Abstract
Purpose.
To investigate the roles of αvβ3 and αvβ5 integrins in phagocytosis in human trabecular meshwork (HTM) cells.
Methods.
Immunofluorescence microscopy and FACS analysis were used to determine levels of αvβ3 and αvβ5 integrins in TM tissue and cultures of normal and immortalized TM cells. Phagocytosis was measured using pHrodo-labeled S. aureus bioparticles followed by FACS analysis. The role of αvβ5 integrin in phagocytosis was evaluated by knocking down αvβ5 integrin expression with siRNA against the human β5 gene. Signaling from focal adhesion kinase (FAK) was blocked using FAK inhibitor 14. The role of αvβ3 integrins in phagocytosis was determined by treating HTM cells with dexamethasone (DEX) or ethanol (EtOH) and by generating stable cell lines that overexpressed either wild type (WT) or constitutively active (CA) β3 integrin subunit.
Results.
Both TM tissue and cell lines expressed αvβ3 and αvβ5 integrins. Knockdown of αvβ5 integrin reduced phagocytosis by ∼60% and FAK inhibition significantly reduced phagocytosis up to 84%, in a dose-dependent manner. DEX treatment increased αvβ3 integrin expression in HTM cells but reduced phagocytosis by ∼50% compared with untreated and EtOH-treated cells. The CA β3 integrin–expressing cell line showed increased αvβ3 integrin levels and decreased phagocytosis by ∼50% compared with the control.
Conclusions.
The αvβ5 integrin-FAK–mediated pathway regulates phagocytosis in TM cells and this pathway is inhibited by activation of αvβ3 integrins. This suggests that changes in integrin expression and activity may be responsible for alterations in phagocytosis observed in steroid induced glaucoma.
Keywords: phagocytosis, trabecular meshwork, steroid-induced glaucoma, integrins
Phagocytosis by trabecular meshwork (TM) cells is important for maintaining normal IOP. The molecular mechanisms regulating TM phagocytosis are unknown. The goal of this study is to determine the mechanism(s) regulating TM phagocytosis in order to potentially identify new treatments for glaucoma.
Introduction
Glaucoma, the second leading cause of blindness worldwide, is a family of eye diseases that results in optic nerve damage. The pathogenesis of glaucoma is multifactorial and is frequently associated with elevated intraocular pressure (IOP) related to decreased cellularity, remodeling of the extracellular matrix, and the restriction of aqueous humor outflow through the trabecular meshwork (TM)–Schlemm's canal system. Phagocytosis is believed to play an essential role in maintaining homeostasis by helping to keep TM channels free of obstructive debris.1–4 In vivo, TM cells phagocytose pigment granules, erythrocytes, and pseudoexfoliative material. They have also been shown to ingest fibrin, bacteria, gold, or India ink and are believed to use the phagocytic pathway to remove degraded matrix components and apoptotic cells.3–7 Recent studies have shown that phagocytosis also regulates the expression and proteolytic activity of extraceullar matrix remodeling genes.5
Phagocytosis is dependent on cytoskeletal interactions and can be inhibited with cytochalasin B or upregulated by TGFβ.6,8 In the latter case, this would contribute to the increased loss of cells observed in glaucoma as TM cells would start phagocytizing apoptotic TM cells induced by TGFβ.9 Phagocytosis is also reduced by treatment with steroids such as dexamethasone (DEX) and this reduction is thought to be another explanation for the increase in IOP observed in primary open angle glaucoma (POAG) and steroid-induced glaucoma (SIG).7,10 The observation that phagocytic TM cells may migrate out of the meshwork after ingesting foreign material is further thought to contribute to cell loss in the TM and increased IOP observed in glaucoma.3 Despite the importance of phagocytosis in the TM, very little is known about the molecular mechanism(s), especially the signaling events, that modulate phagocytosis in the TM.
Integrins are a family of transmembrane heterodimeric cell surface receptors made up of an alpha and a beta subunit that have emerged as key players in regulating phagocytosis in a variety of cell types. Among the integrins found to regulate phagocytosis, outside the immune cells, are αvβ3, αvβ5, α2β1, and α6β1 integrins, with αvβ5 being the best characterized in RPE cells.11,12 In β5−/− mice, the absence of αvβ5 integrin on the apical surface of RPE cells causes inefficient phagocytosis of photoreceptor outer segments and the mice eventually go blind, demonstrating a critical role for αvβ5 integrin in preserving vision.13,14 Recent studies have shown that αvβ5 integrin–mediated phagocytosis required FAK signaling,15–17 and this signaling pathway may play a role in regulating the cytoskeleton events needed for the formation of the phagocytic cup. FAK-αvβ5 integrin–mediated signaling has also been reported to help activate Mer-family tyrosine kinases, which are associated with “eat me” signals during the phagocytosis of apoptotic cells.15–17 Interestingly, recent genomic studies indicate that the β5 integrin gene (3q21.1) is located near the glaucoma loci (GLC1C) and that expression of the gene is downregulated in glaucomatous tissues.18
In the current study, we sought to determine the respective role(s) of the αvβ5 integrin and its other family member, the αvβ3 integrin, in phagocytosis in TM cells. These studies show that the αvβ5 integrin is necessary for phagocytosis and that activation of the αvβ3 integrin inhibits phagocytosis in TM cells.
Methods
Materials
The monoclonal antibodies (mAb) P1F6 (αvβ5 integrin), BV3 (αvβ3), and CRC54 (activated αvβ3 integrin) were purchased from Abcam (Cambridge, MA). mAb LM609 (total surface αvβ3 integrin) and M9 (αv integrin subunit) were purchased from Millipore Corp. (Temecula, CA). Alexa 488-conjugated goat-anti-mouse IgG, Hoescht 33342 nuclear stain, and pHrodo Red S. aureus bioparticles were purchased from Invitrogen (Carlsbad, CA). Mouse IgG1 negative isotype control was purchased from BD Biosciences (San Jose, CA). mAb GAL-13 against β-galactosidase was purchased from Sigma-Aldrich (St. Louis, MO). siRNA against human αvβ5 integrin (ON-TARGETplus SMARTpool, Human ITGB5) and nontargeting siRNA (ON-TARGETplus Nontargeting siRNA#1) were purchased from Dharmacon (Lafayette, CO). Focal adhesion kinase (FAK) inhibitor 14 was purchased from Santa Cruz Biotechnology (Dallas, TX).
Cell Culture
Immortalized human TM-1 cell lines were established by obtaining tissue from a 30–year-old donor and HTM N27TM-2 cell strains were isolated from a 27-year-old donor, as previously described.19–22 Neither donor had a history of ocular diseases. Both cell types were cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich); 2 mM L-glutamine (Sigma-Aldrich); 1% amphotericin B (Mediatech, Herndon, VA); and 0.05% gentamicin (Mediatech). TM-1 cells were grown in 10% fetal bovine serum (FBS) while HTM cells were grown in the presence of 15% FBS and 1 ng/mL FGF-2 (PeproTech, Rocky Hill, NJ). In studies using DEX, HTM cells were differentiated in the absence of FGF-2 for 6 days postconfluency23,24 and then treated for 6 additional days with either 500 nM DEX or EtOH. Monolayers of TM-1 cells were treated for 4 days with either 500 nM DEX or EtOH. Longer treatments resulted in the TM-1 cells overgrowing and lifting off the plates.
