Abstract
Objectives
Delirium is common after cardiac surgery, although underrecognized, and its long-term consequences are likely underestimated. The primary goal of this study was to determine whether patients with delirium after coronary artery bypass graft (CABG) surgery have higher long-term out-of-hospital mortality when compared to CABG patients without delirium.
Methods
We studied 5,034 consecutive patients undergoing CABG surgery at a single institution from 1997–2007. Presence or absence of neurologic complications, including delirium, was assessed prospectively. Survival analysis was performed to determine the role of delirium in the hazard of death, including a propensity score to adjust for potential confounders. These analyses were repeated to determine the association between postoperative stroke and long-term mortality.
Results
Individuals with delirium had an increased hazard of death (adjusted HR 1.65, 95% CI 1.38, 1.97), up to 10 years postoperatively, after adjustment for perioperative and vascular risk factors. Patients with postoperative stroke had a HR of 2.34 (95% CI 1.87–2.92). The effect of delirium on subsequent mortality was the strongest among those without a prior stroke (HR 1.83 vs HR 1.11 (with a prior stroke) (p-interaction=0.02) or who were younger (HR 2.42 (under 65 yo) vs HR 1.49 (65 yo and older) (p-interaction=0.04).
Interpretation
Delirium after cardiac surgery is a strong independent predictor of mortality up to 10 years postoperatively, especially in younger individuals and in those without prior stroke. Future studies are needed to determine the impact of delirium prevention and/or treatment in long-term patient mortality.
Delirium, defined as an acute confusional state with fluctuating impairment in attention and cognition,1 occurs in 15–60% of hospitalized patients, with even higher numbers among those in the ICU.2 It incurs tremendous health care costs, with an estimated national burden of $38 billion to $152 billion per year.3 It has been estimated that between 2% and 57% of patients undergoing cardiac surgery develop delirium, depending on the type of surgery, and the assessment and definition of delirium.4–6
Delirium after cardiac surgery has been reported for years. In one review from the 1970’s, this “postcardiotomy delirium” was felt to be attributable to long periods of cardiopulmonary bypass, arterial hypotension during surgery, emboli, and low postoperative cardiac output, in addition to sensory overload or deprivation in the recovery room or ICU.7 Delirium may be attributed to the long list of medical problems and the resultant long list of medications used to treat postoperative pain, agitation, arrhythmia, and infection. The presence of delirium after CABG can be predicted in individuals who are older, more frequently male, and have preexisting cerebral disease, particularly dementia.4, 6, 8
Compared with other surgical types, coronary artery bypass graft (CABG) and vascular surgery are associated with twice as high rates of postoperative delirium.8 Nonetheless, despite the frequency of delirium in this postoperative setting, the consequence of a transient episode of postoperative delirium on a patient’s long-term outcomes has not been well studied.4, 6, 8 It is likely that its impact on both in-hospital and long-term outcomes may be underestimated. In this study, we sought to determine the role of postoperative delirium in the risk of late postoperative mortality, among a cohort of over 5000 individuals who had CABG surgery at a single institution over a 10-year interval. We hypothesized that mortality would be higher in individuals with an episode of postoperative delirium.
METHODS
Description of cohort
The study was approved by the Johns Hopkins Institutional Review Board. All patients who underwent isolated first time or redo CABG surgery at Johns Hopkins Hospital, from January 1997 through October 2007 were included in the Cardiac Surgery database. On a daily basis, surgical nurses identified possible cases of delirium. Each day, the research staff (MAG or MMB, for the entire duration of the study) queried the charge nurse, for patients in the ICU, or the daily progress sheets, prepared by nurse practitioners (for patients once transferred to the surgical floor), for presence of any neurologic problems. Every surgical patient was thus reviewed on each day of his/her hospitalization. If at any point during the hospitalization, or on a discharge summary, a neurologic problem was identified (stroke, or “neurologic injury”, defined as confusion, agitation, change in mental status, seizure, coma, or slowness to awaken after surgery), this information was communicated to the research staff who reviewed the description and entered this information into the research database. Each individual received a binary yes/no rating for each neurologic injury category, reflecting the presence of these injuries at any point during the postoperative period. The charts and records of each individual with a neurologic injury (in these categories) were reviewed for use of the word “delirium”, and the majority of cases were discussed in person at the time of the event with nursing staff, for clarification of the type of neurologic injury. Medical and demographic information was collected prospectively, at the time of surgery.
