Abstract
Purpose.
Retinal hypoxia is a common pathological condition usually caused by ischemia that may result in alterations in oxidative energy metabolism. We report measurements of oxygen delivery by the retinal circulation (DO2_IR) and inner retinal oxygen metabolism (MO2_IR) under systemic normoxia and hypoxia in rat.
Methods.
Rats were ventilated with fractions of inspired oxygen (FiO2) to induce either normoxia (n = 10), moderate hypoxia (n = 14), or severe hypoxia (n = 10). Oxygen tension was measured in retinal vessels using phosphorescence lifetime imaging and converted to arterial (O2A) and venous (O2V) oxygen contents. Total retinal blood flow (F) was assessed by red-free and fluorescent microsphere imaging. DO2_IR and MO2_IR were calculated as the products of F and O2A, and F and the arteriovenous oxygen content difference (O2A−V), respectively.
Results.
Measurements of O2A, O2V, and O2A−V were significantly reduced with decreased FiO2 (P < 0.001). In response to reduced oxygen availability, F increased under moderate hypoxia (P < 0.001) but did not increase further under severe hypoxia (P = 0.5). DO2_IR was similar under normoxia and moderate hypoxia (P = 0.7), but significantly lower under severe hypoxia (P < 0.001). Likewise, MO2_IR under normoxia and moderate hypoxia was similar (P = 0.1), but significantly reduced under severe hypoxia (P ≤ 0.02).
Conclusions.
DO2_IR and MO2_IR were maintained during moderate hypoxia, but reduced under severe hypoxia, indicating blood flow compensation became insufficient for the reduced oxygen availability. Future studies may aid our understanding of retinal metabolic function in ischemic conditions.
Keywords: retina, delivery, metabolism, oxygen, hypoxia
Measurements of inner retinal oxygen delivery and metabolism arereported in rat under systemic normoxia and hypoxia. Blood flow compensation for the reduced oxygen availability maintained inner retinal oxygen delivery and metabolism during moderate hypoxia, but not under severe hypoxia.
Introduction
The retina requires a continuous and well-regulated supply of oxygen from the retinal and choroidal circulations to meet its high metabolic demand. Impaired oxygen delivery by the retinal circulation can adversely affect oxygen metabolism and lead to loss of visual function. In fact, abnormalities in retinal oxygenation are believed to contribute to the development of several eye diseases, including glaucoma, diabetic retinopathy, retinal vascular occlusion, and retinopathy of prematurity.1–4 Therefore, evaluation of the retinal circulation's capacity to meet the oxygen demand of the retinal tissue under physiological and pathological conditions is important.
Oxygen delivery by the retinal circulation (DO2_IR) has been reported in limited studies of newborn animals using combined measurements of retinal blood flow by a radioactive microsphere technique and arterial oxygen content by sampling of arterial blood.5,6 More commonly, retinal blood flow, an indicator of DO2_IR, has been measured in humans and animals using various techniques, such as Doppler velocimetry,7–11 Doppler optical coherence tomography (OCT),12–17 laser speckle,18,19 microsphere impaction,20,21 cell labeling,22–24 and particle tracking.25,26 However, because DO2_IR is the product of retinal blood flow and arterial blood oxygen content, quantitative measurement of blood flow alone provides only partial information about oxygen delivery to the retina.
Inner retinal oxygen metabolism (MO2_IR) has been previously measured locally in animals using oxygen-sensitive microelectrodes,27 although three-dimensional oxygen gradients from the retinal microvasculature may have influenced the measurements. To circumvent this problem, MO2_IR has been estimated under experimental conditions of retinal vascular occlusion and 100% oxygen inspiration.28–30 Alternatively, MO2_IR has been determined globally based on Fick's principle, by directly sampling the oxygen content of arterial and venous blood and measuring blood flow with labeled microspheres,31,32 as well as using phosphorescence lifetime and fluorescent microsphere imaging techniques.33 In humans, MO2_IR has been derived by oximetry combined with measurements of blood flow using the laser Doppler technique34,35 or mean circulation time using fluorescein angiography.36
Retinal hypoxia is a common pathological condition usually caused by ischemia28,37 that occurs in several major retinal diseases and may lead to derangements in oxidative energy metabolism. Despite an abundance of studies that describe the response of retinal blood flow to hypoxia,5,38–42 there are limited data on DO2_IR and MO2_IR under conditions of reduced oxygen availability.5,36 The purpose of this study was to report measurements of DO2_IR and MO2_IR under normoxia and systemic hypoxia in rats using combined oxygen tension and blood flow imaging.
Methods
Animals
Thirty-four Long Evans pigmented rats (weight: 390 ± 72 g, age: 12 ± 3 weeks, mean ± SD) were used in the study. The rats were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg). Additional injections of ketamine (20 mg/kg) and xylazine (1 mg/kg) were given to maintain anesthesia as required. Rats were mechanically ventilated with three fractions of inspired oxygen (FiO2), either room air (21% oxygen, normoxia), 15% oxygen (moderate hypoxia), or 10% oxygen (severe hypoxia), with the use of an endotracheal tube connected to a small animal ventilator (Harvard Apparatus, Inc., South Natick, MA). Ventilation with 10% O2 was selected based on a previously published study,43 which indicated that this oxygen level produced a maximum or near maximum hypoxic challenge, as evidenced by a significant increase in the retinal oxygen extraction fraction and impairment in the systemic physiological condition. Ventilation with 15% O2 was selected because it represented an intermediate hypoxic challenge between severe hypoxia and normoxia. Rats were ventilated with reduced FiO2 gas mixtures 15 minutes before and continuously during imaging for a duration of 1 to 2 hours. To monitor the animal's physiological condition, the femoral artery was cannulated and a catheter was attached to draw blood and connect a pressure transducer. Systemic arterial oxygen tension (PaO2), carbon dioxide tension (PaCO2), and pH were measured with a blood gas analyzer (Radiometer, Westlake, OH), 5 to 10 minutes after initiation of ventilation. Ventilation parameters, including the respiratory rate and minute volume, were adjusted until the PaCO2 was within the normocapnic range.44 Hemoglobin concentration (HgB) was also measured with a hematology system (Siemens, Tarrytown, NY) from arterial blood. Blood pressure (BP) and heart rate (HR) were monitored continuously with a data acquisition system (Biopac Systems, Goleta, CA) linked to the pressure transducer.