Construction of β3 Integrin Expressing Cell Lines
The full length cDNA for β3 integrin subunit was purchased from ThermoScientific (previously Open Biosystems, Waltham, MA) and cloned into the pLVX-IRES-Puro vector (Clontech, Mountain View, CA) using XbaI and XhoI restriction sites. The CA β3 integrin was created by mutating Thr562 to Asn25 using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA), according to the manufacturer's instructions. The following oligonucleotides were used to introduce the T562N mutation: the forward primer 5′–TGCAACTGTACCAACCGTACTGACACCT–3′ contained a XhoI restriction site and the reverse primer 5′–AGGTGTCAGTACGGTTGGTACAGTTGCA–3′ contained a XbaI restriction site. The mutations were validated by DNA sequencing by the UW-Madison Biotechnology Center. The expression vector was packaged using the Lenti-X HTX packaging system in Lenti-X 293T cells according to the manufacturer's instructions (Clontech). Total viral particle was determined using the Lenti-X p24 Rapid Titer Kit (Clontech) per the manufacturer's instructions. Stable TM-1 cells overexpressing the β3 integrin subunits were created by transducing TM-1 cells with 2.5 × 106 pseudoviral particles/mL expressing wild type (WT) β3 integrin or constitutively active (CA) β3 integrin (MOI = 100). Pseudoviral particles containing the empty vector (EV) were used as a control (MOI = 100). Seventy two hours post-transduction, the medium was changed and 1 μg/mL of puromycin was added to select for cells expressing the transgene. Puromycin was maintained in subsequent cell passages to maintain selective pressure on cells expressing the β3 subunits.
Immunofluorescence Microscopy
Normal human cadaver eyes (normal donor, age 17) were obtained from the Lions Eye Bank of Wisconsin and processed for paraffin embedding as previously described.26 Sections 6-μm thick were cut and mounted onto glass slides. An antigen retrieval procedure was used to maximize antibody binding, as previously described.26 Sections were labeled with either 5 μg/mL mAb P1F6 or 4 μg/mL mAb BV3 and corresponding concentrations of mAb GAL-13 were used as negative controls.26 Monoclonal antibodies were detected using a secondary goat-anti-mouse Alexa 488-conjugated IgG and Hoescht 33342 to identify nuclei.
Cultured TM-1 and HTM cells grown on glass coverslips were washed in PBS and fixed in 1.0% paraformaldehyde, for 20 minutes at room temperature. Cells were labeled with 5 μg/mL mAb P1F6 for 1 hr in PBS containing 2% bovine serum albumin and post-labeled with goat-anti-mouse Alexa 488-conjugated IgG for 1 hour. Afterward cells were stained with the Hoescht 33342 nuclear dye. An epifluorescence microscope (Axioplan 2; Carl Zeiss Inc., Thornwood, NY) and image acquisition software (Axiovision 4.6; Carl Zeiss Inc.) was used to image the tissue sections.
siRNA Transfections
TM-1 cell lines were transfected with either 150 nM siRNA against human β5 integrin or 150 nM nontargeting siRNA. The transfection was done with a transfection reagent (Lipofectamine 2000; Invitrogen) using a reverse transfection procedure, as previously described.21 FACS and PCR were used to validate downregulation of the β5 integrin subunit.
Fluorescence-Activated Cell-Sorting (FACS) Analysis
FACS was performed as previously described,19,21,27 to evaluate surface expression of integrins. Briefly, cell suspensions were incubated on ice for 1 hour with 5 μg/mL mAbs P1F6, LM609, CRC54, or M9 prepared in PBS with 2% BSA. Purified mouse IgG1 was used as a negative isotype control and surface integrin expression was analyzed using the BD FACSCalibur System (BD Biosciences).
qPCR
Total RNA was isolated from cells using the RNA purification mini kit (RNeasy; Qiagen, Valencia, CA). cDNA was generated using either cDNA synthesis kit (Affinity Script cDNA Synthesis Kit; Agilent Technologies) or a high capacity cDNA Reverse transcription kit as per manufacturers' instructions (Invitrogen). Quantitative RT-PCR (qRT-PCR, 7300 Real Time PCR System; Applied Biosciences) was used to evaluate mRNA levels using specific primers for β5, β3, and αv integrin subunits (UW Biotechnology Center, Madison, WI). Results were normalized to the housekeeping gene SDHA. The following primers used were for the qPCR reactions: β5: GGAGCCAGAGTGTGGAAACA and GAAACTTTGCAAACTCCCTC; β3: GTCACCTGAAGGAGAATCTGC and TTCTTCGAATCATCTGGCC; αv: AATCTTATTGAGGATATCAC and AAAACAAGTAGCAACAAT; SHDA: TGGGAACAAGAGGGC ATCTG and CCACCACTGCATCCAATTCATG.
Phagocytosis of S. aureus Bioparticles
Cells were plated at 5 × 105 cells/ 32 mm well into 6-well tissue culture plates. A single vial of lyophilized S. aureus bioparticles (Invitrogen) labeled with pHrodo fluorescent reagent was resuspended in 2 mL of Hank's balanced salt solution (HBSS; Invitrogen) with 5% FBS and 2 mM L-glutamine. Bioparticles were added to a confluent monolayer of cells at a ratio of 1 vial per 2 × 106 cells, as per manufacturer's instructions. All cells were incubated with bioparticles for 4 hours and then processed for FACS analysis using a commercial FACS system (BD FACSCalibur; BD Biosciences) to determine the level of fluorescence. In siRNA transfection experiments, cells were challenged with pHrodo red-conjugated S. aureus bioparticles 48 hours posttransfection and processed for FACS analysis. In some experiments, TM-1 cells were plated at 4 × 105 cells/well into 12-well plates and pretreated for 30 minutes with either 1 μM or 5 μM of FAK 14 inhibitor followed by a 4-hour incubation with pHrodo-labeled bioparticles in the presence of the FAK inhibitor.
Quantification of phagocytosis was done by comparing the geometric mean fluorescence intensity (MFI) of phagocytized pHrodo-labeled S. aureus bioparticles in the different treatment groups relative to their respective control. Percent MFI was calculated by dividing the MFI of phagocytized pHrodo-labeled bioparticles of the treated group by that of the control group × 100%.
Statistical Analysis
Statistical analyses were performed using either a Student's t-test or one-way ANOVA followed by a Tukey HSD posttest. A P value < 0.05 was considered significant. Data are reported as mean percentage of fluorescence intensity (%MFI) ± SEM.
Results
The αvβ5 Integrin Plays a Role in Phagocytosis in TM Cells
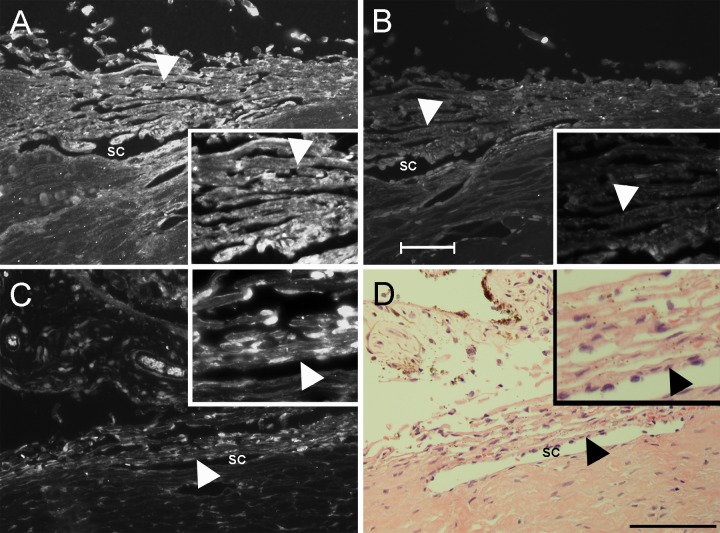
Immunofluorescence microscopy studies demonstrated that human TM tissue expressed both αvβ5 (Fig. 1A) and αvβ3 integrins (Fig. 1C). Labeling for integrins was observed in TM cells surrounding the beams and within the juxtacanilucular (JCT) region of the meshwork. Light labeling for both integrins was occasionally seen along the inner wall of Schlemm's canal as well. In contrast, exposure-matched β-galactosidase negative control (Fig. 1B) exhibited little or no labeling. Light micrograph shows morphological features of the anterior segment (Fig. 1D). Cells were observed within the JCT and lining Schlemm's canal.