The diagnoses of delirium and stroke were mutually exclusive. Thus, a patient who was diagnosed with stroke could not also be classified with delirium, and stroke took precedence over delirium if both were present. Patients who died on the day of or the day immediately after surgery (thus not having a chance for evaluation) were excluded from analysis. Individuals with more than one CABG surgical procedure at Hopkins were each counted only once, at the time of their earlier procedure. The diagnoses of delirium and coma were also mutually exclusive, but other types of neurologic injury could occur in combination with delirium.
Definition of outcome and censoring of individuals
The primary outcome in this study was all-cause mortality. Deaths were recorded in the Cardiac Surgery database. In addition, the National Death Index was searched for all individuals, both to confirm the internal records and to record any additional dates of death through February 1, 2009. Individuals without a known date of death were censored as alive on the date they were last seen at a Johns Hopkins facility. Duration in the study was measured in years, with time contributed calculated from one day preoperatively to the date of death or censoring.
Statistical Analysis
Stata version 8.0 for Macintosh was used for statistical analyses.9 A Kaplan-Meier graph was calculated, both unadjusted and including adjustment for age (centered at 65 years of age), for those with and without delirium. The null hypothesis of no difference in survival functions was tested using the log-rank test. In addition, K-M curves were calculated for the subsets with and without postoperative stroke. All graphs were truncated at 10 years of follow-up. Incidence rates of death, based on total person-years, were calculated for those with and without delirium, and were compared.
To compare the rate of mortality for persons with and without delirium who were otherwise similar with respect to covariates, a propensity score analysis10 was performed. We determined the contribution of multiple medical, demographic, and social factors in the development of delirium. Factors were selected for propensity score analysis based on prior studies or clinical suspicion that these factors might be confounders in the relationship between delirium and death. The propensity scores were estimated using logistic regression. A non-linear age effect was modeled as a cubic spline. The propensity score model was checked for balance, for model fit (with a Hosmer-Lemeshow statistic), and for the ability to differentiate between delirious and not delirious individuals (with an ROC analysis).
The propensity score (between 0 and 1) was stratified into quintiles and entered as a covariate in a Cox proportional hazards model of mortality, in addition to delirium and development of postoperative stroke. Separate models included interaction terms, and also were repeated, but stratified by age and history of prior stroke. The proportional hazards assumption was checked for all models. As a sensitivity analysis, the model was rechecked among those who survived at least one week postoperatively.
RESULTS
A total of 5121 individuals were included in the cohort, all of whom had an isolated CABG procedure. Exclusions included 39 patients who died early in their hospitalization and/or had no neurological injury determined. Of the 437 patients in the cohort population who were identified as having a neurological injury, 48 patients could not be subclassified as having delirium (versus another neurologic injury). These individuals were also excluded from analyses on delirium. A final cohort of 5,034 individuals was used for analyses of delirium, contributing a total of 17,861 person-years to the survival analyses. The 4,748 individuals without delirium contributed 16,931 person-years total, and the 304 individuals with delirium contributed 930 person-years. Of the total cohort, 6% had delirium. Baseline characteristics of individuals with and without delirium are displayed in Table 1.
Table 1.
Baseline characteristics.
| Delirious | Not delirious | p-value* | |
|---|---|---|---|
| Diabetes | 38.0% | 32.3% | 0.04 |
| Redo CABG | 4.9% | 4.4% | 0.69 |
| Sex (% Male) | 69.5% | 73.1% | 0.17 |
| History of MI | 62.0% | 56.8% | 0.08 |
| Hypertension | 79.0% | 73.0% | 0.02 |
| History of smoking | 68.2% | 65.2% | 0.28 |
| History of CHF | 20.3% | 8.7% | <0.001 |
| History of Atrial fibrillation | 12.5% | 5.7% | <0.001 |
| Prior stroke | 17.1% | 8.2% | <0.001 |
| Prior PTCA | 16.1% | 18.8% | 0.13 |
| Prior statin use | 52.8% | 54.7% | 0.51 |
| Carotid bruits | 14.1% | 10.0% | 0.02 |
| Race (% Black) | 13.4% | 11.8% | 0.36 |
| Age, yr † | 70.4 | 63.5 | <0.0001 |
| Mean Cardiopulmonary bypass time (for those with On-pump CABG) | 108.1 min | 103.0 min | 0.008 |
| Off-pump CABG | 11.2% | 6.0% | <0.001 |
| Emergency surgery | 6.9% | 3.4% | 0.001 |
| Mean year of procedure | 2002 | 2001 | <0.0001 |
These variables comprised the factors entered into the propensity score model.