Before imaging, the rat was placed in an animal holder with a copper tubing water heater, which maintained the body temperature at 37°C. The pupils were dilated with 2.5% phenylephrine and 1% tropicamide. A glass cover slip with 1% hydroxypropyl methylcellulose was applied to the cornea to eliminate its refractive power and prevent dehydration. For retinal vascular oxygen tension (PO2) imaging, an oxygen-sensitive molecular probe, Pd-porphine (Frontier Scientific, Logan, UT), was dissolved (12 mg/mL) in BSA solution (60 mg/mL) and administered through the femoral arterial catheter (20 mg/kg). For retinal blood velocity imaging, 2-μm polystyrene fluorescent microspheres (Invitrogen, Grand Island, NY) were injected through the catheter. Typically, three to four injections of the microspheres were given and each injection was approximately 0.3 mL (105 microspheres/mL). One eye of each rat was imaged and data were obtained in 10, 14, and 10 rats under normoxia, moderate hypoxia, and severe hypoxia, respectively.
Retinal Vascular PO2 Imaging
Retinal vascular PO2 was measured using our established optical section phosphorescence lifetime imaging system.45,46 A laser line was projected on the retina after intravenous injection of the Pd-porphine probe and an optical section phosphorescence image was acquired with an intensified charge-coupled device camera. Due to the angle between the excitation laser and imaging path, phosphorescence emissions from the retinal vessels were depth-resolved from the underlying choroid. Phosphorescence lifetimes in the retinal vessels were determined using a frequency-domain approach and converted to PO2 measurements using the Stern-Volmer equation.47,48 PO2 was measured in all major retinal arteries (PO2A) and retinal veins (PO2V), at locations within three optic disc diameters (∼600 μm) from the edge of the optic nerve head. Three repeated PO2 measurements were averaged per blood vessel.
Blood Flow Imaging
Our previously described prototype blood flow imaging system33 was used for red-free and fluorescent microsphere imaging to assess venous blood vessel diameter and velocity, respectively. A slit lamp biomicroscope with standard light illumination (Carl Zeiss, Oberkochen, Germany) was equipped with a green filter (540 ± 5 nm; Edmund Optics, Barrington, NJ) for red-free retinal imaging, and a 488-nm diode laser (excitation; Melles Griot, Carlsbad, CA), coupled with an emission filter (560 ± 60 nm; Spectrotech, Inc., Saugus, MA) for fluorescent microsphere imaging. Images were captured with a high-speed electron multiplier charge coupled device camera (QImaging, Surrey, Canada). Red-free retinal images were obtained using the full resolution of the camera (1002 × 1004 pixels). For fluorescent images, the camera sensor was binned to maximize the frame rate to 108 Hz, allowing the motion of the microspheres to be resolved in time, but with lower spatial resolution (248 × 250 pixels). Multiple image sequences, each 5 seconds in duration, were recorded over several minutes.
Venous diameter (D) was measured from red-free images over a fixed vessel length (200 μm) that spanned between approximately 300 and 500 μm from the center of the optic disk. Diameter measurements were obtained based on the average full width at half maximum of 12 intensity profiles perpendicular to the blood vessel axis. Venous blood velocity (V) was measured by manually tracking displacements of the microspheres over time, following our previously reported methodology.33 Typically, three to five image sequences were analyzed to derive V, which was determined by averaging 27 ± 8 microsphere velocity measurements in each vein. The number of velocity measurements was contingent on the number of microspheres that could be visualized in the image sequences. Blood flow in each major vein was calculated from V and D measurements (V*π*D2/4) and summed over all veins to provide a measure of the total blood flow in the retinal circulation (F). Blood flow was measured in veins because they are less affected by pulsation and have larger diameters as compared with arteries. Measurements of F were obtained approximately 15 minutes following PO2 imaging, while the physiological state of the animals was relatively stable, as indicated by the continuous monitoring of BP, HR, and the use of constant ventilation parameters.
Oxygen Delivery and Metabolism
The O2 content of blood was calculated for each major retinal artery and vein as the sum of oxygen bound to hemoglobin and dissolved in blood49: O2 content = SO2*C*HgB + PO2*k, where SO2 is the oxygen saturation, C is maximum oxygen-carrying capacity of hemoglobin (1.39 mL O2/g),44 HgB is the measured hemoglobin concentration, PO2 is the measured vascular oxygen tension, and k is the oxygen solubility in blood (0.003 mL O2/dL·mm Hg).50 SO2 was calculated from the hemoglobin dissociation curve in rat51 by using the measured PO2 and arterial blood pH values. Last, in each animal, O2A and O2V were determined by averaging the O2 content of all major retinal arteries and veins, respectively.
DO2_IR was determined from the product of F and O2A measurements according to the following equation:
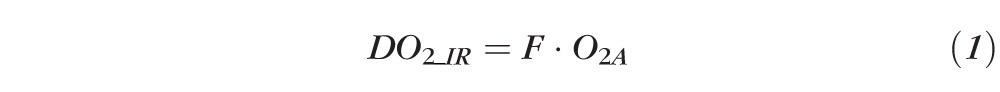 |
MO2_IR was calculated from measurements of F and the arteriovenous oxygen content difference (O2A−V), using Fick's principle52:
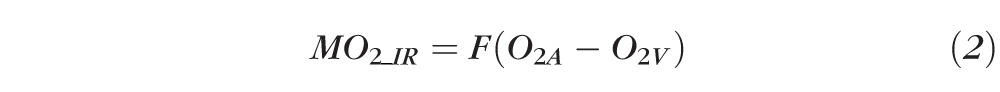 |
Data Analysis
Measurements obtained under normoxia, moderate hypoxia, and severe hypoxia were compared using one-way ANOVA. Linear regression analysis was also performed, demonstrating that PO2A, PO2V, O2A, O2V, V, F, DO2_IR, and MO2_IR were significantly related to BP (P < 0.004; n = 34), while D and O2A−V, were not (P ≥ 0.2). Therefore, to adjust for this source of variability, BP was used as a covariate in a one-way analysis of covariance (ANCOVA). Post hoc analyses using Tukey's method were performed to determine statistically significant pairwise differences. All statistical analyses were performed using Systat (Systat Software, Inc., Chicago, IL) and SAS software (SAS Institute, Inc., Cary, NC). Statistical significance was accepted at P less than 0.05.