Figure 1.
Immunofluorescent localization of αvβ5 and αvβ3 integrin in human TM . Cross-sections of normal human TM tissue were labeled for αvβ5 integrin expression (A) or αvβ3 integrin expression (C). The mAb GAL-13 against β-galactosidase was used as a negative control (B). Images of integrin labeling (A, C) and negative control (B) were taken at matching exposure times. Inserts (A, B) show that the cells along the beams are labeled. H & E staining of human TM tissue shows morphology of the anterior segment (D). The insert in (D) shows the integrity of the cells along the beams. Arrowheads denote the TM. SC, Schlemm's canal; Scale bar (A–C) = 50 μm (×20 magnification); Scale bar (D) = 100 μm; insert magnification = ×40.
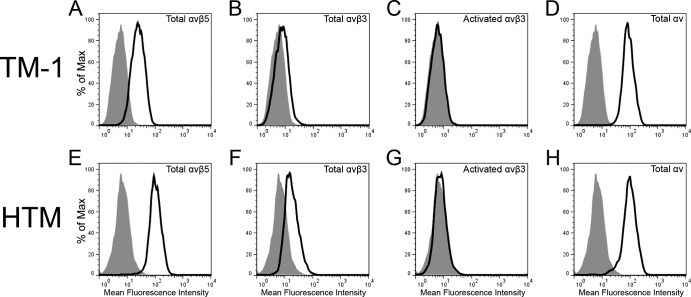
Surface expression of αvβ3 integrin and αvβ5 integrin on cultured TM-1 and HTM cells was then analyzed by FACS. As shown in Figure 2, both the HTM cells and the immortalized TM-1 cells line express moderate levels of αvβ5 integrins (Figs. 2A, 2E). HTM cells expressed modest levels of αvβ3 integrins (Fig. 2F) but TM-1 cells expressed little to no αvβ3 integrins compared with IgG control (Fig. 2B). Both cell lines expressed similar levels of αv integrin subunit (Figs. 2D, 2H). Thus, TM cells both in vivo and in vitro express αvβ3 and αvβ5 integrins.
Figure 2.
FACS analysis of αvβ5 integrin, αvβ3 integrin, and αv integrin subunit expression in immortalized TM-1 cells and HTM cells. Immortalized TM-1 and HTM cells express αvβ5 integrin (A, E) and αv integrin subunit (D, H) at moderate to high levels compared with mouse IgG negative control (tinted). HTM cells express modest levels of αvβ3 integrin (F) compared with the IgG negative control whereas TM-1 cells also show very little αvβ3 integrin expression (B). There was no expression of activated αvβ3 integrin in either cell type (C, G).
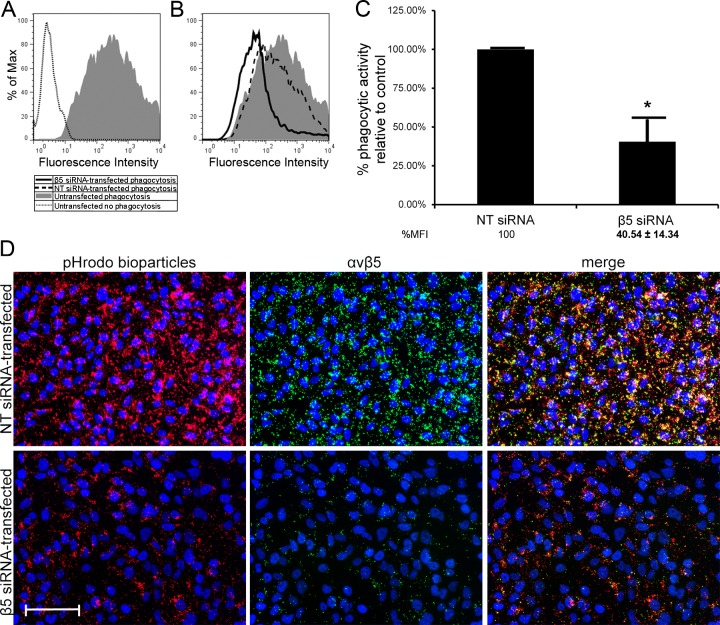
To determine if αvβ5 integrin played a role in TM cell phagocytosis, siRNA against the human β5 integrin gene (β5 siRNA) was used to knockdown expression of the β5 integrin subunit in the immortalized TM-1 cell lines. As shown in Figure 4A, TM-1 cells were able to take in the pHrodo-labeled bioparticles indicating that they had phagocytic activity. pHrodo is a fluorescing dye that increases its fluorescence as the pH of its environment becomes more acidic in the phagosome.5,28 The pHrodo bioparticles have also been recently shown to be a good indicator of the phagocytic activity of TM cells.5 TM-1 cells were used for this experiment since differentiated HTM cells are difficult to transfect.
Figure 4.
Phagocytosis was inhibited in TM-1 cells transfected with siRNA against the human β5 integrin gene. Phagocytic activity of TM-1 cells was assessed by incubating untransfected TM-1 cells with pHrodo-labeled bioparticles. Cells incubated with the bioparticles have higher fluorescence intensity compared with cells not incubated with the bioparticles, suggesting these cells have phagocytic capability (A). Cells transfected with β5 siRNA showed reduced phagocytic activity compared with untransfected cells and NT siRNA-transfected cells (B). Quantification by FACS analysis showed a statistically significant (*) reduction in phagocytic activity, by ∼60%, in β5 siRNA-transfected cells relative to cells transfected with NT siRNA (C); n = 3. Fluorescence micrographs showed a reduction in the uptake of pHrodo-labeled bioparticles (far-left panels, red) and reduced αvβ5 integrin labeling (middle panels, green) in cells transfected with β5 siRNA (bottom panels) compared with NT siRNA-transfected cells (top panels). The merged images (far-right panels) showed localization of pHrodo-labeled bioparticles with αvβ5 integrin (yellow) was more predominant in the NT siRNA-transfected cells compared with β5 integrin siRNA-transfected cells. Cells were counterstained with Hoescht 33342 nuclear dye. Scale bar = 50 μm (×20 magnification).
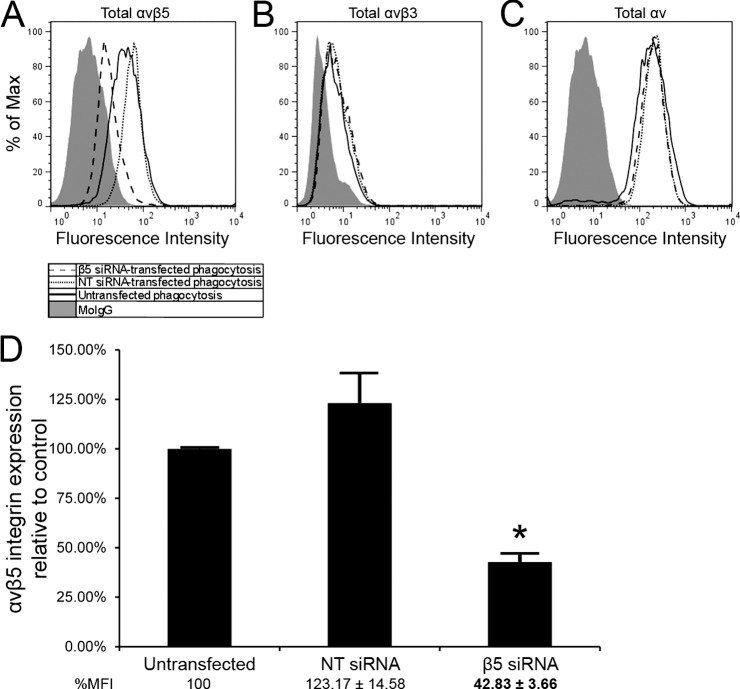
qPCR analysis confirmed that transfection with β5 siRNA knocked down the β5 integrin subunit at the RNA level by 92% compared with cells transfected with nontargeting (NT) siRNA (data not shown). By FACS analysis, cells transfected with β5 siRNA showed a 65% (P < 0.001) reduction in the surface expression of αvβ5 integrin compared with cells transfected with NT siRNA (Figs. 3A, 3D). The slight increase in αvβ5 integrin levels observed in cells transfected with NT siRNA compared with untransfected cells was not statistically significant (Fig. 3A). The transfection did not alter the expression of either αvβ3 integrin or αv integrin subunit (Figs. 3B, 3C).
