Abstract
BACKGROUND
A recent clinical study of patients with inappropriate sinus tachycardia reported that autoantibodies to β-adrenergic receptors (β2ARs) could act as agonists to induce atrial arrhythmias.
OBJECTIVE
To test the hypothesis that activating autoantibodies to the β2AR in the rabbit atrium are arrhythmogenic.
METHODS
Five New Zealand white rabbits were immunized with a β2AR second extracellular loop peptide to raise β2AR antibody titers. A catheter-based electrophysiologic study was performed on anesthetized rabbits before and after immunization. Arrhythmia occurrence was determined in response to burst pacing before and after the infusion of acetylcholine in incremental concentrations of 10 μM, 100 μM, and 1 mM at 1 mL/min.
RESULTS
In the preimmune studies when β2AR antibody titers were undetectable, of a total of 20 events, only 3 episodes of nonsustained (<10 seconds) atrial arrhythmias were induced. In the postimmune studies when β2AR antibody titers ranged from 1:160,000 to 1:1.28 million, burst pacing induced 10 episodes of nonsustained or sustained (≥10 seconds) arrhythmias in 20 events (P = .04 vs preimmune; χ2 and Fisher exact test). Taking into account only the sustained arrhythmias, there were 6 episodes in 20 events in the postimmune studies compared with 0 episodes in 20 events in the preimmune studies (P = .02). Immunized rabbits demonstrated immunoglobulin G deposition in the atria, and their sera induced significant activation of β2AR in transfected cells in vitro compared to the preimmune sera.
CONCLUSIONS
Enhanced autoantibody activation of β2AR in the rabbit atrium leads to atrial arrhythmias mainly in the form of sustained atrial tachycardia.
Keywords: β2-Adrenergic receptor, Activating autoantibodies, Atrial tachyarrhythmias, Acetylcholine, Rabbit
Introduction
Cardiac arrhythmias cause 400,000 sudden deaths annually in the United States. There are several different types of arrhythmias and many occur without known cause. Very recently, a novel pathophysiological concept has emerged connecting the presence of activating autoantibodies to cardiac arrhythmias.1 Contrary to the general understanding of the nature of antibodies, these activating autoantibodies have been shown to have agonist properties.2–4 One report from our laboratory has related the action of activating autoantibodies directed toward the cholinergic and adrenergic receptors to atrial tachyarrhythmias in patients with Graves’ disease. This study found that the majority of these patients have activating autoantibodies to the M2 muscarinic and β1-adrenergic receptors, which were associated with a propensity for atrial fibrillation (AF).5 A recent study also reported that activating autoantibodies to the β1- and β2-adrenergic receptors (β2ARs) are present in a majority of patients with inappropriate sinus tachycardia.6 These studies have engendered a search for similar moieties in other clinical conditions, including cardiac arrhythmias, that might involve autonomic dysfunction.
The present study is based on the hypothesis that selective autoantibody activation of β2AR in the rabbit atrium leads to a variety or specific types of cardiac tachyarrhythmias. We tested this hypothesis in a series of young rabbits.
Methods
This study protocol was approved by the Institutional Animal Care and Use Committee of the Oklahoma City Veterans Affairs Medical Center and Oklahoma University Health Sciences Center and conforms to international standards for animal safety and comfort.
Catheter electrophysiologic study
Five New Zealand white rabbits (3–4 months old) were anesthetized with ketamine/xylazine (35 mg/5 mg/kg) and subjected to a catheter-based electrophysiologic study. Standard electrocardiograms (leads 1-aVF) were continuously monitored. After shaving the neck area and application of betadine antiseptic, the right jugular vein was dissected and cannulated with a 4-F multielectrode catheter. Under electrographic control, the catheter was passed into the right atrium to record atrial potentials in conjunction with the electrocardiogram leads. Atrial tachyarrhythmia susceptibility was tested by bursts of stimuli at a high frequency (20 Hz) and voltages that were at least twice the diastolic pacing threshold. Burst pacing was delivered at least 3 times or up to 10 times before and after the infusion of acetylcholine (Ach) in 3 incremental concentrations (10 μM, 100 μM, and 1 mM) at a rate of 1 mL/min. Sustained (≥10 seconds) arrhythmia occurrence was determined in response to burst pacing at 2× diastolic threshold, at baseline, and then with each of the 3 concentrations of Ach infusion for 2 minutes before initiating at least 3 or more burst pacing intervals. When this study was completed, the wound was closed and antibiotic treatment was instituted. A second electrophysio-logic study was performed after the intervening 6-week immunization interval.
