Abstract
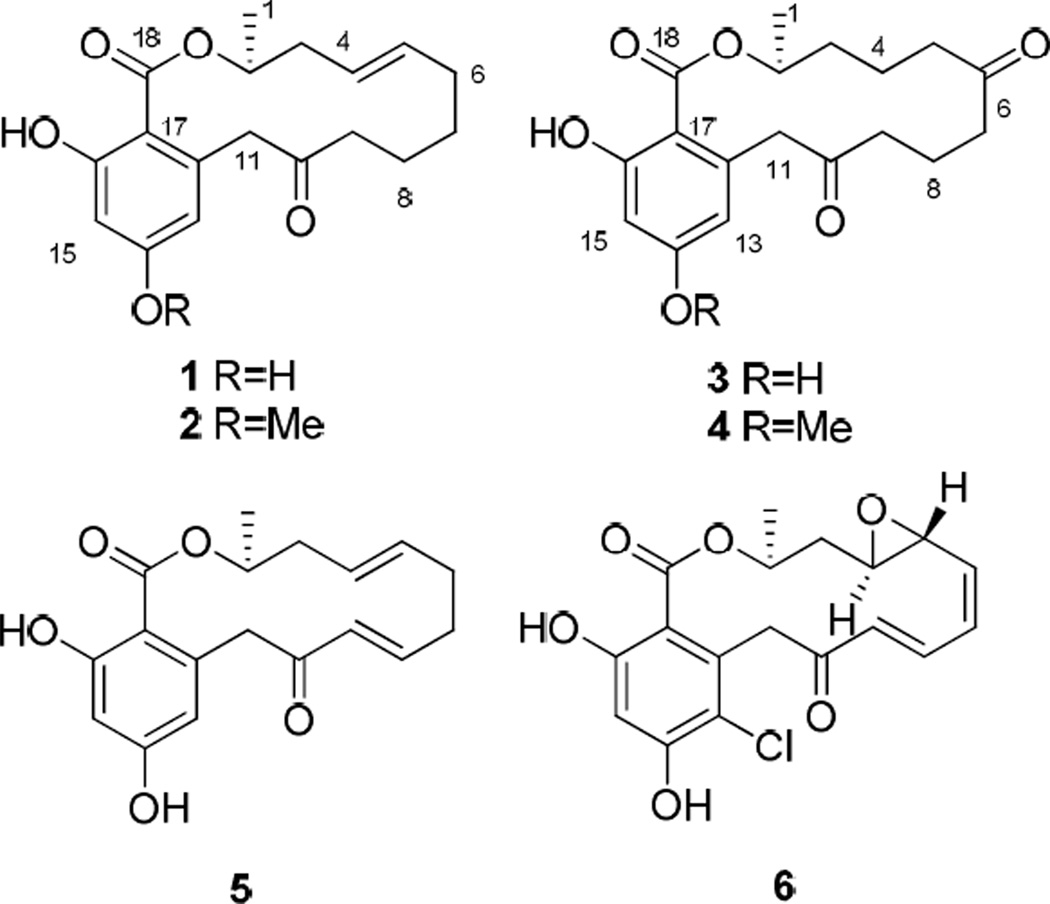
Bioassay-guided fractionation of a fungus Neocosmospora sp. (UM-031509) resulted in the isolation of three new resorcylic acid lactones, neocosmosin A (2), neocosmosin B (3) and neocosmosin C (4). Three known resorcylic acid lactones, monocillin IV (1), monocillin II (5) and radicicol (6) were also isolated and identified. The structures of these compounds were established on the basis of extensive 1D and 2D NMR spectroscopic analysis, mass spectrometric (ESI-MS) data and X-ray crystallography. Compounds 4–6 show good binding affinity for the human opioid receptors. These findings have important implications for evaluating the potential psychoactive effects with this class of compounds.
The scientific literature shows that agonists and antagonists of opioid or cannabinoid receptors, which are G-protein coupled receptors, have long been known to modulate pain.1 During the course of examining fungi for biologically active natural products, we assessed a fungus Neocosmospora sp. (UM-031509) in a high-throughput screen employing a receptor binding assay to find natural products with selective affinity for specific opioid and cannabinoid receptors. The ethyl acetate extract was found to exhibit moderate opioid receptor binding affinity and cannabinoid receptor binding affinity. Successive chromatographic fractionation of extracts from Neocosmospora sp. (UM-031509) yielded three known (1, 5 and 6) and three new resorcylic acid lactones (2–4). We report herein the isolation, structure elucidation and human opioid receptors (subtype δ, κ and μ) and human cannabinoid receptors (subtype CB1 and CB2) binding affinity of six resorcylic acid lactones (1–6).