Results
The systemic physiological status of the rats is summarized according to FiO2 condition in Table 1. As expected, PaO2 was significantly lower under decreased FiO2 (P < 0.001). PaCO2 was also significantly different under FiO2 conditions (P = 0.001), but all values were close to or within the normal range of 35 to 45 mm Hg. Similarly, systemic blood pH differed significantly among FiO2 conditions (P = 0.02), although all values were within the normal range of 7.35 to 7.45. No significant changes in HR and HgB were observed with decreased FiO2 (P ≥ 0.5). However, BP was significantly reduced with decreased FiO2 (P = 0.003).
Table 1.
Systemic Physiological Parameters (Mean ± SD) Under Normoxia and Graded Levels of Hypoxia
|
Systemic Physiologic Parameters |
Normoxia,
n
= 10 |
Moderate Hypoxia,
n
= 14 |
Severe Hypoxia,
n
= 10 |
P
Value |
| Arterial PO2, mm Hg | 91 ± 9 | 46 ± 6 | 31 ± 2 | <0.001 |
| Arterial PCO2, mm Hg | 42 ± 3 | 34 ± 5 | 39 ± 4 | 0.001 |
| pH | 7.36 ± 0.04 | 7.40 ± 0.03 | 7.39 ± 0.05 | 0.02 |
| BP, mm Hg | 101 ± 18 | 84 ± 17 | 68 ± 23 | 0.003 |
| HR, beats/min | 224 ± 35 | 225 ± 31 | 239 ± 27 | 0.5 |
| HgB, g/dL | 14.2 ± 0.8 | 14.2 ± 1.0 | 13.8 ± 0.6 | 0.5 |
values derived by ANOVA are reported.
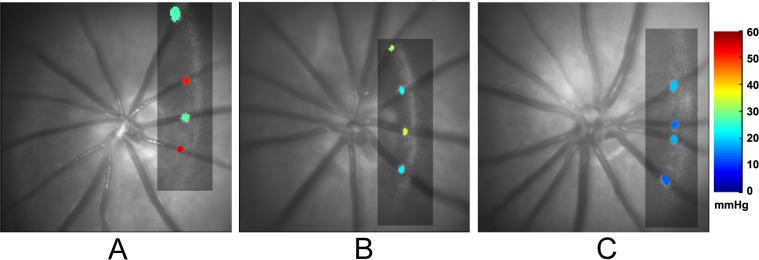
Examples of selected cross-sectional vascular PO2 maps in three rats under different FiO2 conditions, overlaid on the corresponding red-free retinal images, are shown in Figure 1. Each PO2 map depicts measurements in major retinal arteries and veins, demonstrating decreased PO2A and PO2V with lower FiO2. PO2 was measured in all major retinal arteries and veins of each rat in a similar manner and averaged. Mean values of PO2A, PO2V, O2A, O2V, and O2A−V compiled from measurements in all rats are summarized according to FiO2 condition in Table 2. All parameters were significantly different among FiO2 conditions, with and without adjustment for BP (P < 0.001). O2A was significantly reduced with each decreased level of FiO2 (P < 0.001), whereas O2V was similar under moderate hypoxia and normoxia (P = 0.09), and significantly reduced under severe hypoxia (P ≤ 0.01). O2A−V was significantly reduced under moderate hypoxia as compared with normoxia (P < 0.001), but not statistically different between severe and moderate hypoxia (P = 0.2).
Figure 1.
Examples of selected cross-sectional vascular PO2 maps (rectangles) overlaid on red-free retinal images in three rats under (A) normoxia, (B) moderate hypoxia, and (C) severe hypoxia. PO2 values are depicted in two arteries and two veins. Color bar shows PO2 values in mm Hg.
Table 2.
Retinal PO2A, PO2v, O2A, O2V, and O2A−V of Rats (Mean ± SD) Under Normoxia and Graded Levels of Hypoxia
|
Retinal Oxygenation Parameters |
Normoxia,
n
= 10 |
Moderate Hypoxia,
n
= 14 |
Severe Hypoxia,
n
= 10 |
ANOVA
P
Value FiO2 |
ANCOVA
P
Value |
|
|
FiO2 |
BP |
|||||
| PO2A, mm Hg | 44 ± 4 | 29 ± 4 | 21 ± 4 | <0.001 | <0.001 | 0.7 |
| PO2V, mm Hg | 26 ± 3 | 20 ± 3 | 12 ± 6 | <0.001 | <0.001 | 0.001 |
| O2A, mL O2/dL | 11.9 ± 1.4 | 7.3 ± 1.7 | 4.1 ± 1.7 | <0.001 | <0.001 | 0.5 |
| O2V, mL O2/dL | 5.4 ± 1.3 | 3.7 ± 1.2 | 1.5 ± 1.5 | <0.001 | <0.001 | 0.006 |
| O2A−V, mL O2/dL | 6.5 ± 1.5 | 3.6 ± 1.5 | 2.6 ± 1.2 | <0.001 | NA | NA |
values derived by ANOVA and ANCOVA with effects of FiO2 and BP are reported.