Definition of arrhythmias in the rabbit heart
Sinus tachycardia
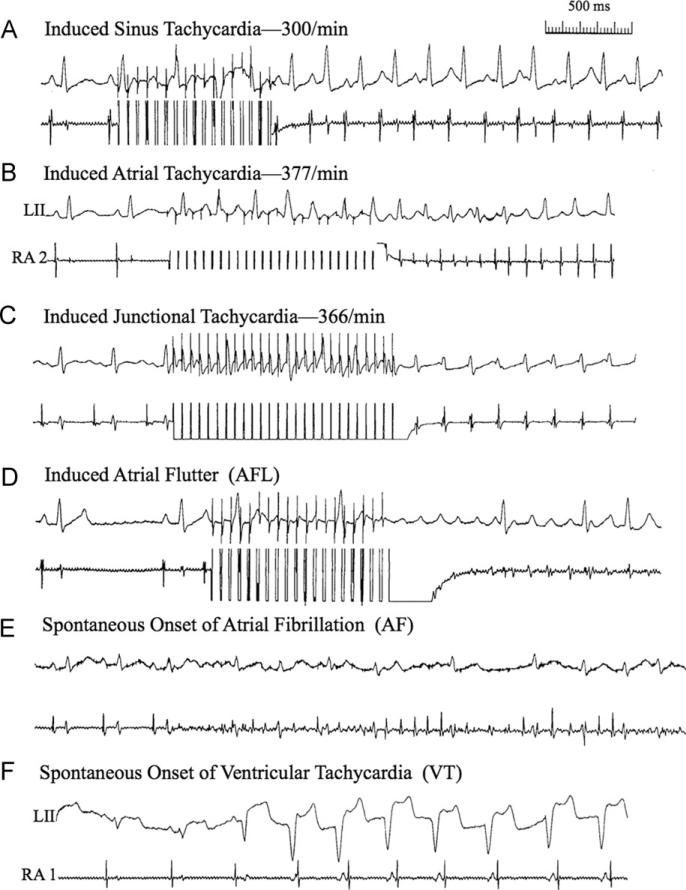
A regular, rapid heart rate ≥250 per minute showing 1:1 atrioventricular (AV) conduction arising from the sinus node as indicated by an unchanged P-wave morphology compared to the baseline state (Figure 1A).
Figure 1.
Examples are shown of the various cardiac arrhythmias that were seen as nonsustained and sustained forms in the present study. See section “Definition of arrhythmias in the rabbit heart” for further descriptions of panels A–F.
Atrial tachycardia
A regular, rapid heart rate ≥250 per minute showing 1:1 or 2:1 AV conduction with P-wave morphology different from the sinus P wave (Figure 1B).
Junctional tachycardia
A regular, rapid heart rate ≥250 per minute showing 1:1 AV conduction in which case the atrial and ventricular potentials are occurring simultaneously or almost at the same time (Figure 1C).
Atrial flutter
A regular, very rapid atrial rate ≥600 per minute with high-grade AV block (Figure 1D).
Atrial fibrillation
Rapid, irregular, fractionated atrial electrograms with a rapid but irregular ventricular response (Figure 1E).
Ventricular tachycardia
Three or more beats arising from the ventricles at a rate ≥200 per minute (Figure 1F).
Nonsustained arrhythmia
Any arrhythmia lasting <10 seconds.
Sustained arrhythmia
Any arrhythmia lasting ≥10 seconds.
Immunization
The 5 rabbits were immunized with 1 mg of the second extracellular loop (ECL2) peptide for β2AR (HWYRATHQEAINCYANETCCDFFTNQ)7 in 0.5 mL of complete Freund's adjuvant. They were boosted with the same peptide plus incomplete Freund's adjuvant (1 mg/0.5 mL) at 2 and 4 weeks. Pre- and postimmune sera were obtained from all animals for enzyme-linked immunosorbent assay (ELISA) and activity assays of the expected antibodies generated during immunization.
ELISA
Antibodies produced in the sera were detected by ELISA, as described previously.7 Briefly, microtiter plates were coated with β2AR ECL2 peptide at 10 μg/mL in coating buffer. To determine antibody titer, sera were diluted 1:10,000 in 1% bovine serum albumin in phosphate buffered saline and thereafter diluted 2-fold. Goat anti-rabbit immunoglobulin G (IgG) conjugated with alkaline phosphatase and its substrate para-nitrophenylphosphate 104 were used to detect antibody binding. Titers were determined as the highest dilution with an optical density value of 0.10 at 60 minutes.
