Abstract
Objectives
The objectives of this in vitro study were to investigate the sensitivity and reliability of the Osstell™ systems (Resonance Frequency Analysis – RFA) compared to the Periotest® system in implant bone simulated conditions. Three conditions were simulated: (1) the direct fixture-bone contact and fibrous tissue fixture contact, (2) The different levels of horizontal bone loss, and (3) The hardening implant–bone interface.
Materials and methods
Forty-nine dental implant fixtures were placed in the center of acrylic cubes. In Part I seven fixtures were placed in direct contact with acrylic and another seven were placed in contact with polyvinyl siloxane impression material as soft interface. Part II: four sets of 0, 2, 4 and 6 mm horizontally exposed fixture samples were made (seven fixtures in each set). Part III: seven fixtures were placed in contact with a thin mix of autopolymerizing resin. The stability of these fixtures was measured using Osstell™ and Periotest® systems.
Results
The mean Periotest® value(PTV) and Osstell™ measurements showed a significant difference between the direct contact and soft interface (P < 0.001). These values also showed statistically significant difference between the different levels of horizontally exposed fixture groups (P < 0.001). The level of horizontal fixture exposure was strongly correlated with the PTVS (r = 0.967) and strongly negatively correlated with Implant Stability Quotient (r = −0.946). A strong correlation was found between the Osstell™ readings and the change in the stiffness of the autopolymerizing resin fixture interface group (r = 0.986).
Conclusions
Both Osstell™ and Periotest® systems proved to be sensitive in measuring dental implant stability in hard and in soft interfaces. Osstell™ also proved to be sensitive in detecting changes in the fixture interface stiffness. Osstell™ system proved to be more reliable compared to Periotest® system in measuring dental implant stability in hard and in soft interfaces.
Keywords: Osseointegration, Implant Stability, Periotest, Osstell, Resonance Frequency Analysis
1. Introduction
While dental implants have become increasingly important in many disciplines in oral rehabilitation, implant treatment still fail. Initial stability at placement (primary stability) and the development of osseointegration in the following healing process (secondary stability) are two important factors for implant success. Clinicians generally agree that it is important to verify the status of implant–bone interface before attachment of prosthetic abutment, after completion and insertion of the definitive prosthesis; and during the maintenance phases (Walker et al., 1997). Implants following the concept of osseointegration are considered failures if mobility is detected (Albrektsson et al., 1986; Schnitman and Shulman, 1979). The soft tissue changes associated with lack of implant osseointegration and/or mechanical failures can also be evaluated but are not easily quantified.
A simple, predictable, noninvasive test to quantify implant stability and osseointegration is highly desirable. It was originally believed that osseointegration of implant can be assessed by tapping an implant and/or abutment with a metal instrument and assessing the nature of the sound. This has proven to be unsuccessful due to the inability of humans to consistently discriminate sound in terms of specific, sensitive criteria (Meredith, 1998).
To evaluate the initial bone quality and the degree of osseointegration, various methods have been proposed (Huang et al., 2003), including histology and histomorphometry (Albrektsson and Jacobsson, 1987; Ericsson et al., 1994; Sennerby et al., 1992a,b), removal torque analysis,(Carlsson et al., 1988; Johansson et al., 1991; Wennerberg et al., 1995) pull- and push-through tests (Dhert et al., 1992) and X-ray examination (Meredith, 1998). However, due to problems of invasiveness and inaccuracy, these methods are not suitable for long-term clinical assessment. To overcome these problems, a noninvasive device called the Periotest® (Periotest®, Siemens AG, Bensheim, Germany) was used to monitor the implant stability (Olive and Aparicio, 1990; Teerlinck et al., 1991). It was originally designed to measure tooth movement in quantitative units. The manufacturer suggests that tooth mobility can be quantitatively ascertained with a high degree of precision in the absence of pathologic radiographic findings (Drago, 2000). The range in Preiotest® values (PTV) shown by clinically immobile dental implants depends on the damping characteristics of the surrounding tissues (bone in successful implants and fibrous tissues in failed implants). Since the slightest clinical mobility of an implant is considered a symptom of failure, the assessment of Periotest® value is of clinical interest (Tricio et al., 1995).