P-values calculated by Chi-square test (categorical variables) and t-test (continuous variables).
Mean ages provided; used as cubic spline with 2 knots for propensity score.
CABG=coronary artery bypass graft; MI=myocardial infarction; CHF=congestive heart failure; PTCA=percutaneous transluminal coronary angioplasty.
Delirium was present in 9% of individuals over 65 years old and in 3% of those less than 65 years old (p<0.001). Individuals without delirium were followed for 3.6 years, on average (range, 0.005–11.7 years), and those with delirium were followed for 3.0 years (0.005–10.8 years) (p<0.0001). The median survival time for individuals with delirium was 10.6 years; for individuals without delirium, it was beyond the range of the follow-up period (>10 years). Postoperative stroke patients had a 5.8 year median survival time.
The overall mortality rate was 7.4 per 100 person-years. The death rate among those with delirium was 16.0 per 100 person-years, and for those without delirium, 7.0 per 100 person-years (p<0.0001). Postoperative length-of-stay was longer for individuals with delirium (15.3 days) than those without delirium (7.3 days, p<0.0001).
Creation of propensity score
Table 1 shows the patient characteristics and historical factors used to create the propensity score. The model fit the data well and the score was balanced. An ROC analysis yielded an area-under-the-curve of 0.75 (95% CI 0.72, 0.78), for prediction of delirium vs no delirium.
