Abstract
To improve outcomes among injection drug users with HIV and/or chronic hepatitis B, it is important to identify drug interactions between antiretroviral and opiate therapies. We report the results of a study designed to examine the interaction between buprenorphine and the nucleos(t)ide reverse transcriptase inhibitors (NRTI) didanosine (ddI), lamivudine (3TC), and tenofovir (TDF). Opioid-dependent, buprenorphine/naloxone-maintained, HIV-negative volunteers (n = 27) participated in two 24-hour sessions to determine (1) pharmacokinetics of buprenorphine alone and (2) pharmacokinetics of both buprenorphine and either ddI, 3TC, or TDF. Among buprenorphine/naloxone-maintained study participants, no significant changes in buprenorphine pharmacokinetics were observed following ddI, 3TC, or TDF administration. Buprenorphine had no significant effect on NRTI concentrations. Concomitant use of buprenorphine with ddI, 3TC, or TDF results in neither a significant pharmacokinetic nor pharmacodynamic interaction.
INTRODUCTION
By the end of 2007, there were approximately 33 million people worldwide living with human immunodeficiency virus (HIV) and an estimated 2.7 million become newly infected annually.1 About 4 million people with HIV are also living with hepatitis B (HBV) infection.2 Among those co-infected with HIV and HBV an estimated 5–10% of these individuals are injection drug users (IDUs).2,3 Because adherence to medical regimens among IDUs is frequently poor,4–6 effective treatment for HIV and/or chronic HBV disease among this population requires successful treatment for substance abuse with a possible opioid therapy. Opioid therapy can prevent the onset of withdrawal symptoms and craving that often lead to opioid-dependent persons spending a significant amount of time participating in activities to gain access to opioids. The lives of opioid-dependent individuals not being treated for their substance dependence can fluctuate daily and decrease their likelihood of adhering to complex therapies for HIV and/or HBV infections. In fact, failure to treat opioid dependence has been associated with poor HIV treatment outcomes,4,7 while chronic hepatitis B co-infection may accelerate HIV progression.8
Important to the success of treating HIV and chronic HBV is establishing steady-state drug concentrations necessary to inhibit viral replication. Likewise, appropriate opioid concentrations are necessary for treating opioid dependence. Drug interactions can lead to subtherapeutic antiretroviral or opioid concentrations that may lead to treatment failure. Conversely, supratherapeutic concentrations of either therapy may cause unwanted side effects leading to treatment discontinuation and more seriously, fatal adverse events.9 For example, methadone, the traditional opioid therapy of choice for opioid dependence, when co-administered with a number of antiretroviral therapeutics has been associated with clinically significant drug interactions related to induction or inhibition of cytochrome P450 (CYP) enzymes involved in methadone metabolism, including CYP 3A4 and 2B6.9–11 The antiretrovirals lopinavir, nevirapine, and efavirenz significantly increase methadone clearance and opiate withdrawal symptoms12–14 while delavirdine decreases methadone clearance, therefore creating a potential risk for opioid toxicity.15 Additionally, methadone reduces bioavailability and the measured areas under the time-concentration curve (AUC) for didanosine (63%) and stavudine (25%).16
Buprenorphine, a mu-opioid receptor partial agonist, has demonstrated efficacy in the treatment of opioid-dependent patients.17 Buprenorphine is primarily converted to an active metabolite, norbuprenorphine, via CYP 3A4 and 2C8.18 Buprenorphine and its metabolite norbuprenorphine are further metabolized by glucuronidation, reducing the potential of competing with other drugs in the CYP system and therefore reducing the likelihood of clinically significant drug interactions when compared to methadone.19 Unique also to buprenorphine is the ceiling effect seen at higher concentrations.20 When the clearance of buprenorphine is obstructed, higher concentrations do not appear to produce typical opioid toxicity-related adverse events such as respiratory depression.20
The use of nucleos(t)ide reverse transcriptase inhibitors (NRTI) remain the backbone of many initial highly active antiretroviral therapy (HAART) regimens for the treatment of HIV.21 Didanosine (ddI) is an older agent that remains a recommended alternative component in a dual-NRTI HAART regimen.22 The bioavailability of ddI is decreased by methadone, possibly leading to subtherapeutic concentrations, although the current enteric-coated tablet formulation is less affected than the earlier buffered tablet formulation.23 Lamivudine (3TC) and tenofovir (TDF) are preferred initial components in a dual-NRTI HAART regimen and have also become important for the treatment of chronic HBV.24–26 3TC and TDF are FDA-approved treatments for chronic HBV treatment and, given its high genetic barrier, TDF is thought to be one of the most effective treatments for chronic HBV.24 Guidelines from the Department of Health and Human Services (DHHS) recommend that all patients who have HIV and chronic HBV co-infection receive two active HBV drugs when both HIV and HBV viruses are advanced enough to require treatment.23,27 The DHHS guidelines specifically cite TDF and 3TC as the preferred agents.
The goals of the current study included the following: (1) to determine whether the pharmacokinetics of the opioid dependence medication, buprenorphine, administered as sublingual buprenorphine/naloxone, are affected by co-administration of the NRTI medications, ddI, 3TC, or TDF; (2) to determine whether the pharmacokinetics of these NRTIs are affected by co-administration of buprenorphine; and (3) to determine whether clinically significant pharmacodynamic effects or toxicities occur when buprenorphine is administered simultaneously with these NRTIs.