Unfortunately, because the Periotest value is strongly related to the excitation direction and position, the reading from the method does not always correspond precisely to a biomechanical parameter (Caulier et al., 1997; Derhami et al., 1995). Due to the need for a nondestructive and noninvasive device to evaluate the conditions of implant–bone interface in vivo, a new device (Osstell™, Integration Diagnostics Ltd., Goteborgsvagen, Sweden) based on Resonance Frequency Analysis (RFA) was developed (Huang et al., 2003). In a series of publications, Meredith and co-workers (1996) reported on the use of transducer that can be directly attached to an implant body or to the abutment on the implant. Clinically, RFA values have been correlated with changes in implant stability during osseous healing, failure of implants, and the supracrestal dimensions of the implant and given a wide range of values (Friberg et al., 1999).
The objectives of this in vitro study were to investigate the sensitivity and reliability of Osstell™ (Resonance Frequency Analysis – RFA) compared to the Periotest® system in implant bone simulated conditions. Three conditions were simulated (1) the direct fixture-bone contact and fibrous-tissue–implant interface, (2) The different levels of horizontal bone loss, and (3) The hardening implant–bone interface during the implant healing phase.
2. Materials and methods
2.1. Samples preparation
The testing media comprised of 49 acrylic resin cubes (15 × 15 mm in cross section and 20 mm in height) which were fabricated with cold cure acrylic resin (Orthoresin, Dentsply, DeguDent GmbH, Postfach, Hanau, Germany). The cold cure resin was poured into a silicon mold after mixing the powder and liquid as per the manufacturer’s recommendations (Flexural modulus = 55 MPa). To minimize voids entrapment, the mix was vibrated on a mechanical vibrator (Vibromaster, Bego Bremer Goldshagerel will GmBA & Co., Bremen, Germany) which was set at frequency of 6000 cycles/min and 0.40 mm amplitude of vibration. The mold was placed in a pressure pot filled with 45 °C water for 10 min at 30 psi. The set acrylic cubes were left for 24 h at room temperature before implant placement.
In order to address the main objectives, the study was methodologically divided into three parts:
Part I: Group A: Seven ITI implants (ITI dental implant system, Straumann Institute, Waldenburg, Switzerland), (Solid screw, SLA, ∅ 4.1 mm diameter, shoulder 4.3 mm, length 14 mm) were placed in the center of the acrylic cubes and in direct contact with the acrylic using the standard surgical protocol described in ITI implant system manual. The implants were placed into the prepared bed mechanically using a hand piece adaptor at 15 rpm. The samples were numbered by a permanent marker (from D1 to D7). This group simulates the fixture bone direct contact. The transfer part was not removed from the implant to serve as standard abutment in the Periotest® measurements.
2.2. Ostell™ and Periotest® measurements
The Periotest® hand piece (Periotest®, Siemens AG, Bensheim, Germany) was mounted on a three-arm clamp and firmly screwed on a vertical stand. For maximum control, the stand position was secured to the bench. The handpiece sleeve was set at a fixed distance from a flat surface of the hexagon and centered perpendicularly to the long axis of the implant. Three measurements were recorded for each sample following the operating instructions recommended by the manufacturer in the clinical manual and registered to a chart particularly designed for this purpose.
The Osstell™ measurements (Osstell™, Integration Diagnostics Ltd., Goteborgsvagen, Sweden) were made by attaching the Osstell™ transducer (No. 100063) at the fixture level using manual torquing. The effect of manual tightening on ISQ (Implant Stability Quotient) was assessed by attaching the transducer manually to an implant and tightened firmly until no more tightening can be accomplished, an ISQ reading was recorded and the transducer was unscrewed. This process was repeated ten times, the ten readings were found to be identical. Three measurements were recorded for each sample in the following experiments using the Osstell™ kit.