Figure 3.
αvβ5 integrin expression was reduced after transfection with siRNA against human β5 integrin gene. Representative FACS analysis showed that the expression of αvβ5 integrin was knocked down in TM-1 cells transfected with 150 nM β5 siRNA compared with untransfected cells, but αvβ5 integrin expression in NT siRNA-transfected cells remained unchanged (A). Transfection with either β5 integrin siRNA or NT siRNA did not change expression of αvβ3 integrin (B) or αv integrin subunit (C) compared with untransfected cells. (D) shows that knockdown of the β5 integrin subunit reduced αvβ5 integrin expression by ∼57% and ∼65% compared with untransfected and NT siRNA-transfected cells, respectively. The change in β5 integrin expression was statistically significant (*); n = 6.
Transfected cells were then incubated with pHrodo-labeled S. aureus bioparticles to determine their phagocytic activity. FACS analysis showed a large shift in geometric mean fluorescence intensity in cells incubated with the pHrodo-labeled bioparticles compared with those that were not, indicating that the TM-1 cells were highly phagocytic (Fig. 4A). Phagocytic activity between untransfected cells and NT siRNA-transfected cells appeared similar, although there was a slight decrease in the phagocytic activity of NT siRNA-transfected cells compared with untransfected cells (Fig. 4B). However, phagocytic activity of β5 siRNA-transfected cells was noticeably lower than untransfected and NT siRNA-transfected cells (Fig. 4B). Compared with cells transfected with NT siRNA (Fig. 4C), phagocytosis was significantly reduced in β5 siRNA-transfected cells, by 60% (P < 0.02). Fluorescence micrographs (Fig. 4D) showed that cells transfected with β5 siRNA appeared to have phagocytosed fewer pHrodo-labeled bioparticles (bottom panel, red label) compared with the NT siRNA-transfected cells (top panel, red label). This suggests that αvβ5 integrin does play a role in phagocytosis in TM-1 cells.
To further demonstrate that αvβ5 integrin was involved in phagocytosis, cells were labeled for β5 integrin expression. As shown in Figure 4D (middle panels), NT siRNA-transfected cells showed labeling for αvβ5 integrin and as expected the intensity of αvβ5 integrin labeling was markedly reduced in β5 siRNA-transfected cells compared with the NT siRNA-transfected controls, suggesting less surface expression of αvβ5 integrin. When images were merged, the pHrodo-labeled bioparticles co-localized with αvβ5 integrin labeling (yellow label) in NT-siRNA-transfected cells indicating that phagocytosis of the bioparticles occurred along regions of αvβ5 integrin localization. A similar co-localization was observed in the β5 siRNA-transfected cells but at a much reduced level.
Phagocytosis in TM Cells Involves FAK Signaling
Since FAK activity had previously been shown to be involved in αvβ5-mediated phagocytosis,15,16 TM-1 cells were incubated with either 1 μM or 5 μM FAK inhibitor 14. FAK inhibitor 14 is a selective inhibitor of FAK that prevents autophosphorylation of tyrosine 397 and does not show any activity toward other tyrosine kinase.29–31 Representative histograms demonstrate that both 1 μM and 5 μM FAK inhibitor 14 impaired phagocytosis in a dose-dependent manner (Fig. 5). Quantitatively, phagocytosis was reduced by ∼38% when cells were incubated with 1 μM FAK inhibitor 14 (P < 0.04) and by ∼84% when cells were incubated with 5 μM FAK inhibitor 14 (P < 0.0001) compared with control cells (Fig. 5C). Taken together, the data suggests that phagocytosis in TM cells involves the αvβ5 integrin–FAK signaling pathway.
Figure 5.
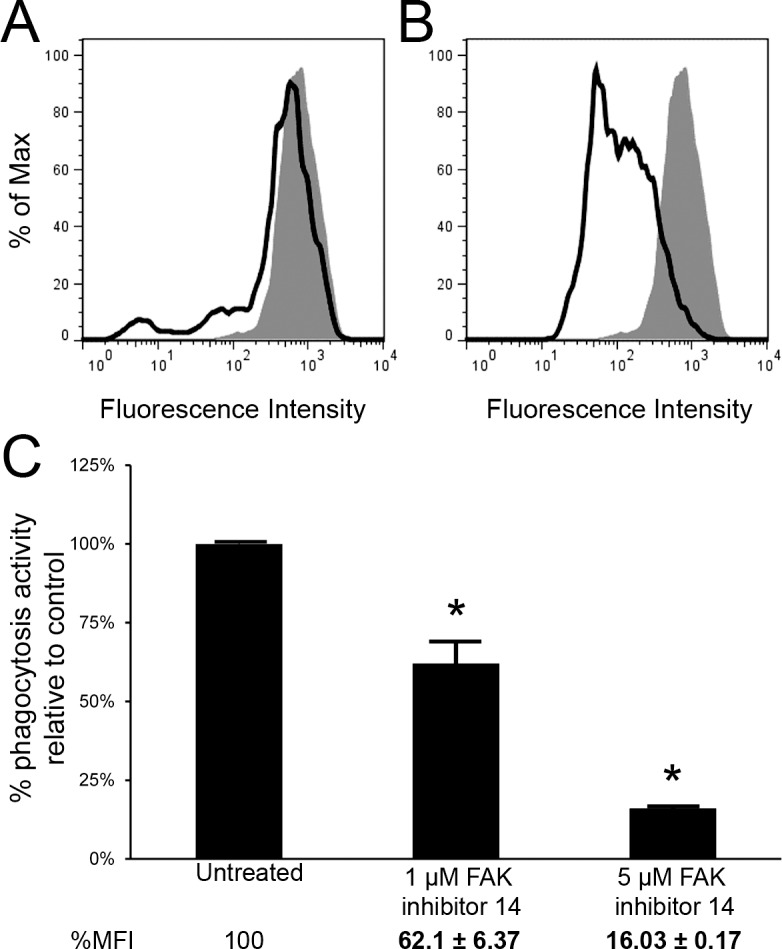
Phagocytosis in TM-1 cells occurred through FAK signaling. FACS analysis show a reduction in phagocytic activity in cells incubated with 1 μM (A) and 5 μM (B) FAK inhibitor 14 (solid lines) compared with untreated (control) cells (filled area). Quantification of phagocytosis (C) showed that 1 μM FAK inhibitor 14 reduced phagocytosis by ∼38% while 5 μM of the inhibitor reduced phagocytosis by ∼84% compared with untreated (control) cells. The reduction was statistically significant (*); n = 3.