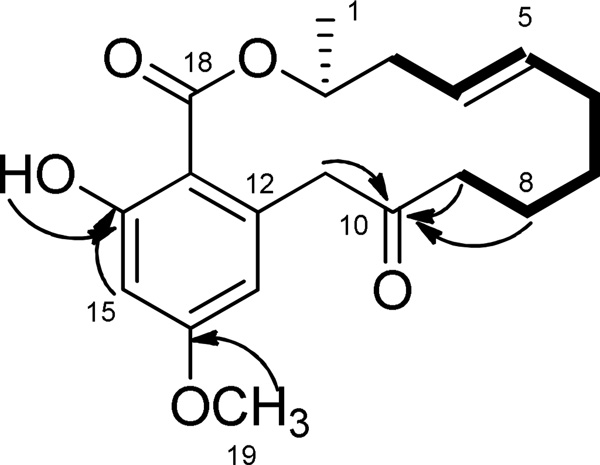
Compound 2 was obtained as a white, amorphous solid and the molecular formula was confirmed by the analysis of positive high-resolution ESIMS (m/z 355.1533, calcd for C19H24O5Na, 355.1521), which is consistent with eight degrees of unsaturation. The 1H NMR spectrum (Table 1) showed signals due to the presence of one secondary methyl group (δH 1.40), twelve methylene protons (δH 1.63–4.42), two olefinic protons (δH 5.46 and 5.51), one hydroxyl group (δH 11.98), one O-methyl group (δH 3.82) and two aromatic protons (δH 6.24 and 6.44) showing a typical pattern of meta-coupling (J=2.5 Hz) consistent with a 1,2,3,5-tetrasubstituted benzene moiety. The 13C and DEPT 135° NMR spectroscopic data of 2 (Table 1) gave signals for 19 carbon atoms, corresponding to methyl and O-methyl groups (δC 18.9 and 55.4), six methylenes (δC 22.1, 25.2, 32.7, 37.7, 40.8 and 50.2), five methines including one O-methine carbon (δC 72.9), two olefinic carbons (δC 124.5 and 135.1), two aromatic carbons (δC 112.1 and 100.1), an ester carbonyl (δC 170.7), one ketone carbonyl (δC 208.2) and four aromatic quaternary carbons (δC 139.1, 163.9, 166.0 and 105.7). The location of the double bond was established by 1H-1H correlations from the COSY experiment, mainly by the correlation of H-4 with H-3 and H-5 with H-6 (Figure 1). The location of the ketone carbonyl was confirmed by the long-range 1H-13C HMBC correlations of H-8, H-9, and H-11 with C-10 (Figure 1). The long-range 1H-13C HMBC correlation of H-2 with C-18 (carbonyl) as well as H-15 with C-18 led to identification of a lactone moiety. The aliphatic chain from H-1 to H-9 was built by the COSY correlations of H-2 with H-1 and H-3, H-4 with H-3 and H-5, H-6 with H-5 and H-7, H-8 with H-7 and H-9. The position of O-methyl group was determined by the long-range 1H-13C HBMC correlations of O-methyl protons with C-14. The highly deshielded resonance at δH 11.98 indicated the presence of an intramolecular hydrogen-bonded phenol, suggesting that the phenol protons are close to strong electronegative atoms or groups, such as carboxylic acids. The location of the phenol group was established by the long-range 1H-13C HBMC correlations of H-15 with C-16, and the correlations of phenol hydrogen with C-16 (Figure 1). Accordingly, compound 2 was given the trivial name neocosmosin A.
Table 1.