Group B: Seven cubes were prepared for seven ITI implants (Solid screw, SLA, ∅ 4.1 mm diameter, shoulder 4.3 mm, length 14 mm); the sockets were widened using a twist drill ∅ 4.2 mm to create 0.1 mm space around the fixture surface. This space was filled by polyvinylsiloxane impression material to act as soft interface between the implant and the acrylic resin, simulating the failure of osseointegration, i.e. healing by fibrous encapsulation. Prior to implant placement, light body polyvinylsiloxane impression material (Zerosil-light, Dreve-Dentamid GMBH, Max-Planck-Straße, Unna, Germany) was dispensed using an injector and a mixing canula. A lentulo spiral was used in the socket at 200 rpm anticlockwise to avoid air bubbles entrapment. The fixture threads were covered with the same mix of impression material and inserted slowly into the socket over the vibrator. The excess material was removed by No. 15 scalpel blade after 4 min (the setting time recommended in the manufacture leaflet). These samples were numbered (S1–S7) and left for 24 h before measurements. The implant stability was measured using the Periotest® and the Osstell™ systems following the same measurement method described earlier for the first group.
Part II: This part of the experiment consisted of four groups, seven implants in each group. The fixtures (ITI, Solid screw, SLA, ∅ 4.1 mm diameter, shoulder 4.3 mm, length 14 mm) were placed directly in the acrylic cubes using the standard ITI surgical (drilling) protocol exposing different heights of the fixture in relation to the horizontal level of the acrylic resin (0 mm, 2 mm, 4 mm and 6 mm) to simulate different stages of horizontal alveolar bone loss. The socket depths were 14, 12, 10 and 8 mm, respectively. These groups were numbered: (A1–A7), (B1–B7), (C1–C7) and (D1–D7), respectively. The measurements of the implant stability using the Periotest® and the Osstell™ systems were made following the method described earlier in Part I.
Part III: In order to evaluate the effect of interface hardening on the Resonance Frequency Analysis (RFA), a thin acrylic mix was placed between the implant surface and the testing acrylic cube. Seven implant beds 4.2 mm in diameter were prepared in the acrylic cubes using ∅ 4.2 twist drill to create 0.1 mm of space around fixture. The Osstell™ transducer (No. 100063) was attached to ∅ 4.1 mm ITI implants (solid screw, SLA, shoulder 4.3 mm, length 14 mm) manually and acrylic resin (Orthoresin, Dentsply, DeguDent GmbH, Postfach, Hanau, Germany) was mixed according to the manufacture instructions. The mix was injected into the acrylic socket and the fixture was coated with the acrylic and placed in the acrylic socket. The excess acrylic material was removed immediately. RF readings were recorded using Osstell™ system every minute for 30 min.
The readings were tabulated and the data were analysed statistically using SPSS software (SPSS software V10.0; SPSS Inc., Chicago, IL).
3. Results
In order to compare the standard deviations of the two devices, the PTVs scale (−8 to +50) was converted to 0–100 scale (the scale used by the Ostell™) using the following equation:
The mean PTV ± standard deviation for the direct contact group was −3.52 ± 0.39 PTV and 5.7 ± 0.24 PTV for the soft interface group. The mean of the Osstell™ measurements ± SD was 70.43 ± 0.00 ISQ (Implant Stability Quotient) for the direct acrylic contact group and 53.95 ± 0.24 ISQ for the soft interface group. A statistically significant difference (using t-test) was found between the direct contact and soft interface (P < 0.001) for PTV and ISQ (Table 1).
Table 1.
Analysis of implant stability measurements in direct and in soft interface samples (N = 7).
| Device | Technique | Mean | SD⁎ | Sig. (t-test) |
|---|---|---|---|---|
| Periotest® (PTV) | Direct contact | −3.52 | 0.39 | ⁎ |
| Soft interface | 5.7 | 0.28 | ⁎ | |
| Osstel™ (ISQ) | Direct contact | 70.43 | 0.00 | ⁎ |
| Soft interface | 53.95 | 0.24 | ⁎ | |
P < 0.001.