DEX-Induced Reduction in Phagocytosis Corresponds With an Increase in αvβ3 Integrin Expression and Activation in Normal HTM Cells
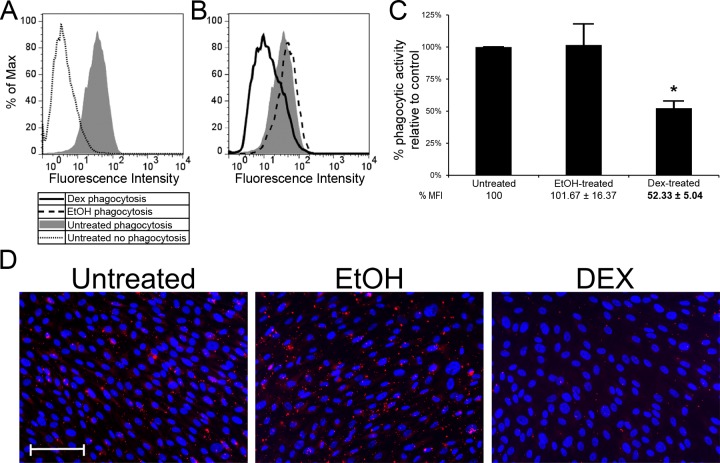
We next examined the role of αvβ5 integrin in phagocytosis after DEX treatment since it has previously been reported that DEX causes a reduction in phagocytosis.7,10 Figure 6A shows that normal HTM cells, like TM-1 cells, were also phagocytic. However, when challenged with pHrodo bioparticles, DEX treatment significantly reduced phagocytic activity by ∼50% (P < 0.05) compared with untreated cells and EtOH- treated (Figs. 6B, 6C). Fluorescence images of EtOH- and DEX-treated HTM cells showed that DEX-treated HTM cells contained less fluorescing pHrodo-labeled bioparticles compared with untreated and EtOH-treated cells (Fig. 6D), demonstrating that the phagocytic activity was reduced.
Figure 6.
DEX treatment significantly reduced the phagocytic activity of differentiated HTM cells. Untreated HTM cells challenged with pHrodo-labeled S. aureus bioparticles showed greater geometric mean fluorescence intensity, indicating that these cells are also capable of phagocytosis (A). The level of phagocytosis between EtOH-treated and untreated cells was similar but DEX-treated cells showed reduced phagocytic activity (B). Quantification by FACS analysis showed a statistically significant (*) reduction in phagocytic activity in DEX-treated cells (∼50%) relative to untreated cells. No statistically significant difference was observed between EtOH-treated cells and untreated cells (C); n = 3. pHrodo-labeled bioparticle uptake in untreated and EtOH-treated cells appeared similar but in DEX-treated cells (far-right panel), bioparticle uptake appeared reduced in comparison to the controls (D). Cells were counterstained with Hoescht 33342 nuclear dye. Scale bar = 50 μm (×20 magnification).
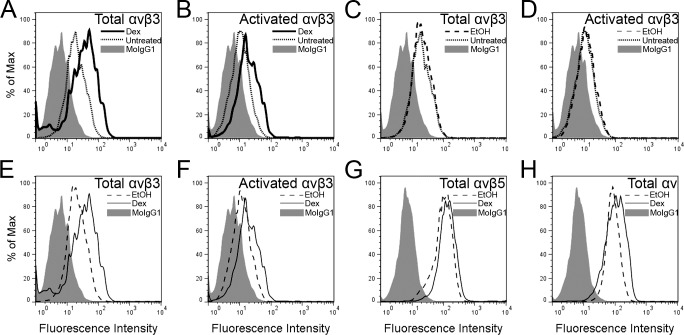
Since phagocytosis was reduced following DEX treatment, we then examined the level of integrin expression in DEX-treated HTM cells. As shown in Figure 7G, DEX treatment did not affect the expression levels of αvβ5 integrin. DEX, however, did cause an increase in total αvβ3 integrin expression compared with untreated and EtOH treated cells (Figs. 7A, 7E). A slight increase in the αv integrin subunit was observed in DEX-treated cells compared with EtOH- treated cells (Fig. 7H). This increase may be due to the increased level of αvβ3 integrin expression in DEX-treated cells. We then investigated whether the level of activated αvβ3 integrin was also increased in DEX-treated cells, since our previous studies have shown that DEX treatment not only upregulates the expression of αvβ3 integrin, but it causes an increase in its activation state.19,20,32,33 Not surprisingly, both total and activated levels of αvβ3 integrin were increased in DEX-treated HTM cells compared with untreated (Figs. 7A, 7B) and EtOH-treated cells (Figs. 7E, 7F). As expected, there was no difference in total or activated levels of αvβ3 integrin between untreated and EtOH-treated cells (Figs. 7C, 7D).
Figure 7.
Treatment with DEX upregulated expression of activated αvβ3 integrins on HTM cells. Total and activated αvβ3 integrin levels increased in DEX-treated cells compared with untreated cells (A, B, respectively) whereas EtOH- treated cells showed no change in the expression of αvβ3 integrins compared with untreated cells (C, D, respectively). The level of total and activated αvβ3 integrin was higher in DEX-treated cells compared with EtOH-treated cells (E, F). DEX treatment did not cause any changes in αvβ5 integrin expression between DEX- and EtOH-treated cells (G) while αv integrin subunit expression was slightly elevated in DEX-treated cells compared with EtOH-treated cells (H).
Expression of Activated αvβ3 Integrin Impairs Phagocytosis in Transduced TM-1 Cells
To investigate if the increase in total αvβ3 integrin or the activation of αvβ3 integrin was responsible for the reduced phagocytosis observed in DEX-treated HTM cells, stable TM-1 cell lines overexpressing either the wild type (WT) β3 integrin subunit or a constitutively active (CA) β3 integrin subunit were generated. The CA β3 integrin subunit was created by mutating Thr562 in the β3 subunit to Asn (T562N) as previously described.25 Changes in the αv integrin subunit were not necessary since this mutation in the β3 integrin subunit has been shown to induce the active conformation of αvβ3 integrin.34–38 Cells transduced with empty vector (EV) were used as controls.
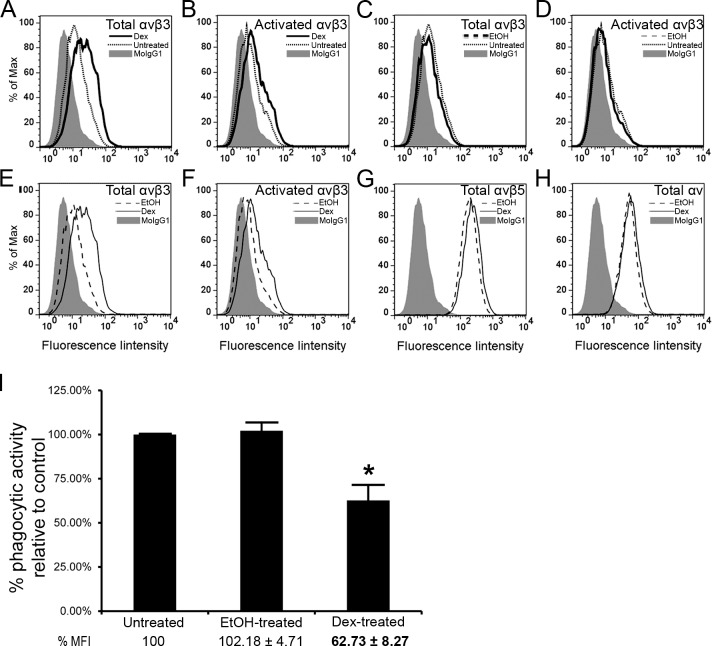
TM-1 cells were used because they express little to no surface αvβ3 integrin (Fig. 2B) and as transformed cells they have a longer life span thereby enabling stable cell lines to be generated. Furthermore, Figure 8 shows their response to DEX treatment was similar to that observed in HTM cells. Like HTM cells, DEX did not affect the expression of αvβ5 integrin in TM-1 cells (Fig. 8G), but it did cause an increase in αvβ3 integrin expression and activation (Figs. 8A, 8B, 8E, 8F). TM-1 cells also showed a statistically significant decrease in phagocytosis after DEX-treatment (Fig. 8I) compared with untreated and EtOH-treated controls.