1H (500Hz) and 13C (150Hz) NMR Data for Compounds 1–4a (CDCl3)
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| position | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 1 | 19.0 | 1.41, d (6.5) | 18.9 | 1.40, d (6.5) | 21.4 | 1.34, d (6.2) | 21.4 | 1.32, d (6.2) |
| 2 | 72.9 | 5.35, m | 72.9 | 5.35, m | 72.8 | 5.18, m | 72.7 | 5.18, dq (12.4, 6.3) |
| 3 | 37.8 | 2.30, m 2.63, m | 37.7 | 2.27, 2.63, m | 35.2 | 1.47, 1.63, m | 35.1 | 1.47, 1.61, m |
| 4 | 124.7 | 5.48, m | 124.5 | 5.51, m | 21.7 | 1.64, m | 21.7 | 1.62, m |
| 5 | 135.2 | 5.49, m | 135.1 | 5.46, m | 43.7 | 2.10, 2.67, m | 43.7 | 2.08, 2.63, m |
| 6 | 32.6 | 2.10, m | 32.7 | 2.09, m | 213.1 | - | 212.9 | - |
| 7 | 25.2 | 1.63, 1.43, m | 25.2 | 1.63, 1.43, m | 37.3 | 3.33, 2.20, dt (17.8, 4.0) | 37.3 | 3.33, 2.17, dt (17.8, 4.0) |
| 8 | 22.3 | 1.65, m | 22.1 | 1.65, m | 17.1 | 1.84, 2.04, m | 17.1 | 1.83, 1.98, m |
| 9 | 40.8 | 2.61, 2.43, m | 40.8 | 2.55, 2.39, m | 38.6 | 2.68, 2.55, m | 38.5 | 2.65, 2.53, m |
| 10 | 210.2 | - | 208.2 | - | 207.5 | - | 206.8 | - |
| 11 | 49.8 | 4.48, 3.55, d (16.8) | 50.2 | 4.42, 3.51, d (16.8) | 50.4 | 4.53, 3.48, d (17.9) | 50.5 | 4.51, 3.48, d (17.9) |
| 12 | 139.2 | - | 139.1 | - | 138.5 | - | 138.0 | - |
| 13 | 112.2 | 6.24, d (2.5) | 112.1 | 6.24, d (2.5) | 113.0 | 6.07, d (2.5) | 112.8 | 6.14, d (2.5) |
| 14 | 160.9 | OH:6.84, s | 163.9 | - | 160.8 | OH:5.96, s | 163.9 | - |
| 15 | 103.0 | 6.40, d (2.5) | 100.1 | 6.44, d (2.5) | 103.0 | 6.37, d (2.5) | 100.2 | 6.43, d (2.5) |
| 16 | 165.7 | OH:11.95, s | 166.0 | OH:11.98, s | 166.1 | OH:12.02, s | 166.2 | OH:12.05, s |
| 17 | 105.7 | - | 105.7 | - | 105.5 | - | 105.6 | - |
| 18 | 170.6 | - | 170.7 | - | 171.0 | - | 171.0 | - |
| 19 | - | - | 55.4 | 3.82, s | - | - | 55.4 | 3.80, s |
Assignments are based on 1D and 2D NMR experiments.
Figure 1.
Key HMBC (arrow) and COSY (bold) correlations of 2
Compound 3 was obtained as a white, amorphous solid with a molecular formula of C18H22O6, which was confirmed by the analysis of positive high-resolution ESIMS (m/z 357.1322, calcd for C18H22O6Na, 357.1314). The 1H and 13C NMR spectra (Table 1) were similar to those of compound 2, suggesting the same skeleton. However, the 1H NMR spectrum showed two hydroxyl groups (δH 12.02 and 5.96) and no O-methyl groups, suggesting that the hydroxyl group replaced the O-methyl group. The 13C and DEPT 135° NMR spectra (Table 1) gave signals for 18 carbon atoms including two ketone carbonyls (δC 207.5 and 213.1). In comparison with compound 2, the above data indicated that compound 3 contained no double bond but had an additional non-conjugated carbonyl. The location of additional ketone carbonyl C-6 (δC 213.1) was confirmed by its long-range 1H-13C HMBC correlations of H-5 and H-7 with C-6. Inspection of 1H-1H correlations from the COSY experiments, 1H-13C correlations from the HSQC and HMBC experiments, and MS data supported the proposed structure for compound 3, which was given the trivial name neocosmosin B.
Compound 4 was obtained as a white, amorphous solid with a molecular formula of C19H24O6, which was confirmed by the analysis of positive high-resolution ESIMS (m/z 371.1461, calcd for C19H24O6Na, 371.1471). The 1H and 13C NMR spectra of 4 (Tables 1) are similar to those of compound 3, differing by a methoxy group that replaces the hydroxyl group. Therefore, compound 4 was given the trivial name neocosmosin C.
Compound 1 was identified to be monocillin IV as shown by its NMR data, optical rotation and X-ray diffraction.2,3 The asymmetric carbon atom C-2 in monocillin IV was determined to have R configuration from single-crystal X-ray diffraction. Therefore, the absolute configuration of the asymmetric carbon atom C-2 in compound 2, 3 and 4 is proposed to be analogous to that of monocillin IV (1) due to the same optical activity sign. Furthermore, the geometry of the double bond (C4–C5) is difficult to determine by coupling constants because of the overlap of H-4 and H-5. The geometry of the double bond (C4–C5) in monocillin IV was E configuration from single-crystal X-ray diffraction. Therefore, we propose the geometry of the double bond (C4–C5) in compound 2 to be E configuration.
