CI, Tsay YG, Yu YH, Huang CY, Lin SC, Liu CJ, Chen YS, Lo JF, Yu CC. Elimination of head and neck cancer initiating cells through targeting glucose regulated protein78 signaling. Mol Cancer. 2010;9:283. doi: 10.1186/1476-4598-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]