Figure 8.
DEX treatment upregulated expression of activated αvβ3 integrins and reduced phagocytic activity of TM-1 cells. Expression of total αvβ3 integrin (A) and activated αvβ3 integrin (B) was elevated in DEX-treated TM-1 cells compared with untreated cells. There was no effect of EtOH treatment on total (C) and activated (D) αvβ3 integrin levels compared with untreated cells. Levels of total (E) and activated (F) αvβ3 integrin in DEX-treated cells were elevated compared with EtOH-treated cells but levels of αvβ5 integrin (G) and αv integrin subunit (H) between DEX-and EtOH-treated cells remained the same. Quantification of phagocytic activity by FACS analysis showed a statistically significant (*) reduction in phagocytosis in DEX-treated TM-1 cells, by 37%, relative to untreated cells (I).
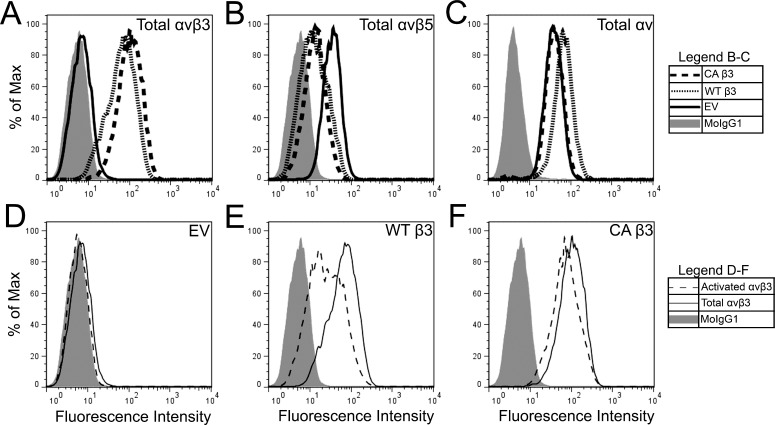
In the EV-transduced TM-1 cell lines, FACS analysis showed that these cells expressed very little αvβ3 integrin (Figs. 9A, 9D). In contrast, overexpression of the WT β3 or CA β3 integrin subunits significantly increased the level of αvβ3 integrin expression compared with the EV-transduced cells (Fig. 9A). Labeling with the CRC54 antibody to detect activated levels of αvβ3 integrin showed that nearly all of the αvβ3 integrin in the CA β3 integrin cell line was activated (Fig. 9F), indicating that the active conformation of β3 integrin subunit was present. A small proportion of the αvβ3 integrin was also activated in WT β3-transduced cells (Fig. 9E). The level of expression for the αv integrin subunit was the same between CA β3- and EV-transduced TM-1 cells (Fig. 9C) indicating there wasn't a corresponding increase in its expression. Not surprisingly, therefore, transduction of TM-1 cells with either β3 integrin transgene reduced the expression of αvβ5 integrin (Fig. 9B). However, the β5 subunit was equally reduced in both WT β3- and CA β3-expressing cells. Presumably, this reduction occurred because the β3 integrin subunit was utilizing the αv integrin subunit normally used by the β5 integrin subunit.
Figure 9.
FACS analysis of total and activated levels of αvβ3 integrin expression on transduced TM-1 cell lines. The top panels show the level of αvβ3 integrin (A), αvβ5 integrin (B), or the αv integrin subunit (C) expression in cells transduced with the empty vector (EV), vectors expressing the WT β3 integrin subunit, or the vector expressing the CA β3 integrin subunit. The bottom panels show level of total and activated αvβ3 integrin expression in the EV-transduced cells (D), the WT β3 integrin expressing cells (E), or the CA β3 integrin-expressing cells (F). Levels of total and activated αvβ3 integrin expression are similar in cells expressing the CA β3 integrin subunit (F), but not in cells expressing the WT β3 intergin subunit. The level of αvβ5 integrin expression is slightly reduced in cells expressing the WT β3 intergin subunit and CA β3 integrin subunit compared with EV-transduced cells (B). Although the total level of expression of the αv integrin subunit was similar between EV-transduced cells and cells expressing the CA β3 integrin subunit, it is higher in cells expressing the WT β3 integrin (C).
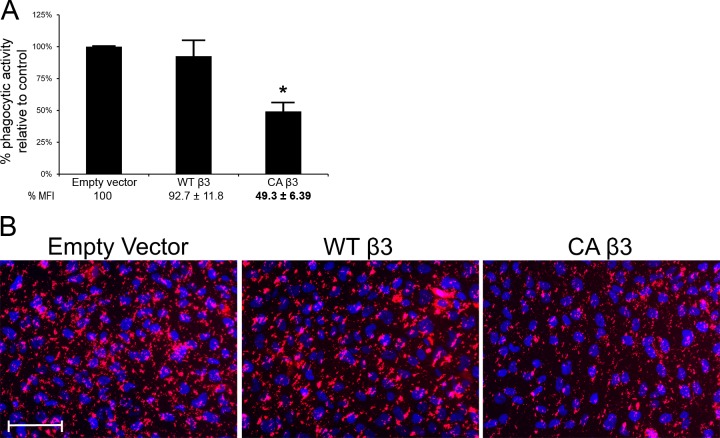
Figure 10A shows that the phagocytic activity between EV- and WT β3 integrin–transduced cells was not different, suggesting that the increased surface expression of αvβ3 integrin (Fig. 9A) did not affect phagocytosis. However, expression of the CA β3 integrin subunit, in which all of the αvβ3 integrin was activated (Fig. 9F), reduced phagocytosis by > 50% (P < 0.0001) relative to empty vector (P < 0.0001) and by ∼47% relative to the WT β3-expressing cell line (P > 0.005; Fig. 10A). Fluorescence microscopy showed that fewer pHrodo-labeled bioparticles were present in CA β3-expressing cell line compared with the EV-transduced and WT β3-transduced cell lines (Fig. 10B). The reduction in phagocytosis could not be attributed to the reduced levels of αvβ5 integrins, since the β5 subunit was equally reduced in both WT β3- and CA β3-expressing cells (Fig. 9B). Rather, this suggests that activation of αvβ3 integrin–inhibited phagocytosis in TM cells.
Figure 10.
Phagocytosis was significantly reduced in cells overexpressing activated αvβ3 integrin. The ∼50% reduction in phagocytosis in cells expressing the CA β3 integrin subunit was statistically significant (*) compared with cells transduced with the empty vector (A); n = 10. The phagocytic activity was similar between EV-transduced cells and cells transduced with the WT β3 integrin transgene. (B) shows fluorescence images of pHrodo-labeled bioparticle (red) taken up by EV cells, WT β3-expressing cells, and CA β3-expressing cells. CA β3-expresssing cells appear to have fewer bioparticles compared with EV cells and WT β3-expressing cells. Cells were counterstained with Hoescht 33342 nuclear dye. Scale bar = 50 μm (×20 magnification).
Discussion
In the present study, we show that phagocytosis in TM cells may be regulated by opposing αvβ3 and αvβ5 integrin–mediated signaling pathways. An αvβ5 integrin-FAK–mediated signaling pathway appeared to facilitate phagocytosis, since knockdown of the β5 subunit or inhibition of FAK significantly reduced phagocytosis. In contrast, αvβ3 integrin appeared to inhibit phagocytosis. The inhibitory effect of αvβ3 integrin was dependent on the activation state of the integrin, since phagocytosis was only impaired when αvβ3 integrins were activated either by DEX-treatment or overexpression of an activated β3 integrin subunit. This suggests that cross-talk between these two integrins may regulate phagocytosis in a transdominant fashion. To the best of our knowledge, this is the first time antagonistic roles for αvβ3 and αvβ5 integrins in phagocytosis has been established in the TM.