For the different levels of fixture exposure group (Table 2), the mean of the PTV ± SD was −3.52 ± 0.39, −1.14 ± 0.0, 1.28 ± 0.0 and 4.33 ± 0.14 PTV for the 0, 2, 4 and 6 mm fixture exposure subgroups, respectively, while the mean ISQ ± SD were 70.43 ± 0.0, 64.14 ± 0.24, 59.47 ± 0.41 and 52.14 ± 0.32 for the four subgroups, respectively. A statistically significant difference (using One-Way ANOVA) was found between the four subgroups (P < 0.001) for the PT and ISQ tests. Spearman’s Rho correlation test (Fig. 1 and 2) revealed that the level of horizontal fixture exposure strongly correlate with the PTVs (r = 0.967) and strongly reversibly correlate with ISQ (r = −0.946).
Table 2.
Analysis of implant stability measurements in different levels of implant length exposure samples. (N = 7).
| Device | Implant exposure length (mm) | Mean | SD∗ | Correlation (specimen’s rho) |
|---|---|---|---|---|
| Periotest® (PTV) | 0 | −3.52 | 0.39 | r = 0.967 |
| 2 | −1.14 | 0.00 | ||
| 4 | 1.28 | 0.00 | ||
| 6 | 4.33 | 0.14 | ||
| Osstel™ (ISQ) | 0 | 70.43 | 0.00 | r = −0.946 |
| 2 | 64.14 | 0.24 | ||
| 4 | 59.47 | 0.41 | ||
| 6 | 52.14 | 0.32 | ||
Figure 1.
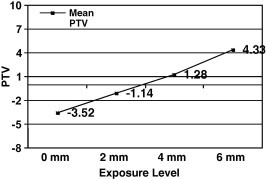
The correlation between mean PTV and level of implant exposure.
Figure 2.
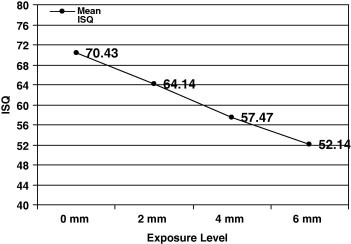
The correlation between ISQ and level of implant exposure.
The changes in resonance frequency measured during polymerization of acrylic resin around the fixture were plotted against the curing time in Figs. 3 and 4. The ISQ is strongly correlated with the change in the elasticity of the acrylic during polymerization (r = 0.986).
Figure 3.
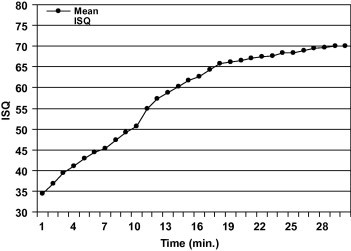
Mean ISQ of implant in hardening interface.
Figure 4.
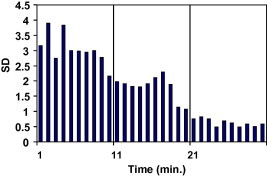
Standard deviation for auto polymerizing resin interface samples in relation to time.
4. Discussion
Both Periotest® and Osstell™ systems detected the change in implant stability in hard and in soft fixture interfaces, and this is demonstrated by the significant statistical difference (P < 0.001) between the direct contact and the soft interface groups. The mean ISQ reported in this study for the direct contact group (70 ± 0.0) was close to what has been reported in the literature for successfully integrated implants 69 ± 6.5 ISQ and 70 ISQ (Glauser et al., 2003).
The average of the PTV for the osseointegrated implant reported in the literature ranged from −8 to ±5.5 (Aparicio, 1997; Salonen et al., 1997; Tricio et al., 1995) the mean PTV for the direct acrylic contact group was −3.52 ± 0.39. The Osstell™ showed better reliability compared to Periotest® in measuring the implant stability in hard and in soft interface (Periotest® SD = 0.24 and 0.28 compared to 0.00 and 0.24). The mean value in this study for the soft interface using the Osstell™ was 53.95 ± 0.24 ISQ which coincide with Sennerby’s observation who stated that “the risk of failure starts to increase below 55 ISQ” and with the manufacturer statement that “implant with ISQ less than 50 is more prone to fail” (Sennerby et al., 1992a).