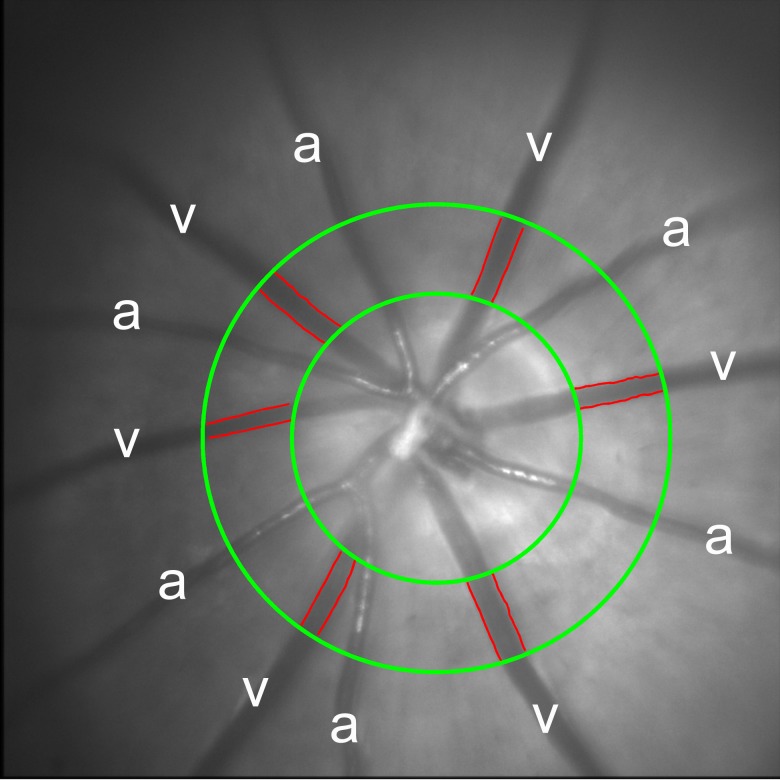
An example of a red-free retinal image obtained in one rat under normoxia, displaying major retinal arteries and veins, is shown in Figure 2. Measurements of D, V, and blood flow were obtained in each vein, and the average D and V and the summed total retinal blood flow (F) were calculated. Mean values of D, V, and F compiled from measurements in all rats are summarized according to FiO2 condition in Table 3. Measurements of D were similar (P = 0.5), whereas measurements of V and F were significantly different among FiO2 conditions, with and without adjustment for BP (P ≤ 0.03). V was significantly higher under moderate hypoxia as compared with normoxia (P = 0.003). F was significantly higher under both moderate and severe hypoxia as compared with normoxia (P ≤ 0.03), but not statistically different between moderate and severe hypoxia (P = 0.5).
Figure 2.
An example of a red-free retinal image displaying six major retinal arteries (a) and veins (v) in a rat under normoxia. The outlined edges of the retinal veins (red lines) were identified from multiple-diameter measurements over a fixed vessel span at a defined distance from the center of the optic nerve head, as delineated by green circles.
Table 3.
Retinal Venous D, V, and F of Rats (Mean ± SD) Under Normoxia and Graded Levels of Hypoxia
|
Blood Flow Parameters |
Normoxia,
n
= 10 |
Moderate Hypoxia,
n
= 14 |
Severe Hypoxia,
n
= 10 |
ANOVA
P
Value FiO2 |
ANCOVA
P
Value |
|
|
FiO2 |
BP |
|||||
| D, μm | 51 ± 6 | 54 ± 6 | 53 ± 6 | 0.5 | NA | NA |
| V, mm/s | 11.8 ± 2.8 | 14.3 ± 2.0 | 9.7 ± 5.2 | 0.01 | 0.002 | <0.001 |
| F, μL/min | 7.9 ± 1.7 | 10.9 ± 2.5 | 7.9 ± 4.5 | 0.03 | <0.001 | <0.001 |
values derived by ANOVA and ANCOVA with effects of FiO2 and BP are reported.
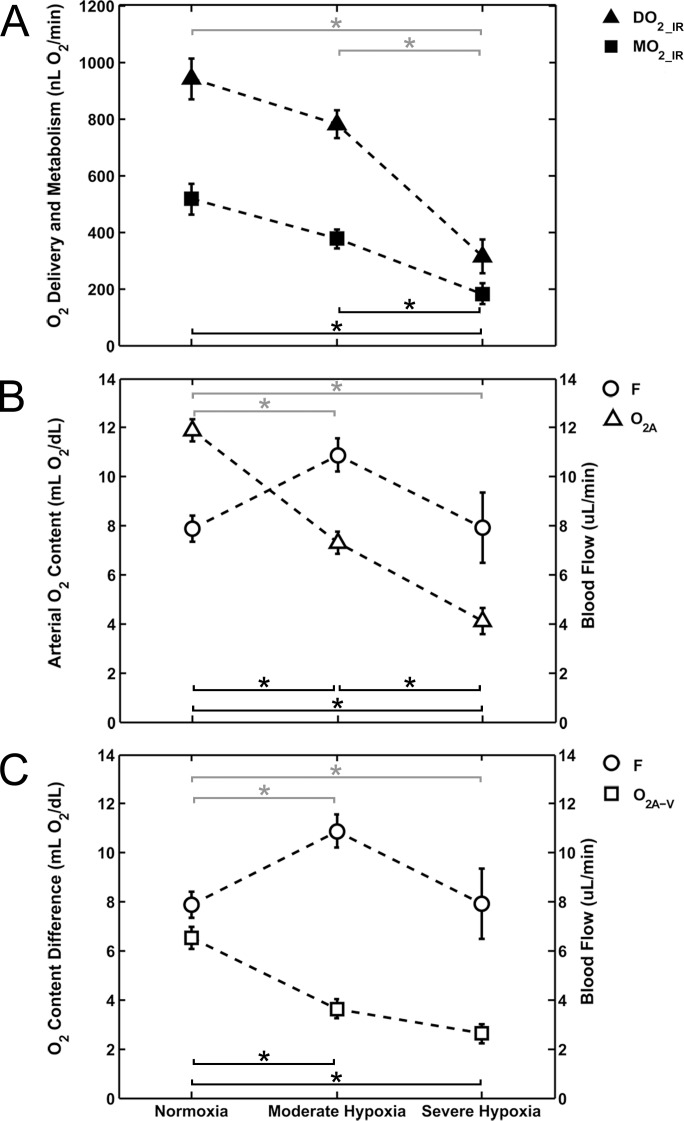
Mean values of DO2_IR and MO2_IR according to FiO2 condition are shown in Figure 3A. DO2_IR and MO2_IR were significantly different among FiO2 conditions, with and without adjustment for BP (P ≤ 0.002). There was a significant effect of BP on DO2_IR (P < 0.001), but not on MO2_IR (P = 0.3). DO2_IR under normoxia (941 ± 231 nL O2/min) and moderate hypoxia (781 ± 186 nL O2/min) were similar (P = 0.7). Under severe hypoxia, DO2_IR (313 ± 189 nL O2/min) was significantly lower than under normoxia and moderate hypoxia (P < 0.001). Likewise, MO2_IR under normoxia (516 ± 175 nL O2/min) and moderate hypoxia (377 ± 125 nL O2/min) were similar (P = 0.1). Under severe hypoxia, MO2_IR (182 ± 115 nL O2/min) was significantly lower than under normoxia and moderate hypoxia (P ≤ 0.02).