Immunostaining of atrial tissue
Immunostaining was performed on paraffin-embedded atrial tissue sections, as described previously.8 Briefly, the sections were incubated with biotin-conjugated goat anti-rabbit IgG (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 minutes, followed by incubation with alkaline phosphatase-conjugated streptavidin (1 μg/mL; Jackson ImmunoResearch Laboratories) for 30 minutes at room temperature. IgG autoantibody binding was detected with Fast Red substrate (BioGenex, Fremont, CA) against a counterstain of Mayer's hematoxylin.
Cyclic adenosine monophosphate assay
Sera were tested for the activation of β2AR by using the cyclic adenosine monophosphate (cAMP) HuntereXpress GPCR Kit (DiscoveRx, Fremont, CA), as described previously.7 Briefly, 30,000 β2AR-transfected Chinese hamster ovary cells were dispensed into each well of 96-well culture plate and incubated overnight. The medium was removed, and assay buffer containing the cAMP antibody and sera in the presence and absence of the β2 selective blocker ICI-118551 were sequentially added and incubated for 30 minutes. Preincubation of sera with a 10-fold excess of β2AR ECL2 peptide was also tested for neutralization studies. cAMP standard, negative (buffer), and positive (isoproterenol 100 nM) controls were included in each assay. Samples were tested in triplicate. Following sample reatment, the detection reagent was added and the luminescence signal quantitated (Victor 3V, PerkinElmer) to determine cAMP levels.
Statistical analysis
Data are expressed as mean ± SD. χ2 analysis (2 × 2 contingency table) followed by a 2-tailed Fisher exact test was used to determine the difference in occurrence of atrial arrhythmias, either nonsustained or sustained for each rabbit at baseline and at all concentrations of Ach infusion. Differences in cAMP production were assessed by a paired or unpaired Student t test as appropriate. A Bonferroni correction was applied to adjust for multiple comparisons. A P value of <.05 was considered statistically significant.
Results
Studies before peptide immunization
In Table 1, during the preimmune studies, only nonsustained (<10 seconds) arrhythmias could be induced by burst pacing either at baseline or at any infused concentration of Ach. If no arrhythmia could be elicited, no response was registered. Two episodes of nonsustained AF and 1 episode of nonsustained atrial tachycardia (AT) were transiently induced during the 20 burst pacing events consisting of the baseline state and 3 incremental concentrations of infused Ach.
Table 1.
Rabbit response to acetylcholine and burst pacing—preimmune and postimmune studies
| Rabbit # | Acetylcholine | Preimmune | Postimmune | Anti-β2AR titer |
|---|---|---|---|---|
| 19 | 0 | N/R | N/R | 1:160,000 |
| 10 μM | N/R | NS/AT | ||
| 100 μM | N/R | NS/JT | ||
| 1 mM | N/R | NS/AT/AF | ||
| 20 | 0 | N/R | N/R | 1:1.28 million |
| 10 μM | N/R | N/R | ||
| 100 μM | N/R | sus AT | ||
| 1 mM | NS/AF | sus AF | ||
| 21 | 0 | N/R | N/R | 1:160,000 |
| 10 μM | N/R | N/R | ||
| 100 μM | N/R | N/R | ||
| 1 mM | N/R | sus AT | ||
| 22 | 0 | N/R | N/R | 1:1.28 million |
| 10 μM | N/R | N/R | ||
| 100 μM | N/R | sus AT | ||
| 1 mM | NS/AT | sus AT | ||
| 23 | 0 | N/R | N/R | 1:1.28 million |
| 10 μM | N/R | N/R | ||
| 100 μM | N/R | sus AT | ||
| 1 mM | NS/AT | NS/VPC |
AF = atrial fibrillation; AT = atrial tachycardia; β2AR = β2-adrenergic receptor; JT = junctional tachycardia; N/R = no response; NS = nonsustained; sus = sustained; VPC = ventricular premature contraction.
Studies after peptide immunization to produce β2AR antibodies
Table 1 shows the arrhythmias induced during the Ach infusion protocol in response to burst pacing in the right atrium. Examples of the various cardiac arrhythmias including nonsustained and sustained forms are shown in Figure 1. Of the total 20 events in the postimmune studies, there were 10 episodes of nonsustained or sustained (≥10 seconds) arrhythmias induced compared to 3 of 20 in the preimmune state (P = .04; χ2 and Fisher exact test). Taking into account only the sustained arrhythmias, there were 6 episodes (mainly AT) in 20 events in the postimmune studies compared with 0 episodes in 20 events in the preimmune studies (P = .02). β2AR antibody titers ranged from 1:160,000 to 1:1.28 million in postimmune studies and were undetectable in the preimmune studies.