Survival analysis
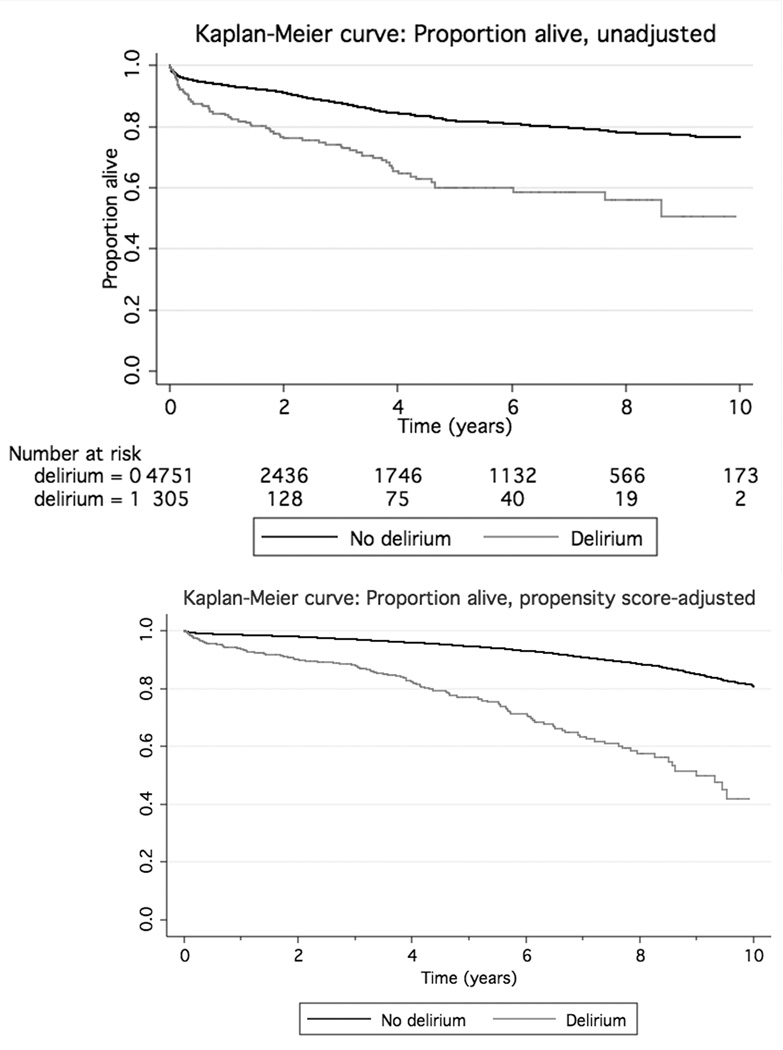
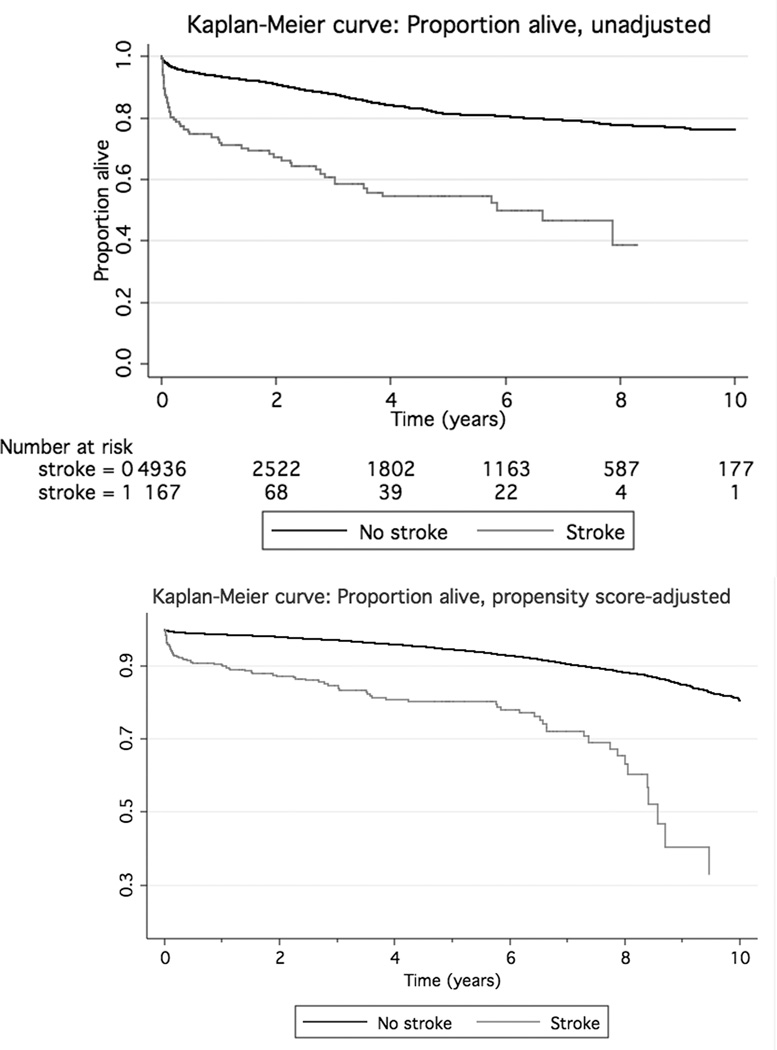
Unadjusted and age-adjusted Kaplan-Meier curves were created for those with and without delirium. Figure 1 shows these curves. The relationship between presence/absence of new postoperative stroke and long-term mortality is shown in Figure 2. Each of the four graphs had a significant (p<0.0001) log-rank test for comparing each pair of curves.
Figure 1.
Kaplan-Meier curve for individuals without (solid upper line) and with (dashed, lower line) postoperative delirium. The unadjusted graph (upper) is displayed, with numbers at risk, as well as the same graph adjusted for age (lower; centered at median age of 65 years old, median cardiopulmonary bypass time, and for male without risk factors). The log-rank p<0.0001 for the differences between each pair of curves.
Figure 2.
Kaplan-Meier curves for individuals without (solid upper line) and with (dashed, lower line) postoperative stroke. The unadjusted graph (upper) is displayed, as well as the same graph adjusted for propensity score (centered at age 65, median cardiopulmonary bypass time, and for male without risk factors). The log-rank p<0.0001 for comparison between the pair of curves.