Figure 3.
(A) Mean values of DO2_IR and MO2_IR plotted under normoxia and graded levels of hypoxia. (B) The determinants of DO2_IR: F and O2A. (C) The determinants of MO2_IR: F and O2A−V. Error bars represent the SEM. Black asterisks (MO2_IR, O2A, O2A−V) and gray asterisks (DO2_IR, F) indicate statistically significant pairwise differences (P < 0.05) between FiO2 conditions based on post hoc analysis.
Following Equations 1 and 2, the determinants of DO2_IR and MO2_IR are plotted according to FiO2 condition in Figures 3B, 3C, respectively. Under moderate hypoxia, DO2_IR and MO2_IR approximated normoxic levels because the reduction in O2A and O2A−V, respectively, was compensated by the increase in F. Under severe hypoxia, DO2_IR was significantly reduced as compared with moderate hypoxia, owing to a further reduction in O2A without an additional compensatory increase in F, presumably due to maximized blood flow compensation. MO2_IR was also significantly reduced under severe hypoxia as compared with moderate hypoxia due to the combined trends of F and O2A−V, even though no significant changes were found in these two parameters individually.
Discussion
Assessment of inner retinal oxygen delivery and metabolism is important for evaluating alterations in retinal metabolic function under hypoxic conditions. In the present study, measurements of DO2_IR and MO2_IR were reported for the first time in rats and a significant reduction in these parameters was established in response to severe systemic hypoxia.
Hypoxia was induced in rats with controlled ventilation of gases with variable oxygen content. The PaCO2 and pH levels were significantly altered, but remained close to their normal ranges due to controlled ventilation parameters. Decreased FiO2 also resulted in reduced levels of PaO2 and BP, consistent with previously published studies in hypoxic animals.38,53,54 ANCOVA was used to differentiate the effects of FiO2 and BP, assuming a linear relationship between the measured parameters and the covariate (BP). Although this assumption is not entirely valid, particularly in the presence of autoregulation, highly significant linear regressions of the parameters with BP were determined. Accordingly, using BP as a covariate in ANCOVA allowed elimination of a substantial component of the variability so as to better isolate the effect of FiO2.
In the current study, measurements obtained under normoxia corresponded with previously published data. Mean PO2A measurements (44 mm Hg) were in general agreement with previously reported values obtained with microelectrodes near the surface of retinal arteries in rats (32 mm Hg),55 pigs (37 mm Hg),56 miniature pigs (47 mm Hg),57 and cats (56 mm Hg).58 Mean PO2V measurements (26 mm Hg) were also comparable to measurements recorded near retinal veins in rats (19 mm Hg).55 Mean D measurements (51 μm) were similar to values obtained by in vivo fundus imaging (45 μm)59 and a corrosion cast technique (53 μm).60 Similarly, mean V measurements (11.8 mm/s) were in general agreement with previously reported measurements using labeled red blood cells (15.5 and 17.4 mm/s),24,22 and smaller than a value obtained from microsphere tracking (34.9 mm/s).25 Although the accuracy of our V measurements may have been limited due to the distribution of velocities that occur with laminar flow, by averaging velocities of multiple microspheres in each blood vessel, this effect was minimized. Mean F measurements (7.9 μL/min) were also similar to values reported using Doppler OCT (6.4 μL/min)15 and a fluorescent microsphere impaction technique (11.6 μL/min).61
To compare DO2_IR and MO2_IR measurements with other published studies, these values were converted to per-unit mass, assuming a retinal mass of 16 mg in rat62,63 and an inner retinal volume of 50%. DO2_IR was estimated to be 11.8 mLO2/min*100 g, higher than 5.6 mL O2/min*100 g reported in newborn lambs.5 MO2_IR was calculated to be 6.5 mL O2/min*100 g, slightly higher than values reported in pigs (4.6 and 3.8 mL O2/min*100 g),31,32 cats (3.7 mL O2/min*100 g),28 and rats (2.7 mL O2/min*100 g),30 and lower than whole retinal consumption values determined in humans (9.7 and 8.3 mL O2/100 mL tissue*100 g).64,65 These differences may be attributed to the assumed mass and volume of the inner retinal tissue, as well as dissimilarities in methodologies, species, or ages. Another factor that may have affected our estimation of MO2_IR was calculation of O2V from the oxygen dissociation curve using the measured arterial blood pH. Because blood pH tends to be lower in veins as compared with arteries,66 the actual O2V was probably lower due to a right shift of the oxygen dissociation curve. Therefore, the actual MO2_IR was likely slightly higher than the reported value in the current study.
In response to moderate systemic hypoxia and the consequent reduction in retinal vascular PO2, F increased significantly due to blood flow compensation for the reduced oxygen availability. This finding is consistent with previously reported retinal blood flow increases in humans,42 monkeys,38 cats,41,67 and newborn lambs5 during acute systemic hypoxia. The increase in F was predominately due to an increase in V, as a significant change in D was not detected. In accord with our findings, blood velocity has been previously reported to increase under hypoxia,42,67,68 presumably due to an overall reduction in flow resistance resulting from vasodilation in the retinal microvascular network. Although previous studies have reported dilation of major retinal vessels in response to hypoxia in cats,67 monkeys,38 and humans,69 the relatively large interanimal variability of D likely precluded detection of a statistically significant change in the current study. Under severe hypoxia, F did not increase further as compared with moderate hypoxia, suggesting blood flow compensation was near or at its full capacity.