Known compounds 5 and 6 were identified as monocillin II and radicicol by comparing their spectroscopic data with the reported data.4
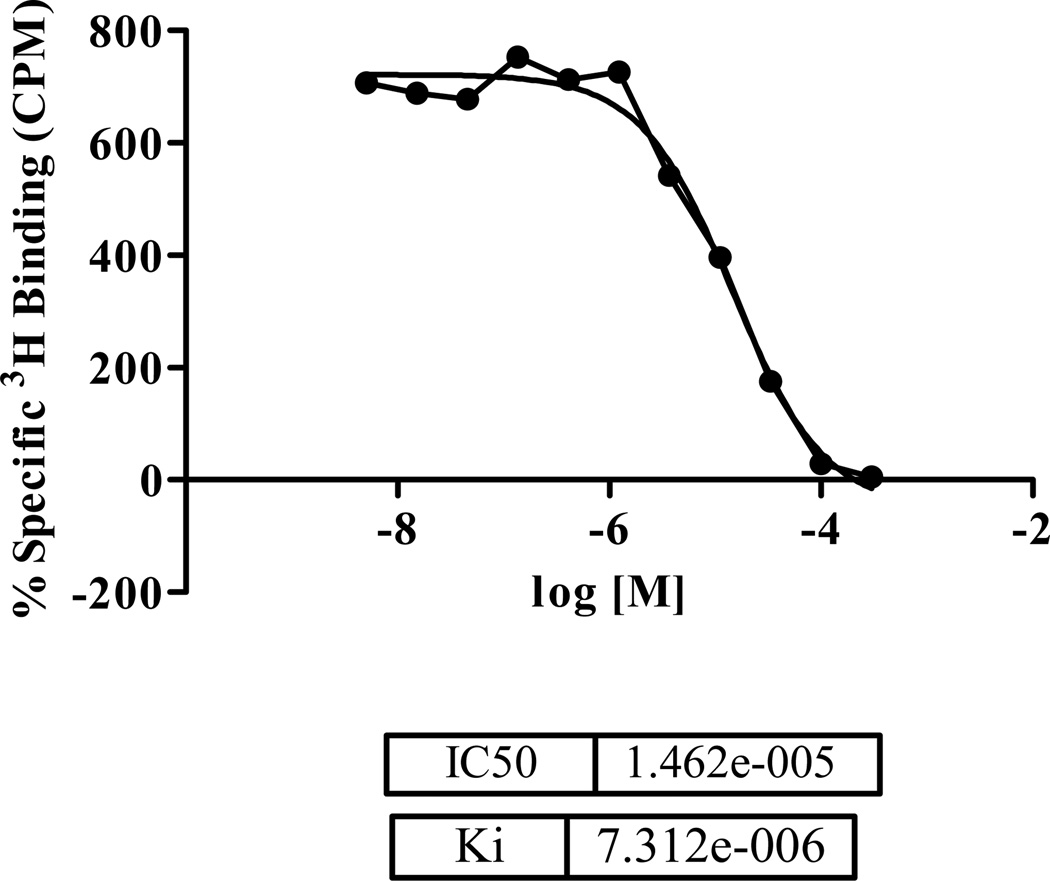
All pure compounds (1–6) were submitted for in vitro binding assays using opioid receptors (subtype δ, κ and μ) and cannabinoid receptors (CB1 and CB2). The results are shown in Table 2. Compound 4 selectively inhibited 54.9 % the specific binding of [3H]-enkephalin to CHO-K1 cell membranes expressing human δ–opioid receptors at the concentration of 10 µM (IC50 values of 14.82 µM) (Figure 2 and Table 2). Compound 5 and 6 selectively inhibited 63.5% and 84.9% the specific binding of [3H]-[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) to CHO-K1 cell membranes expressing human μ–opioid receptors at the concentration of 10 µM (Table 2).
Table 2.
Binding Affinity Assay of the Compounds (1–6) for Human Opioid Receptors (subtype δ, κ and μ) and Cannabinoid Receptors (subtype CB1 and CB2)
| Opioid Receptors (%) | Cannabinoid Receptors (%) | ||||
|---|---|---|---|---|---|
| Compound | δ | κ | μ | CB1 | CB2 |
| 1 | 45.7 | 24. 7 | 8.7 | n.t.a | n.t. |
| 2 | 44.7 | 11.4 | 22.4 | n.t. | 44.5 |
| 3 | −60.3 | 8.1 | 41.3 | 47.4 | 27.7 |
| 4 | 54.9 | 32.7 | 24.0 | n.t. | 46.3 |
| 5 | −3.1 | 9.0 | 63.5 | 26.2 | 14.8 |
| 6 | −27.8 | −56.0 | 84.9 | 30.7 | 13.0 |
| Naloxone | 103.4 | 105.6 | 100.1 | n.t. | n.t. |
| CP 55,940 | n.t. | n.t. | n.t. | 88.8 | 90.3 |
Not Tested
Figure 2.