In a Cox proportional hazards analysis, presence of delirium predicted death (adjusted HR 1.65, 95% CI 1.38, 1.97 (Table 2)). The proportional hazards assumptions were not violated. The hazard ratios differed substantially by the presence or absence of each of these factors (older age and past stroke) (Table 3); the p-value for interaction for age and delirium was 0.05, and for prior stroke and delirium was 0.03. The effect of delirium was less in the presence of either older age or prior stroke, and completely null in the presence of both of these together. The combination of younger age and no prior stroke further increased the HR.
Table 2.
Hazard ratios (HR) in multivariate model predicting death.
| HR | 95% CI | |
|---|---|---|
| Delirium | 1.65 | 1.38–1.97 |
| Propensity scorea | 1.58 | 1.51–1.65 |
| Postoperative stroke | 2.34 | 1.87–2.92 |
Per quintile increase in propensity score.
HR=hazard ratio; CI=confidence interval.
Table 3.
Adjusted hazard ratios (HR) for presence of delirium, dependent on status of prior stroke history and age group.
| HR | 95% CI | |
|---|---|---|
| With prior stroke | 1.11 | 0.74–1.68 |
| Without prior stroke | 1.83 | 1.49–2.23 |
| 65 yo and older | 1.49 | 1.21–1.82 |
| Under 65 yo | 2.42 | 1.65–3.56 |
| Age<65 and no prior stroke | 2.57 | 1.67–3.93 |
| Age>65 and prior stroke | 0.98 | 0.61–1.56 |
When the primary analyses were repeated for the subset of individuals who survived the first week after surgery and had complete data on delirium status and for propensity scoring (N=4,677), relationships were even stronger between delirium and long-term mortality (HR 1.74, 95% CI 1.45, 2.09). The interaction terms remained similar, with higher HR in younger individuals and individuals without prior stroke.
Results for stroke patients
The HR for a new postoperative stroke in the multivariate model was 2.34 (table 2). This shows that the effect size is similar for postoperative stroke in prediction of subsequent death as it is for delirium. This relationship persists in the sensitivity analysis of those who survived beyond one week postoperatively (HR 2.40 for stroke (95% CI 1.91, 3.00)). Although the interaction terms were not significant between new postoperative stroke and either age or prior stroke history, the HR’s associated with a new postoperative stroke were higher in stratified models for younger individuals (adjusted HR 3.11, 95% CI 1.96–4.95) than older individuals (adjusted HR 2.18, 95% CI 1.69–2.80). For individuals with a prior stroke, however, the additional injury of a new postoperative stroke was associated with a slightly higher hazard of death (adjusted HR 2.61, 95% CI 1.60–4.25) than for individuals without a prior stroke (adjusted HR 2.26, 95% CI 1.76–2.90), in contrast to the findings for delirium.
INTERPRETATION
In this single-center cohort, individuals with delirium after CABG surgery had a higher hazard of death, continuing over 10 years after surgery, compared with those without delirium. This association persisted after adjustment for many covariates (including age, multiple vascular risk factors, and cardiopulmonary bypass duration), which might confound the relationship between delirium and death.
The relationship between delirium and death in our study was modified based on age and prior stroke history of the individual. Individuals who were either older at the time of surgery or who had a history of prior stroke had less additional effect from delirium in prediction of death. For both groups, mortality was already increased in the absence of delirium, and delirium was only marginally associated with a further increase in mortality.
The reasons for these interactions are unclear. It is likely that the mechanism of the association between delirium and death differs within the populations. In older patients, or in patients with a prior stroke, a relatively minor event (such as a wound infection, the use of a centrally-acting medication, or a drug interaction) is more likely to trigger delirium. Thus, an episode of delirium may be less likely to further increase in mortality in these individuals. Similarly, delirium might be assigned more quickly to these individuals, who may be perceived to be at higher risk for delirium. In younger individuals, however, or others without a clear predisposition to delirium, it is more likely that multiple brain stresses may be required to induce delirium, such as the combination of general anesthesia, psychoactive medications,1 cerebral embolization or hypoperfusion. Detailed information about these and other intraoperative and postoperative complications would help clarify the circumstances surrounding the development of delirium. The addition of neuroimaging information might also help elucidate the observed difference in younger versus older individuals. Finally, dementia is a very important and well-recognized risk factor for subsequent delirium,11 and could be associated both with delirium and with subsequent mortality, independent of increased age; information on cognitive status was unfortunately not available for this study. It is likely that some patients in our population would have had some impairment in cognitive performance,12 given the probable role of vascular disease in development of dementing illnesses.