Alterations in DO2_IR under systemic hypoxia resulted from the opposing effects of the reduced oxygen content of blood and the compensatory increase in blood flow. Under moderate hypoxia, DO2_IR remained relatively stable because the increase in blood flow counteracted the reduction in oxygen availability. This finding is consistent with the previously reported constant DO2_IR values in lambs5 and relatively unchanged inner retinal tissue PO2 in cats during acute hypoxia.70 Under severe hypoxia, the significant decrease in DO2_IR was attributed to a further reduction of available oxygen, without an increase in blood flow, suggesting maximized blood flow compensation capacity under this extreme level of hypoxia.
In the current study, MO2_IR measurements were reported for the first time under hypoxia. Under moderate hypoxia, MO2_IR remained relatively unchanged as compared with normoxia, because DO2_IR did not decline significantly. This finding is in agreement with previously reported steady levels of cerebral oxygen metabolism in rats during moderate levels of hypoxia.71 However, under severe hypoxia, MO2_IR was significantly reduced due to a considerable decrease in DO2_IR. In fact, a reduction in MO2_IR was inevitable because DO2_IR fell below the normoxic MO2_IR level. Consequently, it was impossible to maintain some energy-requiring processes, which may lead to reduced retinal function and viability.
The severe hypoxic condition investigated in the current study may have initiated metabolic cascades resulting in retinal injury. For example, the expressions of HIF-1α and VEGF in ex vivo retinal tissue of rodents have been shown to increase under short-term hypoxia and ischemia.72,73 Therefore, it is likely these hypoxic and angiogenic factors would have become elevated in the retinal tissue following severe hypoxia. Furthermore, the observed reduction in MO2_IR under severe hypoxia may have initiated cell apoptosis, as previously demonstrated by elevated levels of proapoptotic markers (Bax and caspase 3) in rats following an ischemic insult.74,75
The findings of the current study may have relevance to retinal ischemic conditions associated with hypoxia. For example, in diabetic retinopathy, capillary nonperfusion in localized retinal regions is known to result in reduced DO2_IR, tissue hypoxia, and presumably impaired MO2_IR. The status of the retinal tissue under the severe hypoxic condition in the current study likely bears similarities to ischemic retina in diabetic retinopathy and vascular occlusions. Future studies are needed to investigate the presence and degree of alterations in DO2_IR and MO2_IR in animal models of retinal ischemia.
In summary, combined measurements of inner retinal oxygen delivery and metabolism were reported for the first time in rats under systemic normoxia and graded levels of hypoxia. Blood flow compensation for the reduced oxygen availability maintained inner retinal oxygen delivery and metabolism during moderate hypoxia, but not under severe hypoxia. Future studies with more incremental levels of inspired oxygen are needed to determine the minimum level of oxygen delivery that is required to maintain normal retinal energy metabolism. Because hypoxia is a major consequence of retinal ischemia, findings from such studies will further elucidate the pathophysiology of the retinal ischemic conditions commonly encountered in clinical settings.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland, EY017918 (MS) and EY001792 (UIC); Research to Prevent Blindness, New York, New York, senior scientific investigator award (MS) and an unrestricted departmental award; and University of Illinois at Chicago's Center for Clinical and Translational Science supported by National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, Maryland, UL1TR000050.
Disclosure: J. Wanek, None; P.-Y. Teng, None; N.P. Blair, None; M. Shahidi, P
References
- 1. Mozaffarieh M, Grieshaber MC, Flammer J. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis. 2008; 14: 224–233 [PMC free article] [PubMed] [Google Scholar]
- 2. Stefansson E. Oxygen and diabetic eye disease. Graefes Arch Clin Exp Ophthalmol. 1990; 228: 120–123 [DOI] [PubMed] [Google Scholar]
- 3. Yoneya S, Saito T, Nishiyama Y, et al. Retinal oxygen saturation levels in patients with central retinal vein occlusion. Ophthalmology. 2002; 109: 1521–1526 [DOI] [PubMed] [Google Scholar]
- 4. Zhang W, Ito Y, Berlin E, Roberts R, Berkowitz BA. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003; 44: 3119–3123 [DOI] [PubMed] [Google Scholar]
- 5. Milley JR, Rosenberg AA, Jones MD Jr. Retinal and choroidal blood flows in hypoxic and hypercarbic newborn lambs. Pediatr Res. 1984; 18: 410–414 [DOI] [PubMed] [Google Scholar]
- 6. Bottoli I, Beharry K, Modanlou HD, et al. Effect of group B streptococcal meningitis on retinal and choroidal blood flow in newborn pigs. Invest Ophthalmol Vis Sci. 1995; 36: 1231–1239 [PubMed] [Google Scholar]
- 7. Feke GT, Tagawa H, Deupree DM, Goger DG, Sebag J, Weiter JJ. Blood flow in the normal human retina. Invest Ophthalmol Vis Sci. 1989; 30: 58–65 [PubMed] [Google Scholar]
- 8. Garcia JPS, Garcia PT, Rosen RB. Retinal blood flow in the normal human eye using the canon laser blood flowmeter. Ophthalmic Res. 2002; 34: 295–299 [DOI] [PubMed] [Google Scholar]
- 9. Grunwald JE, Riva CE, Baine J, Brucker AJ. Total retinal volumetric blood-flow rate in diabetic-patients with poor glycemic control. Invest Ophthalmol Vis Sci. 1992; 33: 356–363 [PubMed] [Google Scholar]
- 10. Riva CE, Grunwald JE, Sinclair SH, Petrig BL. Blood velocity and volumetric flow-rate in human retinal-vessels. Invest Ophthalmol Vis Sci. 1985; 26: 1124–1132 [PubMed] [Google Scholar]