IC50 and Ki value of compound 4.
The [35S]-GTPγS functional assay can be applied to assess agonism, antagonism, and inverse agonism of ligands of G-protein-coupled receptors.5 The principle of [35S]-GTPγS functional assays is that agonists stimulate the binding of [35S]-GTPγS, a stable analogue of GTP, to the G-protein, inverse agonists reduce [35S]-GTPγS binding, and antagonists have no effect.
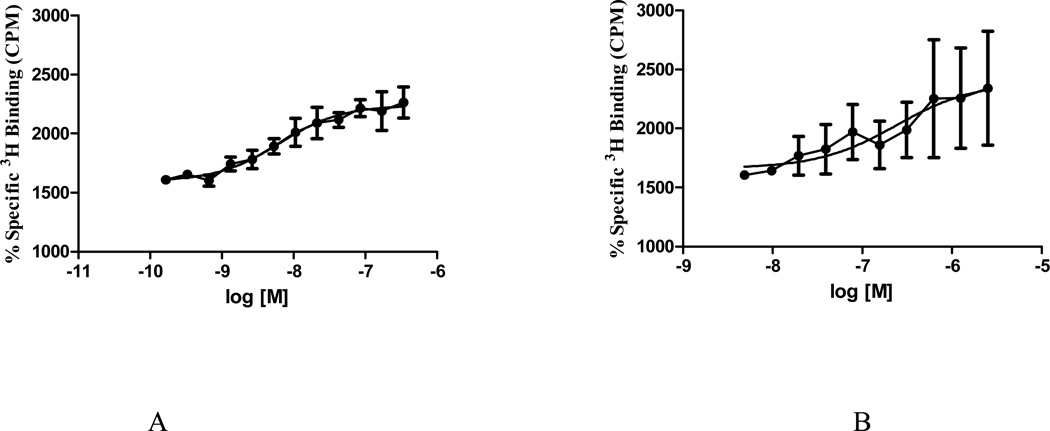
Figure 3 showed the effects of the standard agonist [2-D-penicillamine, 5-D-penicillamine]-enkephalin (DPDPE) on [35S]-GTPγS binding to CHO-K1 cell membranes, which contain a high density of δ-opioid receptors. The agonist DPDPE clearly enhanced [35S]-GTPγS binding in a dose-dependent manner (EC50=6.34 nM, maximal effect 2200 ± 100%) (Figure 3A). Compound 4 increased [35S]-GTPγS binding (EC50=239 nM) (Figure 3B). However, the maximal stimulation (2400 ± 600%) was similar to that of the full agonist DPDPE. Thus, compound 4 can be classified as a full agonist at the human δ-opioid receptor.
Figure 3.
[35S]-GTPγS functional assay data for DPDPE (A) (IC50=6.34 nM) and compound 4 (B) (IC50=239 nM).
In conclusion, we have isolated six resorcylic acid lactone derivatives, three of which are new natural products (2–4). Compound 4 was found to be a potent, full agonist at the δ-opioid receptor. Furthermore, compounds 5 and 6, showed potent binding affinities at the μ-opioid receptor. This is the first report on resorcylic acid lactone derivatives with strong affinities for human opioid receptors suggesting that this series of compounds can serve as leads in the development of structure and activity relationship (SAR) studies of this class of compounds.