The association between delirium and an increased hazard of death may reflect a more substantial intraoperative injury (if this is the cause of the delirium) or worse pre-existing cerebrovascular disease, both among those who experience delirium and among those who die. The associations were still present, however, after adjustment for intraoperative and preoperative factors, but there still could be some unmeasured confounders in this association (primarily, preoperative cognitive dysfunction). Thus, if delirium is a marker of an injured brain (either preoperatively or intraoperatively), prevention of delirium, or more importantly, the management of those factors that may have contributed to the delirium, would be the most reasonable way to decrease mortality. Successful reduction in delirium has occurred with the use of a proactive geriatrics consultation before hip surgery (RR 0.64 for delirium),13 the use of postoperative risperidone in CABG patients,14 and the use of intensive screening of sleep patterns, visual and hearing impairment, immobility, cognitive impairment, and hydration status (OR 0.60).15 Whether prevention of this type or treatment of delirium, once it has occurred, is associated with reductions in long-term increases in mortality is less clear. Prevention and control of factors that lead to subsequent delirium (such as embolization or hypoperfusion during surgery, as well as appropriate selection of postoperative medications) is a more likely means by which the long-term consequences of delirium could be minimized. An alternative but less likely possibility is that delirium itself may alter long-term brain functions. If the latter is correct, the frequency and consequences of delirium make it a potential target for an intervention that could reduce these short and long-term negative outcomes.
The primary limitations of this study were: 1) the lack of a standardized assessment of delirium, and, 2) the lack of baseline cognitive assessment. Delirium was reported by the nursing staff, without standard definitions or without a standardized testing battery like the Confusion Assessment Method (CAM).20 There might be bias in the ascertainment of delirium, particularly in individuals who are older or had preexisting cerebrovascular disease. Relying on nurses for identification of delirium has been previously identified as having a low sensitivity and high specificity,21 which would indicate that we may have underdiagnosed delirium, which would be consistent with the higher rates reported in other studies. In addition, it could be hypothesized that the more severe cases that were most likely to be identified. Thus, the associations we found might be most important in individuals with relatively severe delirium. Similarly, by requiring the diagnoses of delirium and stroke to be mutually exclusive, we may have missed an important population with delirium, further leading to an underestimation of the frequency of delirium. We are also limited by the monocentric nature of the study, as our institution has relatively high acuity patients and might bias towards a sicker population, or a population more likely to become delirious or to have earlier mortality. In addition, the lack of baseline cognitive assessment makes it impossible to assess for potential confounding by underlying cognitive dysfunction. Because this is a primary risk factor for both delirium and mortality, this could be an unmeasured confounder in our observed associations.
Despite these limitations, the strengths of our study include the large sample size, the longitudinal information on participants, and the standardized measurement of basic demographic and vascular risk factors, recorded prospectively. Delirium outcome data were collected prospectively by the same research team, which interfaced with the surgical nursing staff on a daily basis. The techniques and staff used were consistent for the duration of the study. Because each patient was discussed on a daily basis with nursing staff, this might provide an advantage over a one-time CAM measurement in its likelihood of detecting an episode of delirium. Because of the large sample size, the estimates in this study were fairly precise, and allowed for study of interactions.
Our study provides a longer follow-up period than most preceding studies on delirium and mortality. In other smaller studies using standardized delirium assessment in nonsurgical patients, delirium has been associated with increased 12-month mortality.17, 18 Among surgical patients, results are inconsistent; in one study, delirium has not been shown to even increase in-hospital mortality at all.19 In our study, the strongest associations were in the first few years, but, even in the subgroup surviving beyond 3 years, mortality was still significantly higher (by log-rank test) in individuals with postoperative delirium.