- 11. Riva CE, Feke GT, Eberli B, Benary V. Bidirectional Ldv system for absolute measurement of blood speed in retinal-vessels. Appl Optics. 1979; 18: 2301–2306 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D. Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol. 2009; 93: 634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang YM, Bower BA, Izatt JA, Tan O, Huang D. Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2008; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werkmeister RM, Dragostinoff N, Pircher M, et al. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008; 33: 2967–2969 [DOI] [PubMed] [Google Scholar]
- 15. Zhi ZW, Cepurna W, Johnson E, Shen T, Morrison J, Wang RKK. Volumetric and quantitative imaging of retinal blood flow in rats with optical microangiography. Biomed Opt Express. 2011; 2: 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang YM, Bower BA, Izatt JA, Tan O, Huang D. In vivo total retinal blood flow measurement by Fourier domain Doppler optical coherence tomography. J Biomed Opt. 2007; 12:041215. [DOI] [PubMed] [Google Scholar]
- 17. Leitgeb RA, Schmetterer L, Drexler W, Fercher AF, Zawadzki RJ, Bajraszewski T. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt Express. 2003; 11: 3116–3121 [DOI] [PubMed] [Google Scholar]
- 18. Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010; 88: 723–729 [DOI] [PubMed] [Google Scholar]
- 19. Nagahara M, Tamaki Y, Tomidokoro A, Araie M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci. 2011; 52: 87–92 [DOI] [PubMed] [Google Scholar]
- 20. Alm A, Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972; 84: 306–319 [DOI] [PubMed] [Google Scholar]
- 21. Alm A, Ocular Bill A. and optic-nerve blood-flow at normal and increased intraocular pressures in monkeys (Macaca irus). Study with radioactively labeled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973; 15: 15–29 [DOI] [PubMed] [Google Scholar]
- 22. Nishiwaki H, Ogura Y, Kimura H, Kiryu J, Honda Y. Quantitative evaluation of leukocyte dynamics in retinal microcirculation. Invest Ophthalmol Vis Sci. 1995; 36: 123–130 [PubMed] [Google Scholar]
- 23. Le Gargasson JF, Paques M, Guez JE, et al. Scanning laser ophthalmoscope imaging of fluorescein-labelled blood cells. Graefes Arch Clin Exp Ophthalmol. 1997; 235: 56–58 [DOI] [PubMed] [Google Scholar]
- 24. Wajer SD, Taomoto M, McLeod DS, et al. Velocity measurements of normal and sickle red blood cells in the rat retinal and choroidal vasculatures. Microvasc Res. 2000; 60: 281–293 [DOI] [PubMed] [Google Scholar]
- 25. Lorentz K, Zayas-Santiago A, Tummala S. Kang Derwent JJ. Scanning laser ophthalmoscope-particle tracking method to assess blood velocity during hypoxia and hyperoxia. Adv Exp Med Biol. 2008; 614: 253–261 [DOI] [PubMed] [Google Scholar]
- 26. Khoobehi B, Shoelson B, Zhang YZ, Peyman GA. Fluorescent microsphere imaging: a particle-tracking approach to the hemodynamic assessment of the retina and choroid. Ophthalmic Surg Lasers. 1997; 28: 937–947 [PubMed] [Google Scholar]
- 27. Cringle SJ, Yu DY, Yu PK, Su EN. Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci. 2002; 43: 1922–1927 [PubMed] [Google Scholar]
- 28. Alder VA, Ben-Nun J, Cringle SJ. PO2 profiles and oxygen consumption in cat retina with an occluded retinal circulation. Invest Ophthalmol Vis Sci. 1990; 31: 1029–1034 [PubMed] [Google Scholar]
- 29. Braun RD, Linsenmeier RA, Goldstick TK. Oxygen consumption in the inner and outer retina of the cat. Invest Ophthalmol Vis Sci. 1995; 36: 542–554 [PubMed] [Google Scholar]
- 30. Yu DY, Cringle SJ, Yu PK, Su EN. Intraretinal oxygen distribution and consumption during retinal artery occlusion and graded hyperoxic ventilation in the rat. Invest Ophthalmol Vis Sci. 2007; 48: 2290–2296 [DOI] [PubMed] [Google Scholar]
- 31. Tornquist P, Alm A. Retinal and choroidal contribution to retinal metabolism in vivo. A study in pigs. Acta Physiol Scand. 1979; 106: 351–357 [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Tornquist P, Bill A. Glucose metabolism of the inner retina in pigs in darkness and light. Acta Physiol Scand. 1997; 160: 71–74 [DOI] [PubMed] [Google Scholar]
- 33. Wanek J, Teng PY, Albers J, Blair NP, Shahidi M. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomed Opt Express. 2011; 2: 2562–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sebag J, Delori FC, Feke GT, Weiter JJ. Effects of optic atrophy on retinal blood flow and oxygen saturation in humans. Arch Ophthalmol. 1989; 107: 222–226 [DOI] [PubMed] [Google Scholar]
- 35. Siesky B, Harris A, Kagemann L, et al. Ocular blood flow and oxygen delivery to the retina in primary open-angle glaucoma patients: the addition of dorzolamide to timolol monotherapy. Acta Ophthalmol. 2010; 88: 142–149 [DOI] [PubMed] [Google Scholar]
- 36. Hickam JB, Frayser R. Studies of the retinal circulation in man: observations on vessel diameter, arteriovenous oxygen difference, and mean circulation time. Circulation. 1966; 33: 302–316 [DOI] [PubMed] [Google Scholar]
- 37. Pournaras CJ, Tsacopoulos M, Strommer K, Gilodi N, Leuenberger PM. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990; 97: 1321–1328 [DOI] [PubMed] [Google Scholar]
- 38. Eperon G, Johnson M, David NJ. The effect of arterial PO2 on relative retinal blood flow in monkeys. Invest Ophthalmol. 1975; 14: 342–352 [PubMed] [Google Scholar]
- 39. Pournaras C, Tsacopoulos M, Chapuis P. Studies on the role of prostaglandins in the regulation of retinal blood flow. Exp Eye Res. 1978; 26: 687–697 [DOI] [PubMed] [Google Scholar]
- 40. Odden JP, Bratlid D, Hall C, Farstad T, Roll E, Stiris T. Effect of hypoxemia and hypovolemia on retinal and choroidal blood flow in the newborn piglet. Biol Neonate. 1993; 64: 140–150 [DOI] [PubMed] [Google Scholar]
- 41. Ahmed J, Pulfer MK, Linsenmeier RA. Measurement of blood flow through the retinal circulation of the cat during normoxia and hypoxemia using fluorescent microspheres. Microvasc Res. 2001; 62: 143–153 [DOI] [PubMed] [Google Scholar]