A significantly positive correlation was found between increased exposed implant lengths (EIL) and the PTV, while strong negative correlation was found with Osstell™ system which is in agreement with Meredith’s (1998) observation. This reflects the ability of both systems to detect any changes in exposed implant length. The positive and negative correlations are due to different measurement scales used in the two systems i.e. the PTV decreases as the implant stability increases while the ISQ increases as the implant stability increases. The reliability of Osstell™ in this study decreased as the implant stability decreased in this group (SD: 0.0, 0.24, 0.4 and 0.32 for the 0, 2, 4 and 6 mm fixture exposure, respectively) while, in contrary, the Periotest® becomes more reliable as the implant stability decreased (SD; 0.39, 0.0, 0.0 and 0.14).
Derhami et al. (1995) observed that varying the vertical measuring point on the implant-abutment assembly markedly influenced the PTV value when using the Periotest®. However, the increased reliability of Periotest® demonstrated in this study might be attributed to the standardisation of the Periotest® vertical level, angulation and distance between the Periotest® tip and the fixture.
Changes in stiffness that may occur at the implant–tissue interface during bone formation and healing were modeled using the change in the state from liquid to solid that occurs during the polymerization of polymethyl methacrylate. This allows any change in stiffness to be monitored over a convenient period of time. The significant rise in mean RF when plotted against curing time indicates that the Osstell™ system transducer is sensitive to changes in stiffness as the resin hardens progressively. The row data for the seven samples, when the ISQ plotted against time, show a maximum data scattering in the first ten minutes (SD: 2–4), less scattering in the second ten minutes (SD: 1–2) and least scattering in the last ten minutes (SD: 0.5–1). This obviously support the pervious finding in this study that the reliability of Osstell™ system increased as the implant stability increased or the stiffness of the surrounding media increased.
5. Conclusions
Within the limitations of this study, the following conclusions can be drawn:
Osstell™ and Periotest® systems proved to be sensitive in measuring dental implant stability in hard and in soft interfaces.
Osstell™ proved to be sensitive in detecting changes in the fixture interface stiffness.
Osstell™ system proved to be more reliable compared to Periotest® system in measuring dental implant stability in hard and in soft interfaces.
The reliability of the Osstell™ system increases as the implant stability increases.
The reliability of the Periotest® system increases as the implant stability decreases.
References
- Albrektsson T., Jacobsson M. Bone–metal interface in osseointegration. J. Prosthet. Dent. 1987;57:597–607. doi: 10.1016/0022-3913(87)90344-1. [DOI] [PubMed] [Google Scholar]
- Albrektsson T., Zarb G., Worthington P., Eriksson A.R. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int. J. Oral Maxillofac. Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- Aparicio C. The use of the Periotest value as the initial success criteria of an implant: 8-year report. Int. J. Periodontics Restorative Dent. 1997;17:150–161. [PubMed] [Google Scholar]
- Carlsson L., Rostlund T., Albrektsson B., Albrektsson T. Removal torques for polished and rough titanium implants. Int. J. Oral Maxillofac. Implants. 1988;3:21–24. [PubMed] [Google Scholar]
- Caulier H., Naert I., Kalk W., Jansen J.A. The relationship of some histologic parameters, radiographic evaluations, and Periotest measurements of oral implants: an experimental animal study. Int. J. Oral Maxillofac. Implants. 1997;12:380–386. [PubMed] [Google Scholar]
- Derhami K., Wolfaardt J.F., Faulkner G., Grace M. Assessment of the Periotest device in baseline mobility measurements of craniofacial implants. Int. J. Oral Maxillofac. Implants. 1995;10:221–229. [PubMed] [Google Scholar]