Delirium in the post-CABG setting appears to increase hazard for late mortality, even after adjustment for a number of covariates, including prior medical problems, gender, age, and other potential confounders. The relationship is modified among those who are older and those with prior stroke, with less effect from delirium in these two groups, despite delirium being more prevalent in each of these groups. These results emphasize the importance of delirium in the perioperative setting, as it is often ignored and overlooked by clinicians, particularly in combination with other comorbidities that are common in postoperative patients. Delirium should also be taken seriously in the postoperative setting as it may be a marker for poor long-term prognosis, and future studies should continue to search for causes of delirium in the intraoperative and postoperative setting. The strengths of the associations found in this study emphasize the importance of future studies in preventing delirium and managing factors that might contribute to delirium, with the goal of decreasing mortality.
Acknowledgements
This study was supported by the Johns Hopkins Clinician Scientist Award (RG) and RO1-NS035610 (GM) and the Dana Foundation (GM).
Footnotes
The authors have no financial disclosures.
Contributor Information
R.F. Gottesman, Email: rgottesm@jhmi.edu.
M.A. Grega, Email: mgrega@jhmi.edu.
M.M. Bailey, Email: mmbailey@jhsph.edu.
L.D. Pham, Email: lpham@jhsph.edu.
S.L. Zeger, Email: szeger@jhsph.edu.
W.A. Baumgartner, Email: wbaumgar@jhmi.edu.
O.A. Selnes, Email: oselnes@jhmi.edu.
G.M. McKhann, Email: guy.mckhann@jhu.edu.
References
- 1.Inouye SK. Delirium in older persons. N. Engl. J. Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: An underrecognized syndrome of organ dysfunction. Seminars in Respiratory and Critical Care Medicine. 2001;22:115–126. doi: 10.1055/s-2001-13826. [DOI] [PubMed] [Google Scholar]
- 3.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Archives of Internal Medicine. 2008;168:27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan MC, Felde A, Kuskowski M, et al. Incidence and predictors of postcardiotomy delirium. Am. J. Geriatr. Psychiatry. 2008;16:575–583. doi: 10.1097/JGP.0b013e318172b418. [DOI] [PubMed] [Google Scholar]
- 5.van der Mast RC, Roest FHJ. Delirium after cardiac surgery: A critical review. J. Psychosom. Res. 1996;41:13–30. doi: 10.1016/0022-3999(96)00005-0. [DOI] [PubMed] [Google Scholar]
- 6.Loponen P, Luther M, Wistbacka JO, et al. Postoperative delirium and health related quality of life after coronary artery bypass grafting. Scand. Cardiovasc. J. 2008;42:337–344. doi: 10.1080/14017430801939217. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez E, Chitwood WR., Jr Postcardiotomy delirium: an overview. International Journal of Psychiatry Medicine. 1975;6:373–383. doi: 10.2190/03FJ-9QJE-NNBN-B0WC. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph JL, Jones RN, Rasmussen LS, et al. Independent vascular and cognitive risk factors for postoperative delirium. Am. J. Med. 2007;120:807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Stata Corp. Stata Statistical Software: Release 10.1. College Station, TX: Stata Corporation; 2008. [Google Scholar]
- 10.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 11.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Annals of Surgery. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 12.Selnes OA, Grega MA, Bailey MM, et al. Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: A controlled study. Ann. Thorac. Surg. 2007;84:1885–1896. doi: 10.1016/j.athoracsur.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: A randomized trial. J. Am. Geriatr. Soc. 2003;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 14.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth. Intensive Care. 2007;35:714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J. Am. Geriatr. Soc. 2005;53:462–466. doi: 10.1111/j.1532-5415.2005.53165.x. [DOI] [PubMed] [Google Scholar]
- 17.McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Archives of Internal Medicine. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 18.Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent delirium predicts greater mortality. J. Am. Geriatr. Soc. 2009;57:55–61. doi: 10.1111/j.1532-5415.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yildizeli B, Ozyurtkan O, Batirel HF, et al. Factors associated with postoperative delirium after thoracic surgery. Ann. Thorac. Surg. 2005;79:1004–1009. doi: 10.1016/j.athoracsur.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Foreman MD, Mion LC, et al. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Archives of Internal Medicine. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]