- 42. Strenn K, Menapace R, Rainer G, Findl O, Wolzt M, Schmetterer L. Reproducibility and sensitivity of scanning laser Doppler flowmetry during graded changes in PO2. Br J Ophthalmol. 1997; 81: 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teng PY, Wanek J, Blair NP, Shahidi M. Inner retinal oxygen extraction fraction in rat. Invest Ophthalmol Vis Sci. 2013; 54: 647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. West JB. Pulmonary Physiology and Pathophysiology: An Integrated, Case-Based Approach. 2nd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007: vii, 150. [Google Scholar]
- 45. Shahidi M, Shakoor A, Blair NP, Mori M, Shonat RD. A method for chorioretinal oxygen tension measurement. Curr Eye Res. 2006; 31: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shahidi M, Wanek J, Blair NP, Mori M. Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Invest Ophthalmol Vis Sci. 2009; 50: 820–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lakowicz JR, Szmacinski H, Nowaczyk K, Berndt KW, Johnson M. Fluorescence lifetime imaging. Anal Biochem. 1992; 202: 316–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shonat RD, Kight AC. Oxygen tension imaging in the mouse retina. Ann Biomed Eng. 2003; 31: 1084–1096 [DOI] [PubMed] [Google Scholar]
- 49. Shapiro BA, Peruzzi WT, Kozelowski-Templin R. Clinical Application of Blood Gases. 5th ed. St. Louis, MO: Mosby; 1994: xviii, 427. [Google Scholar]
- 50. Costanzo LS. Physiology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2007: xii, 334. [Google Scholar]
- 51. Cartheuser CF. Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comp Biochem Physiol Comp Physiol. 1993; 106: 775–782 [DOI] [PubMed] [Google Scholar]
- 52. Berne RM, Levy MN. Physiology. 2nd ed. St. Louis, MO: Mosby; 1988. [Google Scholar]
- 53. Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007; 1135: 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johannsson H, Siesjo BK. Cerebral blood flow and oxygen consumption in the rat in hypoxic hypoxia. Acta Physiol Scand. 1975; 93: 269–276 [DOI] [PubMed] [Google Scholar]
- 55. Yu DY, Cringle SJ, Alder V, Su EN. Intraretinal oxygen distribution in the rat with graded systemic hyperoxia and hypercapnia. Invest Ophthalmol Vis Sci. 1999; 40: 2082–2087 [PubMed] [Google Scholar]
- 56. Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986; 27: 722–726 [PubMed] [Google Scholar]
- 57. Pournaras CJ, Riva CE, Tsacopoulos M, Strommer K. Diffusion of O2 in the retina of anesthetized miniature pigs in normoxia and hyperoxia. Exp Eye Res. 1989; 49: 347–360 [DOI] [PubMed] [Google Scholar]
- 58. Buerk DG, Shonat RD, Riva CE, Cranstoun SD. O2 gradients and countercurrent exchange in the cat vitreous-humor near retinal arterioles and venules. Microvasc Res. 1993; 45: 134–148 [DOI] [PubMed] [Google Scholar]
- 59. Link D, Strohmaier C, Seifert BU, et al. Novel non-contact retina camera for the rat and its application to dynamic retinal vessel analysis. Biomed Opt Express. 2011; 2: 3094–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamakawa K, Bhutto IA, Lu ZY, Watanabe Y, Amemiya T. Retinal vascular changes in rats with inherited hypercholesterolemia. Corrosion cast demonstration. Curr Eye Res. 2001; 22: 258–265 [DOI] [PubMed] [Google Scholar]
- 61. Shih YY, Wang L, De La Garza BH, et al. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr Eye Res. 2013; 38: 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brotherton J. Studies on the metabolism of the rat retina with special reference to retinitis pigmentosa . I. Anaerobic glycolysis. Exp Eye Res. 1962; 1: 234–245 [DOI] [PubMed] [Google Scholar]
- 63. Citirik M, Dilsiz N, Batman C, Zilelioglu O. Comparative toxicity of 4 commonly used intravitreal corticosteroids on rat retina. Can J Ophthalmol. 2009; 44: e3–e8 [DOI] [PubMed] [Google Scholar]
- 64. Anderson B Jr, Saltzman HA. Retinal oxygen utilization measured by hyperbaric blackout. Arch Ophthalmol. 1964; 72: 792–795 [DOI] [PubMed] [Google Scholar]
- 65. Carlisle R, Lanphier EH, Rahn H. Hyperbaric oxygen and persistence of vision in retinal ischemia. J Appl Physiol. 1964; 19: 914–918 [DOI] [PubMed] [Google Scholar]
- 66. Oshima T, Karasawa F, Satoh T. Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol Scand. 2002; 46: 831–835 [DOI] [PubMed] [Google Scholar]
- 67. Nagaoka T, Sakamoto T, Mori F, Sato E, Yoshida A. The effect of nitric oxide on retinal blood flow during hypoxia in cats. Invest Ophthalmol Vis Sci. 2002; 43: 3037–3044 [PubMed] [Google Scholar]
- 68. Sponsel WE, DePaul KL, Zetlan SR. Retinal hemodynamic effects of carbon dioxide, hyperoxia, and mild hypoxia. Invest Ophthalmol Vis Sci. 1992; 33: 1864–1869 [PubMed] [Google Scholar]
- 69. Hickam JB, Sieker HO, Frayser R. Studies of retinal circulation and A-V oxygen difference in man. Trans Am Clin Climatol Assoc. 1959; 71: 34–44 [PMC free article] [PubMed] [Google Scholar]
- 70. Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J Gen Physiol. 1992; 99: 177–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoffman WE, Albrecht RF, Miletich DJ. Cerebrovascular response to hypoxia in young vs aged rats. Stroke. 1984; 15: 129–133 [DOI] [PubMed] [Google Scholar]
- 72. Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci. 2006; 47: 1126–1141 [DOI] [PubMed] [Google Scholar]
- 73. Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999; 40: 182–189 [PubMed] [Google Scholar]
- 74. Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci. 1999; 40: 967–975 [PubMed] [Google Scholar]
- 75. Kaneda K, Kashii S, Kurosawa T, et al. Apoptotic DNA fragmentation and upregulation of Bax induced by transient ischemia of the rat retina. Brain Res. 1999; 815: 11–20 [DOI] [PubMed] [Google Scholar]