β2AR antibody production and activity
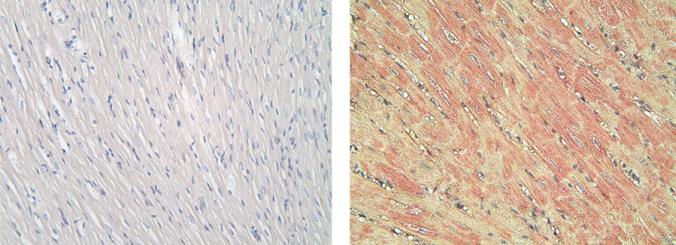
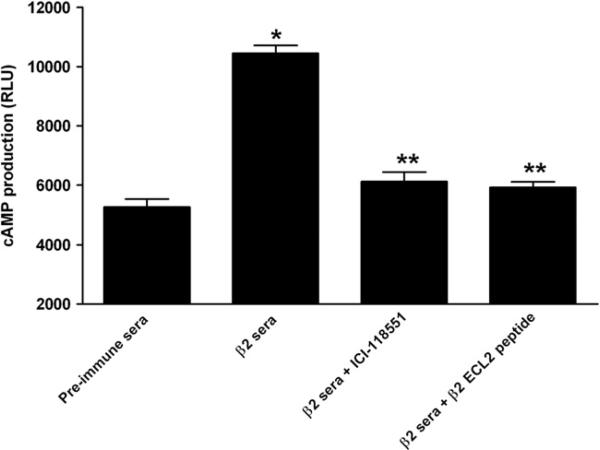
All 5 rabbits developed high antibody titers to β2AR ranging from 1:160,000 to 1:1.28 million after peptide immunization. IgG deposition was observed in the atrial myocytes of immunized rabbits (Figure 2). Preimmune rabbits did not demonstrate any deposition of IgG. Rabbit antisera were able to activate β2AR production of cAMP in β2AR-transfected Chinese hamster ovary cells in vitro (Figure 3). Sera-induced β2AR activation was abolished by the β2 selective blocker ICI-118551 or by preincubation with β2AR ECL2 peptide. The β1 selective antagonist CGP-20712A failed to block any activity of the rabbit sera (data not shown).
Figure 2.
In vivo immunoglobulin G (IgG) deposition in the rabbit atria. Rabbits immunized with β2-adrenergic receptor peptide demonstrated IgG deposition in the atrial myocytes (right), while no atrial tissue-bound IgG was detected in the preimmune rabbits (left) (20× magnification).
Figure 3.
Rabbit sera-induced cyclic adenosine monophosphate (cAMP) production in Chinese hamster ovary cells transfected with β2-adrenergic receptor (β2AR). Rabbit anti-β2AR sera significantly increased cAMP production (*β < .01 vs preimmune sera; n = 3), while β2 selective blocker ICI-118551 and preincubation with the second extracellular loop (ECL2) peptide for β2AR both effectively blocked the sera-induced β2AR activation (**P < .01, n = 3). RLU = relative luminescence unit.