Phagocytosis is an actin-mediated event and FAK is an important signaling molecule that regulates actin cytoskeleton remodeling.39,40 Our studies show that the role of αvβ5 integrin–FAK in phagocytosis in TM cells (Fig. 11) is consistent with its role in other cell types, particularly in RPE cells.15,16 In these cells, phagocytosis of photoreceptor outer segment fragments required an αvβ5 integrin–FAK signaling event in conjugation with a secondary receptor, Mer. Activation of the αvβ5 integrin–FAK pathway was necessary for the formation of a F-actin-rich phagocytic cup that controls the engulfment of the photoreceptor outer-segment fragments and activation of MerTK15,16,41,42 and FAK was specifically required for enhanced internalization.15 Hence, the αvβ5 integrin is not needed to directly interact with the ingested material but rather to govern the cytoskeleton and Mer receptor recognition events required for phagocytosis. The demonstration that an αvβ5 integrin is involved in phagocytosis in the TM is not surprising, since phagocytosis is regulated by cytoskeleton-mediated events.43,44
Figure 11.
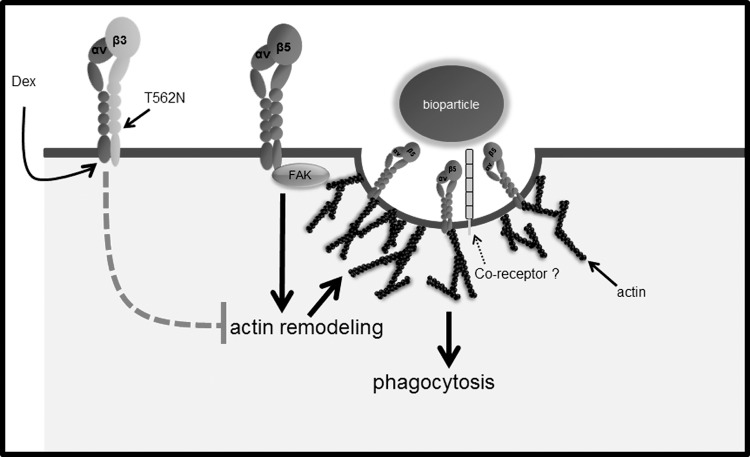
Schematic diagram showing phagocytic roles of αvβ5 and αvβ3 integrins. Model of how αvβ5 integrin–FAK signaling events could regulate phagocytosis in TM cells is based on the pathway known to be active in RPE cells.15 In this paradigm, activation of αvβ5 integrin leads to actin remodeling events which promotes the formation of the phagocytic cup and possibly activation of coreceptor (or coreceptors) such as Mer.41 How activation of αvβ3 integrin interferes with αvβ5 integrin-mediated phagocytosis in unknown. One possibility is that αvβ3 integrins redirect the signaling events required for F-actin remodeling during phagocytosis.
The decrease in αvβ5 integrin-mediated phagocytosis appeared to require the activation of the β3 integrin subunit and not merely a downregulation of αvβ5 integrin expression. In the DEX-treated HTM cell strains and TM-1 cell lines, the level of αvβ5 integrin remained unchanged compared with EtOH controls. Furthermore, a reduction in phagocytosis was only observed in the CA β3-transduced cells. Thus, a reduction in the level of αvβ5 expression could not explain the decrease in phagocytosis since the stable cell lines overexpressing either the WT β3 or CA β3 integrin subunit both showed a similar reduction in αvβ5 integrin levels. Hence, it appears that the activation of the αvβ3 integrin (which also occurs in DEX-treated cells) may be responsible for the decrease in phagocytosis.
Although both DEX-treated HTM and TM-1 cells showed a statistically significant reduction in their phagocytic activity, DEX appeared to have a slightly greater effect on phagocytosis in the HTM cells then the TM-1 cells. Whereas DEX reduced phagocytosis in the HTM cells by 50%, it only reduced phagocytosis in the TM-1 cells by 37%. The reason for this is unclear. It could be due to the length of time the cells were treated with DEX. HTM cells were treated for 6 days with DEX whereas TM-1 cells only saw 4 days of DEX treatment in order to prevent the cells from overgrowing. Alternatively, it could be due to the level of αvβ3 expression relative to the level of αvβ5 expression. TM-1 cells express considerably lower levels of αvβ3 integrin compared with HTM cells.
Demonstration that activation of the αvβ3 integrin inhibits phagocytosis offers the first molecular explanation for why phagocytosis in the TM may be reduced in steroid-induced glaucoma. But how this activation of αvβ3 integrin specifically inhibits phagocytosis is presently unknown. Our laboratory has previously shown that activation of αvβ3 integrins resulted in the formation of an unusual cross-linked actin network called CLANs, a structure found in higher frequency in glaucomatous and steroid treated TM tissue.45–47 Since phagocytosis is mediated by cytoskeleton events, αvβ3 integrin could be inhibiting phagocytosis by triggering this change in the organization of the actin cytoskeleton and impairing the recruitment of actin filaments to the phagocytic cup (Fig. 11). Alternatively, activation of αvβ3 integrins could be altering the Rac1 signaling pathway involved in phagocytosis. Previous studies have shown that αvβ5 integrin uses a Rac1 mediated pathway to control phagocytosis48–50 and our studies have shown that the formation of CLANs was dependent on a Trio/Rac1 signaling pathway.19,20 Hence, utilization of Rac1 by αvβ3 integrin could be preventing the utilization of Rac1 at the phagocytic site.
The observation that expression of an activated αvβ3 integrin could regulate αvβ5 integrin–FAK signaling events during phagocytosis indicates that the phagocytic activities of αvβ5 integrin does not necessarily require an outside ligand for activation and can be regulated by inside-out signaling events. For example, proteins talin-1 and paxillin, which are known to directly bind the β3 integrin subunit, regulate the activity of integrins.51,52 Interestingly, a number of the molecules that could control the activity of αvβ5 and αvβ3 integrin signaling events have been reported to be upregulated in DEX treated TM cells.32 Among them are filamin-B, caveolin-1, talin-1, paxillin, Src, and EPS8. Upregulation of caveolin-1 is particularly interesting since it has been reported to be a risk factor in glaucoma,53–55 suggesting that alterations in caveolin-1/integrin signaling pathways could be involved in some glaucomas. Clearly, additional studies are needed to determine which proteins are responsible for the activation of αvβ3 integrin in TM cells. In summary, these studies suggest that the activation state of αvβ3 integrin regulates αvβ5-mediated phagocytosis in TM cells and that DEX-induced activation of αvβ3 integrin could be partly responsible for the decrease in phagocytosis in steroid-treated TM cells or SIG patients. Elucidating the molecular mechanisms that promote or inhibit phagocytosis in TM cells will allow us to target new and/or alternative pathways that help maintain outflow facility and develop new therapies for managing IOP.
Acknowledgments
Supported by NEI Grants EY06665-01, EY017006, EY020490 (D.M.P.), a Core grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665).