- Dhert W.J., Verheyen C.C., Braak L.H., de Wijn J.R., Klein C.P., de Groot K., Rozing P.M. A finite element analysis of the push-out test: influence of test conditions. J. Biomed. Mater. Res. 1992;26:119–130. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- Drago C.J. A prospective study to assess osseointegration of dental endosseous implants with the Periotest instrument. Int. J. Oral Maxillofac. Implants. 2000;15:389–395. [PubMed] [Google Scholar]
- Ericsson I., Johansson C.B., Bystedt H., Norton M.R. A histomorphometric evaluation of bone-to-implant contact on machine-prepared and roughened titanium dental implants. A pilot study in the dog. Clin. Oral Implants Res. 1994;5:202–206. doi: 10.1034/j.1600-0501.1994.050402.x. [DOI] [PubMed] [Google Scholar]
- Friberg B., Sennerby L., Meredith N., Lekholm U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999;28:297–303. [PubMed] [Google Scholar]
- Glauser R., Lundgren A.K., Gottlow J., Sennerby L., Portmann M., Ruhstaller P., Hammerle C.H. Immediate occlusal loading of Branemark TiUnite implants placed predominantly in soft bone: 1-year results of a prospective clinical study. Clin. Implant Dent. Relat. Res. 2003;5 (Suppl. 1):47–56. doi: 10.1111/j.1708-8208.2003.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Huang H.M., Chiu C.L., Yeh C.Y., Lin C.T., Lin L.H., Lee S.Y. Early detection of implant healing process using resonance frequency analysis. Clin. Oral Implants Res. 2003;14:437–443. doi: 10.1034/j.1600-0501.2003.00818.x. [DOI] [PubMed] [Google Scholar]
- Johansson C.B., Sennerby L., Albrektsson T. A removal torque and histomorphometric study of bone tissue reactions to commercially pure titanium and Vitallium implants. Int. J. Oral Maxillofac. Implants. 1991;6:437–441. [PubMed] [Google Scholar]
- Meredith N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998;11:491–501. [PubMed] [Google Scholar]
- Meredith N., Alleyne D., Cawley P. Quantitative determination of the stability of the implant–tissue interface using resonance frequency analysis. Clin. Oral Implants Res. 1996;7:261–267. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- Olive J., Aparicio C. Periotest method as a measure of osseointegrated oral implant stability. Int. J. Oral Maxillofac. Implants. 1990;5:390–400. [PubMed] [Google Scholar]
- Salonen M.A., Raustia A.M., Kainulainen V., Oikarinen K.S. Factors related to Periotest values in endosseal implants: a 9-year follow-up. J. Clin. Periodontol. 1997;24:272–277. doi: 10.1111/j.1600-051x.1997.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Schnitman P.A., Shulman L.B. Recommendations of the consensus development conference on dental implants. J. Am. Dent. Assoc. 1979;98:373–377. doi: 10.14219/jada.archive.1979.0052. [DOI] [PubMed] [Google Scholar]
- Sennerby L., Thomsen P., Ericson L.E. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int. J. Oral Maxillofac. Implants. 1992;7:62–71. [PubMed] [Google Scholar]
- Sennerby L., Thomsen P., Ericson L.E. Ultrastructure of the bone–titanium interface in rabbits. J. Mater. Sci.: Mater. Med. 1992;3:262–271. [Google Scholar]
- Teerlinck J., Quirynen M., Darius P., van Steenberghe D. Periotest: an objective clinical diagnosis of bone apposition toward implants. Int. J. Oral Maxillofac. Implants. 1991;6:55–61. [PubMed] [Google Scholar]
- Tricio J., van Steenberghe D., Rosenberg D., Duchateau L. Implant stability related to insertion torque force and bone density: an in vitro study. J. Prosthet. Dent. 1995;74:608–612. doi: 10.1016/s0022-3913(05)80313-0. [DOI] [PubMed] [Google Scholar]
- Walker L., Morris H.F., Ochi S. Periotest values of dental implants in the first 2 years after second-stage surgery: DICRG interim report no. 8. Dental implant clinical research group. Implant Dent. 1997;6:207–212. doi: 10.1097/00008505-199700630-00007. [DOI] [PubMed] [Google Scholar]
- Wennerberg A., Albrektsson T., Andersson B., Krol J.J. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin. Oral Implants Res. 1995;6:24–30. doi: 10.1034/j.1600-0501.1995.060103.x. [DOI] [PubMed] [Google Scholar]