Experimental Section
General Experimental Procedures
Optical rotations were recorded using a Rudolph Research Analytical Autopol V polarimeter. UV was respectively obtained using a Perkin-Elmer Lambda 3B UV/vis-spectrophotomer. 1H and 13C NMR spectra were obtained on Bruker model AMX 500 NMR spectrometers with standard pulse sequences, operating at 500 MHz in 1H and 125 MHz in 13C. The chemical shift values were reported in parts per million units (ppm) from trimethylsilane (TMS) using known solvent chemical shifts. Coupling constants were recorded in Hertz (Hz). Standard pulse sequences were used for COSY, HMQC, HMBC, TOCSY, NOESY and DEPT. High-resolution mass spectra (HRMS) were measured on a Micromass Q-Tof Micro mass spectrometer with a lock spray source. Column chromatography was carried out on silica gel (70–230 mesh, Merck) and Sephadex LH-20 (Mitsubishi Kagaku, Tokyo, Japan). TLC (silica gel 60 F254) was used to monitor fractions from column chromatography. Preparative TLC was carried out on silica gel 60 PF254+366 plates (20 × 20 cm, 1 mm thick). Visualization of the TLC plates was achieved with a UV lamp (λ=254 and 365 nm) and anisaldehyde/acid spray reagent (MeOH-acetic acid-anisaldehyde-sulfuric acid, 85:9:1:5). All HPLC analyses were performed on a Waters LC Module I equipped with a UV detector 486 utilizing the Millenium 32 Chromatography Manager software (Waters). An ODS column (Phenomenex Luna C18, 10 × 250 mm, 5 µm) was used. All HPLC solvents were HPLC grade, filtered through appropriate filters (water through 0.45 µm and organic solvents through 0.22 µm filters) and purged prior to and during analysis with nitrogen gas at a flow rate of 50 mL/min. If possible, a single crystal was used for data collection using a Bruker Smart Apex II system with Cu Kα radiation with a graphite monochromator, fine-focus sealed tube. The crystal was stored at 100 K under cooled nitrogen gas with a KRYO-FLEX low-temperature device. APEX II software was used to perform data collection, indexing, and initial cell refinements. All chemicals used were purchased from Sigma-Aldrich (Poole, Dorset, U.K.) with the following exceptions: for the binding experiments, [3H]-CP-55,940 (174.8 Ci/mmol), [3H]-DAMGO (53.4 Ci/mmol), [3H]-U-69,593 (42.7 Ci/mmol), and [3H]-enkephalin (45 Ci/mmol) were purchased from Perkin-Elmer Life Sciences Inc. (Boston, MA, U.S.A.). CP-55,940, DAMGO, DPDPE, nor-binaltorphimine, and WIN 55,212-2 mesylate were purchased from Tocris Bioscience (Ellisville, Missouri, U.S.A.).
Fungal Materials
A strain of Fusarium solani (UM-031509) was collected from a piece of orange peel in Tifton, Georgia, and the membership of the isolate in this species was confirmed by the United States Department of Agriculture (USDA). The isolate produced Fusarium-like colonies and microconidia. Bt2a and Bt2b primers were used for amplification of beta-tubulin DNA sequences,6 and EF-1 and EF-2 primers for elongation factor-1α partial gene sequences.7 Amplification was performed on a Stratagene RoboCycler® thermal cycler. Sequencing reactions and primer synthesis were performed by Eurofins MWG/Operon (Huntsville, AL). Sequence data were assembled and aligned using Sequencher™ 4.9 (Gene Codes Corp. Ann Arbor, MI). Sequences were compared to those in GenBank and published sequences belonging to the Fusarium solani species complex (FSSC) using BLASTN 2.2.21 software.8 Beta-tubulin gene sequence analysis indicated that the isolate belongs to the genus Fusarium. The isolate EF-1α had 98–99% identity to members of Clade 3, haplotype 8, identifying it as a Neocosmospora species.9,10 The EF-1α gene sequence is 100% homologous to a single Neocosmospora sp. isolate, NRRL 22472 (the only sequence in the gene bank with 100% homology). NRRL 22472, although well documented as belonging to Neocosmospora (FSSC), has not been assigned a species name. A voucher specimen (UM-031509) has been deposited in the culture collection of the Medicinal Chemistry Department, University of Mississippi.
Fungus Culture
The fungus was plated-out on potato-dextrose agar (PDA) that was maintained at 24 °C until discrete fungal colonies appeared. Samples were taken from colonies and kept on PDA slants in test tubes at 24 °C, then placed in a 4 °C refrigerator until used. The fungus was inoculated in 50 mL of potato-dextrose broth and kept for two weeks in stationary phase at 24 °C. Then the fungus was seeded into a medium consisting of 100 g of shredded wheat, 200 mL of low-pH Oxoid mycological broth, 2% yeast extract, and 20% sucrose in 2.0 L Fernbach flasks (10 flasks were used) followed by incubation for 22 days at 24 °C.