Discussion
Cardiac arrhythmias, including tachyarrhythmias, are associated with significant morbidity and mortality. Numerous pathophysiological conditions are potentially involved in arrhythmogenesis.9 In a recent review, Lazzerini et al10 suggested that cardiac arrhythmias, many of which have been classified as “idiopathic” (ie, of unknown origin), may have their basis in an immune disorder. Indeed, patients with autoimmune diseases commonly manifest abnormal electrocardiographic abnormalities.11 Circulating autoantibodies targeting the cardiac autonomic nervous system are frequently observed in several pathological conditions characterized by rhythm disturbances, including idiopathic dilated cardiomyopathy,12–14 Chagas’ disease,15–17 myocarditis,18,19 and primary electrical cardiac abnormalities.6,20 These autoantibodies exert agonist-like activity in vitro and primarily target the ECL2s of their respective receptors. A high prevalence of sympathomimetic anti-βAR autoantibodies has been documented and associated with primary ventricular arrhythmias20 and inappropriate sinus tachycardia,6 and a high incidence of ventricular tachycardia and sudden death has been reported in dilated cardiomyopathy.21 Parasympathomimetic anti-M2R autoantibodies have been reported to be associated with both bradyarrhythmias and tachyarrhythmias, such as idiopathic sinus node dysfunction22 and AF.23
In the present study, we were able to use each rabbit as its own control. The potential arrhythmogenic response of young rabbits to an intravenous infusion of an increasing concentration of Ach combined with burst pacing could be compared in the preimmune studies and after immunization, which greatly increased the activation of the β2AR. Importantly, rabbit anti-β2AR sera demonstrated significant activation of β2AR in transfected cells in vitro and could be effectively blocked by the β2 selective antagonist ICI-118551. In the preimmune studies, there was no instance of an induced sustained arrhythmia. In the postimmune studies, AT was observed in 5 of 6 sustained arrhythmias induced by burst pacing (Figure 1B and Table 1). A review of the literature on adrenergic receptor density in the rabbit indicated that β2AR density occurs predominantly in the peripheral atria whereas β1AR density is greater in the sinus node.24 Therefore, it would appear that there is a correspondence between the marked expression of β2AR activity and inducibility of sustained AT in the rabbit.
Clinical implications
The density of β receptors has been shown to vary in different areas of the atrium. For example, β1 receptor density in mammalian atria is significantly higher in the sinoatrial node than in the working atrial myocardium. β2 receptor density is higher in atrial myocardial cells than in the specialized tissues.25,26 In rabbits, this situation is reversed.24,27 The present study and those cited above1–6 support the concept that some cardiac arrhythmias may have an immunological component based on the presence of 1 or more activating autoantibodies that can serve as agonists to the autonomic cardiac regulatory system. This would be more likely in regard to those arrhythmias that are designated as idiopathic, that is, of unknown origin.
Limitations
It would seem counterintuitive that the infusion of Ach, a parasympathetic neurotransmitter, with burst pacing would provoke arrhythmias associated with adrenergic receptor agonist activity. Previous studies in ambulatory dogs with a propensity for inducing either paroxysmal AF or AT was based on the simultaneous interaction of the neural activity of both arms of the autonomic nervous system.28 Immunohistochemical studies of cardiac ganglia have shown the colocalization of cholinergic and adrenergic neurotransmitters.29 Our future studies will test the provocative actions of both cholinergic and adrenergic activating autoantibodies on inducing a variety of cardiac arrhythmias in the rabbit heart. It is important to differentiate the short-term effects of β2AR-activating autoantibodies and their arrhythmogenic effects from those of humans with endogenous immune disorders. Longer exposure to b agonists may produce a cardiomyopathy and resultant adaptation/recruitment of additional factors that might alter the propensity to arrhythmias. The present study was intended to provide a prototypical model to examine the specific role of the β2AR in the absence of β1AR activation and/or other receptors that may be activated in more complex circumstances present in chronic human autoimmune diseases.
Conclusions
In a series of young rabbits before and after peptide immunization that induced high titers of activating auto-antibodies to β2AR, burst pacing in combination with Ach infusions regularly induced sustained AT. These findings are consistent with previous reports of a significantly higher density of β2AR in the atria than in the sinus node in the rabbit heart.
Acknowledgments
This study was supported in part by grants from the Heart Rhythm Institute at the University of Oklahoma Health Sciences Center, the Helen and Will Webster Arrhythmia Research Fund of the University of Oklahoma Foundation (to B.J.S. and D.C.K.), National Heart, Lung, and Blood Institute (R01 HL56267; to M.W.C. and D.C.K.), American Heart Association Postdoctoral Fellowship (to H.L.), and VA Merit Review (to D.C.K. and X.Y.).