Disclosure: D. Gagen, None; M.S. Filla, None; R. Clark, None; P. Liton, None; D.M. Peters, None
References
- 1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91: 564–579 [DOI] [PubMed] [Google Scholar]
- 2. Bill A. Editorial: the drainage of aqueous humor. Invest Ophthalmol. 1975; 14: 1–3 [PubMed] [Google Scholar]
- 3. Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990; 31: 2156–2163 [PubMed] [Google Scholar]
- 4. Rohen Javd ZE The phagocytic activity of the trabecular meshwork endothelium: Electron microscopic study of the vervet (Cercopithecus aethiops). Graefes Arch Clin Exp Ophthalmol. 1968; 175 [DOI] [PubMed] [Google Scholar]
- 5. Porter KM, Epstein DL, Liton PB. Up-regulated expression of extracellular matrix remodeling genes in phagocytically challenged trabecular meshwork cells. PLoS One. 2012; 7: e34792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirato S, Murphy CG, Bloom E, et al. Kinetics of phagocytosis in trabecular meshwork cells. Flow cytometry and morphometry. Invest Ophthalmol Vis Sci. 1989; 30: 2499–2511 [PubMed] [Google Scholar]
- 7. Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007; 84: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao Y, Wei H, Da B, Huang Y. Effect of transforming growth factor-beta 2 on phagocytosis in cultured bovine trabecular meshwork cells. J Tongji Med Univ. 2001; 21: 318–320 [DOI] [PubMed] [Google Scholar]
- 9. Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013; 97: 680–686 [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci. 1997; 38: 1902–1907 [PubMed] [Google Scholar]
- 11. Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008; 121: 1773–1783 [DOI] [PubMed] [Google Scholar]
- 12. Finnemann SC. Role of alphavbeta5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv Exp Med Biol. 2003; 553: 337–342 [DOI] [PubMed] [Google Scholar]
- 13. Nandrot EF, Anand M, Sircar M, Finnemann SC. Novel role for alphavbeta5-integrin in retinal adhesion and its diurnal peak. Am J Physiol Cell Physiol. 2006; 290: 1256–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nandrot EF, Finnemann SC. Altered rhythm of photoreceptor outer segment phagocytosis in beta5 integrin knockout mice. Adv Exp Med Biol. 2006; 572: 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003; 22: 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin S, Rodrigues GA. Roles of alphavbeta5, FAK and MerTK in oxidative stress inhibition of RPE cell phagocytosis. Exp Eye Res. 2012; 94: 63–70 [DOI] [PubMed] [Google Scholar]
- 17. Sayedyahossein S, Dagnino L. Integrins and small GTPases as modulators of phagocytosis. Int Rev Cell Mol Biol. 2013; 302: 321–354 [DOI] [PubMed] [Google Scholar]
- 18. Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006; 12: 774–790 [PMC free article] [PubMed] [Google Scholar]
- 19. Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Invest Ophthalmol Vis Sci. 2011; 52: 2952–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct beta1 and beta3 integrin pathways. Invest Ophthalmol Vis Sci. 2009; 50: 5723–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwinn MK, Gonzalez JM Jr, Gabelt BT, Sheibani N, Kaufman PL, Peters DM. Heparin II domain of fibronectin mediates contractility through an alpha4beta1 co-signaling pathway. Exp Cell Res. 2010; 316: 1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filla MS, Liu X, Nguyen TD, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002; 43: 151–161 [PubMed] [Google Scholar]
- 23. Polansky JR, Weinreb R, Alvarado JA. Studies on human trabecular cells propagated in vitro. Vision Res. 1981; 21: 155–160 [DOI] [PubMed] [Google Scholar]
- 24. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18: 1043–1049 [PubMed] [Google Scholar]
- 25. Kashiwagi H, Tomiyama Y, Tadokoro S, et al. A mutation in the extracellular cysteine-rich repeat region of the beta3 subunit activates integrins alphaIIbbeta3 and alphaVbeta3. Blood. 1999; 93: 2559–2568 [PubMed] [Google Scholar]
- 26. Filla MS, David G, Weinreb RN, Kaufman PL, Peters DM. Distribution of syndecans 1-4 within the anterior segment of the human eye: expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp Eye Res. 2004; 79: 61–74 [DOI] [PubMed] [Google Scholar]
- 27. Peterson JA, Sheibani N, David G, Garcia-Pardo A, Peters DM. Heparin II domain of fibronectin uses alpha4beta1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J Biol Chem. 2005; 280: 6915–6922 [DOI] [PubMed] [Google Scholar]
- 28. Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011; 186: 3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beierle EA, Ma X, Stewart J, et al. Inhibition of focal adhesion kinase decreases tumor growth in human neuroblastoma. Cell Cycle. 2010; 9: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golubovskaya VM, Nyberg C, Zheng M, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008; 51: 7405–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hochwald SN, Nyberg C, Zheng M, et al. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009; 8: 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark R, Nosie A, Walker T, et al. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013; 12: 194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filla MS, Woods A, Kaufman PL, Peters DM. Beta1 and beta3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bodeau AL, Berrier AL, Mastrangelo AM, Martinez R, LaFlamme SE. A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J Cell Sci. 2001; 114: 2795–2807 [DOI] [PubMed] [Google Scholar]
- 35. Green LJ, Mould AP, Humphries MJ. The integrin beta subunit. Int J Biochem Cell Biol. 1998; 30: 179–184 [DOI] [PubMed] [Google Scholar]
- 36. Kieffer N, Melchior C, Guinet JM, Michels S, Gouon V, Bron N. Serine 752 in the cytoplasmic domain of the beta 3 integrin subunit is not required for alpha v beta 3 postreceptor signaling events. Cell Adhes Commun. 1996; 4: 25–39 [DOI] [PubMed] [Google Scholar]
- 37. Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J Biol Chem. 1998; 273: 2379–2383 [DOI] [PubMed] [Google Scholar]
- 38. Zhao R, Pathak AS. Stouffer GA. beta(3)-Integrin cytoplasmic binding proteins. Arch Immunol Ther Exp (Warsz). 2004; 52: 348–355 [PubMed] [Google Scholar]
- 39. Tse SM, Furuya W, Gold E, et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003; 278: 3331–3338 [DOI] [PubMed] [Google Scholar]
- 40. Zhu Z, Bao Z, Li J. MacMARCKS mutation blocks macrophage phagocytosis of zymosan. J Biol Chem. 1995; 270: 17652–17655 [DOI] [PubMed] [Google Scholar]
- 41. Finnemann SC, Nandrot EF. MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv Exp Med Biol. 2006; 572: 499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005; 118: 539–553 [DOI] [PubMed] [Google Scholar]
- 43. May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001; 114: 1061–1077 [DOI] [PubMed] [Google Scholar]
- 44. Monastyrska I, Rieter E, Klionsky DJ, Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc. 2009; 84: 431–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci. 2009; 50: 1255–1263 [DOI] [PubMed] [Google Scholar]
- 46. Read AT, Chan DW, Ethier CR. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp Eye Res. 2006; 82: 974–985 [DOI] [PubMed] [Google Scholar]
- 47. Wade NC, Grierson I, O'Reilly S, et al. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp Eye Res. 2009; 89: 648–659 [DOI] [PubMed] [Google Scholar]
- 48. Akakura S, Singh S, Spataro M, et al. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004; 292: 403–416 [DOI] [PubMed] [Google Scholar]
- 49. Albert ML, Kim JI, Birge RB. Alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000; 2: 899–905 [DOI] [PubMed] [Google Scholar]
- 50. Kim S, Park SY, Kim SY, et al. Cross talk between engulfment receptors stabilin-2 and integrin alphavbeta5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol Cell Biol. 2012; 32: 2698–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009; 122: 187–198 [DOI] [PubMed] [Google Scholar]
- 52. Singh S, D'Mello V. van Bergen en Henegouwen P, Birge RB. A NPxY-independent beta5 integrin activation signal regulates phagocytosis of apoptotic cells. Biochem Biophys Res Commun. 2007; 364: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Surgucheva I, Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol Vis. 2011; 17: 2878–2888 [PMC free article] [PubMed] [Google Scholar]
- 54. Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010; 42: 906–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuehn MH, Wang K, Roos B, et al. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. Mol Vis. 2011; 17: 430–435 [PMC free article] [PubMed] [Google Scholar]