Extraction and Isolation
Following incubation, 300 mL of acetone were added to each flask, and the mycelia and the substrate were homogenized (Super Dispex, Tekmark Co., SD-45). The suspension was filtered and the filtrate was concentrated under vacuum at 40 °C. The residue was mixed with H2O (200 mL), then extracted with EtOAc (500 mL × 3). The EtOAc extracts were combined, dried using anhydrous Na2SO4, and concentrated under vacuum. The EtOAc extract (10.0 g) was chromatographed on silica gel 60, 70–230 mesh (400 g), with fractions stepwise from hexane to methanol (90% hexane in EtOAc, 60 % hexane in EtOAc, 40% hexane in EtOAc, 10% hexane in EtOAc, 80% EtOAc in MeOH, 50% EtOAc in MeOH, and 100% MeOH), yielding seven fractions. The second and third fractions showed good binding affinities for opioid and cannabinoid receptors and were combined. The fraction was rechromatographed using a silica gel column eluted with a hexane-EtOAc gradient to yield 47 fractions. Based on TLC, using the solvent system hexane-EtOAc (1:1), these 47 fractions were combined into three subfractions. The first subfraction was purified by HPLC using an RP C18 column and a gradient elution from H2O/MeOH (4:6) to afford 2 (10 mg) and 4 (3 mg). The second subfraction was purified by using an RP C18 column and a gradient elution from H2O/MeOH (4:6) to afford 3 (8 mg) and 1 (25 mg). The third subfraction was purified by using an RP C18 column and a gradient elution from H2O/MeOH (4:6) to afford 5 (3 mg) and 6 (4 mg).
Cell Culture
HEK293 cells (ATCC) were stably transfected via electroporation with full length human recombinant cDNA for cannabinoid receptor subtypes 1 and 2 (obtained from Origene). These cells were maintained at 37 °C and 5% CO2 in a Dulbecco’s Modified Eagles’s medium (DMEM) nutrient mixture F-12 HAM supplemented with 2 mM L-glutamine, 10% fetal bovine serum, penicillin–streptomycin, and G418 antibiotic solutions. CHO-K1 cells stably transfected with opioid receptor subtypes V, δ, and κ, were used to perform the opioid receptor binding assays. These cells were maintained at 37°C and 5% CO2 in a DMEM nutrient mixture supplemented with 2 mM L-glutamine, 10% fetal bovine serum, penicillin–streptomycin, and either G418 or hygromycin B antibiotic solutions. Membranes for the radio-ligand binding assays were prepared by scraping the cells in a 50 mM Tris-HCl buffer, homogenation via sonication, and then centrifuged for 40 minutes at 13650 rpm at 4 °C. These were kept at −80 °C until used for bioassays. Protein concentration was found via Bio-Rad Protein Assay.11
Radio-ligand Binding for Cannabinoid Receptor Subtypes
Compounds evaluated in the assay were run in competition binding against both the cannabinoid receptor subtypes, CB1 and CB2.12 Cannabinoid receptor binding assays were performed under the following conditions: 10 µM of each compound was incubated with 0.5 nM [3H]-CP 55,940, the potent cannabinoid agonist with affinity to both receptor subtypes, and 10 µg CB1 or CB2 membrane prepared in the above section for 90 minutes in a 96-well plate. The reaction was terminated via rapid vacuum filtration through GF/C filters presoaked with 0.3% BSA using a Perkin Elmer 96-well Unifilter (Perkin Elmer Life Sciences Inc., Boston, Mass. U.S.A.) followed by 10 washes with 50 mM Tris-EDTA buffer containing 0.2% BSA. Plates were read using a Perkin Elmer Topcount (Perkin Elmer Life Sciences Inc., Boston, Mass. U.S.A.). Total binding was defined as binding in the presence of 1.0% DMSO. Nonspecific binding was the binding observed in the presence of 1.0 µM CP-55,940. Specific binding was defined as the difference between total and nonspecific binding. Percent binding was calculated using the following formula:
100-(binding of compound- nonspecific binding)*100/specific binding.
Ki and IC50 values were calculated using Graph-Pad Prism 5.
Radio-ligand Binding for Opioid Receptor Subtypes
All compounds evaluated in the assay were run in competition binding against the opioid receptor subtypes (δ, κ, μ), as well as the cannabinoid receptor subtypes mentioned above. Saturation experiments were performed after each batch of membrane was scraped for each of the three opioid cell lines (δ, κ, μ) to determine the optimal tritium and membrane concentration to be used in the assay. Saturation experiments determine the receptor number and radioligand affinity for the membrane. Opioid binding assays were performed under the following conditions: 10 µM of each compound was incubated with [3H]-DAMGO (μ), [3H]-U-69,593 (κ), or [3H]-enkephalin (δ) for 60 minutes in a 96-well plate. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked with 0.3% BSA using a Perkin Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris-HCl. Plates were read using a Perkin Elmer Topcount. Total binding was defined as binding in the presence of 1.0% DMSO. Nonspecific binding was the binding observed in the presence of 10 µM DAMGO (μ), nor-binaltorphimine (κ), or DPDPE (δ). Specific binding was defined as the difference between total and nonspecific binding. Percent binding was calculated using the following formula:
100-(binding of compound- nonspecific binding)*100/specific binding.