ABBREVIATIONS
- Ach
acetylcholine
- AF
atrial fibrillation
- AT
atrial tachycardia
- AV
atrioventricular
- β2AR
β2-adrenergic receptor
- ECL2
second extracellular loop
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
References
- 1.Lee HC, Huang KT, Wang XL, Shen WK. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011;8:1788–1795. doi: 10.1016/j.hrthm.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dragun D, Philippe A, Catar R, Hegner B. Autoimmune mediated G-protein receptor activation in cardiovascular and renal pathologies. Thromb Haemost. 2009;101:643–648. [PubMed] [Google Scholar]
- 3.Herda LR, Felix SB, Boege F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br J Pharmacol. 2012;166:847–857. doi: 10.1111/j.1476-5381.2012.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: preeclampsia and beyond. Expert Rev Clin Immunol. 2011;7:659–674. doi: 10.1586/eci.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stavrakis S, Yu X, Patterson E, et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–1316. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiale PA, Garro HA, Schmidberg J, et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006;3:1182–1186. doi: 10.1016/j.hrthm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Kem DC, Reim S, et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59:402–408. doi: 10.1161/HYPERTENSIONAHA.111.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 9.Shah M, Akar FG, Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005;112:2517–2529. doi: 10.1161/CIRCULATIONAHA.104.494476. [DOI] [PubMed] [Google Scholar]
- 10.Lazzerini PE, Capecchi PL, Guideri F, et al. Autoantibody-mediated cardiac arrhythmias: mechanisms and clinical implications. Basic Res Cardiol. 2008;103:1–11. doi: 10.1007/s00395-007-0686-8. [DOI] [PubMed] [Google Scholar]
- 11.Lazzerini PE, Capecchi PL, Guideri F, Acampa M, Galeazzi M, Laghi Pasini F. Connective tissue diseases and cardiac rhythm disorders: an overview. Autoimmun Rev. 2006;5:306–313. doi: 10.1016/j.autrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–1968. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–2767. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 14.Wallukat G, Nissen E, Morwinski R, Muller J. Autoantibodies against the beta-and muscarinic receptors in cardiomyopathy. Herz. 2000;25:261–266. doi: 10.1007/s000590050017. [DOI] [PubMed] [Google Scholar]
- 15.Borda ES, Sterin-Borda L. Antiadrenergic and muscarinic receptor antibodies in Chagas’ cardiomyopathy. Int J Cardiol. 1996;54:149–156. doi: 10.1016/0167-5273(96)02592-2. [DOI] [PubMed] [Google Scholar]
- 16.Labovsky V, Smulski CR, Gomez K, Levy G, Levin MJ. Anti-beta1-adrenergic receptor autoantibodies in patients with chronic Chagas heart disease. Clin Exp Immunol. 2007;148:440–449. doi: 10.1111/j.1365-2249.2007.03381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum MB, Chiale PA, Schejtman D, Levin M, Elizari MV. Antibodies to beta-adrenergic receptors disclosing agonist-like properties in idiopathic dilated cardiomyopathy and Chagas’ heart disease. J Cardiovasc Electrophysiol. 1994;5:367–375. doi: 10.1111/j.1540-8167.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 18.Caforio AL, Daliento L, Angelini A, et al. Autoimmune myocarditis and dilated cardiomyopathy: focus on cardiac autoantibodies. Lupus. 2005;14:652–655. doi: 10.1191/0961203305lu2193oa. [DOI] [PubMed] [Google Scholar]
- 19.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoanti-bodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 20.Chiale PA, Rosenbaum MB, Elizari MV, et al. High prevalence of antibodies against beta 1- and beta 2-adrenoceptors in patients with primary electrical cardiac abnormalities. J Am Coll Cardiol. 1995;26:864–869. doi: 10.1016/0735-1097(95)00262-2. [DOI] [PubMed] [Google Scholar]
- 21.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–424. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 22.Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 23.Baba A, Yoshikawa T, Fukuda Y, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–1115. doi: 10.1016/j.ehj.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Tenner TE, Jr., Young JA, Earley KJ, Yen YC. Functional characterization of beta-adrenoceptor subtypes in rabbit right atria. Life Sci. 1989;44:651–660. doi: 10.1016/0024-3205(89)90469-4. [DOI] [PubMed] [Google Scholar]
- 25.Beau SL, Hand DE, Schuessler RB, et al. Relative densities of muscarinic cholinergic and beta-adrenergic receptors in the canine sinoatrial node and their relation to sites of pacemaker activity. Circ Res. 1995;77:957–963. doi: 10.1161/01.res.77.5.957. [DOI] [PubMed] [Google Scholar]
- 26.Rodefeld MD, Beau SL, Schuessler RB, Boineau JP, Saffitz JE. Beta-adrenergic and muscarinic cholinergic receptor densities in the human sinoatrial node: identification of a high beta 2-adrenergic receptor density. J Cardiovasc Electrophysiol. 1996;7:1039–1049. doi: 10.1111/j.1540-8167.1996.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 27.Molenaar P, Summers RJ. Characterization of beta-1 and beta-2 adrenoceptors in guinea pig atrium: functional and receptor binding studies. J Pharmacol Exp Ther. 1987;241:1041–1047. [PubMed] [Google Scholar]
- 28.Shen MJ, Choi EK, Tan AY, et al. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]