Ki and IC50 values were calculated using Graph-Pad Prism 5.
[35S]-GTPγS Functional Assay
The following conditions were used for the functional assay: 10 µL of diluted test compounds were incubated with a predetermined protein concentration of 300 µl membrane and 40 µl of 0.05 nM 35S-labelled GTP, giving a final reaction volume of 350 µl per well. Plates were incubated for 60 minutes at room temperature. The reaction was terminated via rapid vacuum filtration through GF/C filters with a Perkin Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris-HCl (pH=7.4). Plates were read using a Perkin Elmer Topcount. Basal binding was defined as binding in the presence of GTPγS buffers. Nonspecific binding was the binding observed in the presence of 10 µM unlabelled GTPγS salt. Emax binding was defined as binding in the presence of 10 µM of DAMGO (μ), nor-binaltorphimine (κ), or DPDPE (δ). Percent stimulation was calculated using the following formula:
(binding of compound- nonspecific binding)/Emax) * 100.
IC50 or EC50 values were calculated using Graph-Pad Prism 5.
Neocosmosin A (2): white, amorphous solid; mp 160–161 °C; -43 (c 0.6, CHCl3); λmax 215 nm 1H NMR (Table 1, 500 MHz, CDCl3); 13C NMR (Table 1, 150 MHz, CDCl3); HRESIMS m/z 355.1533 [M+Na]+ (calcd for C19H24O5Na, 355.1521).
Neocosmosin B (3): white crystals; mp 148–149 °C; -78 (c 0.4, CHCl3); λmax 210 nm 1H NMR (Table 1, 500 MHz, CDCl3); 13C NMR (Table 1, 150 MHz, CDCl3); HRESIMS m/z 357.1322 [M+Na]+ (calcd for C18H22O6Na, 357.1314).
Neocosmosin C (4): white, amorphous solid; mp 148–149 °C; -52 (c 0.3, CHCl3); λmax 210 nm 1H NMR (Table 1, 500 MHz, CDCl3); 13C NMR (Table 1, 150 MHz, CDCl3); HRESIMS m/z 371.1461 [M+Na]+ (calcd for C19H24O6Na, 371.1471).
Supplementary Material
ACKNOWLEDGMENTS
The project described was supported by Grant Number 5P20RR021929 from the National Center for Research Resources (NCRR) and 9P20GM104932-06 from the National Institute of General Medical Sciences, components of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Furthermore, this investigation was conducted in a facility constructed with support from research facilities improvement program C06 RR-14503-01 from the NIH National Center for Research Resources. The authors are grateful to Corey Gemelli for his assistance in performing the membrane preparations for the opioid assays. The opioid receptors were a generous gift from Dr. Bryan Roth, University of North Carolina at Chapel Hill, Chapel Hill, N.C., U.S.A.
Footnotes
ASSOCIATED CONTENT
Supporting Information.
HRESIMS, 1D- and 2D-NMR spectra of 2–4. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES AND NOTES
- 1.Ahmad S, Dray A. Curr. Opin. Investig. Drugs. 2004;5:67–70. [PubMed] [Google Scholar]
- 2.Proisy N, Sharp SY, Boxall K, Connelly S, Roe SM, Prodromou C, Slawin AM, Pearl LH, Workman P, Moody CJ. Chem. Biol. 2006;13:1203–1215. doi: 10.1016/j.chembiol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Ayer WA, Pena-Rodriguez L, Vederas JC. Can. J. Microbiol. 1981;27:846–847. doi: 10.1139/m81-131. [DOI] [PubMed] [Google Scholar]
- 4.Hellwig V, Mayer-Bartschmid A, Muller H, Greif G, Kleymann G, Zitzmann W, Tichy HV, Stadler M. J. Nat. Prod. 2003;66:829–837. doi: 10.1021/np020556v. [DOI] [PubMed] [Google Scholar]
- 5.Harrison C, Traynor JR. Life Sci. 200374:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Glass NL, Donaldson GC. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Schwartz S, Wagner L. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM. J. Clin. Microbiol. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogale M, Steenkamp ET, Wingfield MJ, Wingfield BD. Eur. J. Plant Pathol. 2009;124:369–378. [Google Scholar]
- 11.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Ross RA, Gibson TM, Stevenson LA, Saha B, Crocker P, Razdan RK, Pertwee RG. Brit. J. Pharmacol. 1999;128:735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.