METHODS
Procedures
Forty-seven individuals participated in this study that was reviewed and approved by the Institutional Review Board at Virginia Commonwealth University (VCU) in Richmond, VA. Participants received treatment for opioid dependence with buprenorphine/naloxone in the Addiction Psychiatry Treatment Research Program at VCU and provided voluntary, written, informed consent for study participation. Study samples were based on a power analysis showing that seven opioid-dependent individuals were needed for within-subjects analyses aimed at determining the effect of NRTIs on buprenorphine, while 10 individuals per group (buprenorphine-maintained and controls) were needed to determine the effect of buprenorphine on NRTI medications (see section “Statistical Analysis”). Twenty-seven opioid-dependent individuals stable for at least 2 weeks on a daily dose of sublingual 4:1 buprenorphine/naloxone (14–16 mg buprenorphine) participated in a 24-hour blood sampling study to determine buprenorphine pharmacokinetics. This was followed by oral administration of either ddI 400 mg daily (enteric-coated formulation) for 5 days, 3TC 300 mg daily for 5 days, or TDF 300 mg daily for 5 days and a second 24-hour pharmacokinetic study in which blood sampling for buprenorphine and antiretroviral (ARV) plasma concentrations was obtained. In addition, 20 age-, weight-, race-, and gender-matched control volunteers participated in the ddI and TDF studies in which they received ddI 400 mg daily for 5 days or TDF 300 mg daily for 5 days followed by a 24-hour blood sampling study to determine ddI and TDF pharmacokinetics. Each dose of study medication was administered by clinical staff in the research program to assure adherence to the study protocol. A control sample was not obtained for the 3TC study because a previous methadone trial revealed no effect on 3TC pharmacokinetics28 and therefore it seemed unlikely that buprenorphine would have an effect on 3TC pharmacokinetics. As an alternative, data on AUC, maximum concentration in plasma (Cmax), time of Cmax (Tmax), and clearance (CL/F) available in published literature29 were used to estimate the effect of buprenorphine on 3TC pharmacokinetics.
Study procedures included standardized and validated measures of opioid withdrawal by clinician rating (Objective Opioid Withdrawal Scale [OOWS], scores ≥ 3 indicate moderate withdrawal symptoms)30 and cognitive impairment using the Mini-Mental State Examination31 for opioid-dependent participants (maximum score = 30; scores of <27 indicate cognitive impairment). Adverse symptoms were recorded for all participants using an Adverse Symptoms Checklist that queried for a wide range of adverse experiences including changes in energy, gastrointestinal symptoms, central nervous system effects, genitourinary symptoms, and other somatic complaints scored for severity on an ordinal scale (0–3, with 0 = not present, 1 = mild, 2 = moderate, and 3 = severe, maximum possible, score = 87). These ratings were administered at baseline, following stabilization on buprenorphine (prior to ARV administration), and at the completion of the ARV dosing period; for control subjects prior to and at the completion of antiretroviral administration.
Analytical Assays
Buprenorphine Assay
Plasma buprenorphine and metabolite concentrations were determined using a recently described liquid chromatography-tandem mass spectrometry (LC-MS/MS) method32 except that buprenorphine-d4 and norbuprenorphine-d3 were used as the internal standards for their respective glucuronides.
Didanosine Assay
Didanosine plasma concentrations were determined using reversed phase high-performance liquid chromatography (HPLC), using a modification as previously described.33
Lamivudine Assay
Lamivudine plasma concentrations were determined using a recently developed HPLC method.34
Tenofovir Assay
Tenofovir plasma concentrations were measured using a published LC-MS/MS method modified and applied in our laboratory.35 Internal standard (lamivudine) was added to 0.3 ml of sample prior to sample preparation.
Pharmacokinetics Analysis
The pharmacokinetic parameters of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, norbuprenorphine-3-glucuronide, ddI, 3TC, and TDF were evaluated as appropriate for each subject. Buprenorphine pharmacokinetics were calculated following sublingual administration of buprenorphine/naloxone alone and again following administration of either ddI, 3TC, or TDF. In the control group, ddI and TDF pharmacokinetic parameters were evaluated after administration as described above. AUC, minimum plasma concentration (Cmin), Cmax, Tmax, and sublingual (buprenorphine) or oral (NRTIs) clearance (CL/F) were determined using the noncompartmental analysis module of WinNonLin Professional Version 5.2 (Pharsight Corp., Mountain View, CA). For metabolites, CL/F was calculated based on the administered dose of parent compound. The F term represents the fraction of parent drug ultimately converted to the metabolite. Cmax, Cmin, and Tmax were estimated by inspection of the raw data. For purposes of non-compartmental analysis, drug concentrations that were less than the limit of quantitation were expressed as one-half of the detection limit. All pharmacokinetic parameters were summarized and displayed by treatment period.
Statistical Analysis
Previous experience with drug interaction studies examining methadone in combination with antiretroviral medications indicated that the between-subject coefficient of variation (CV) for the AUC would be approximately 30%. It was also assumed that a 40% change or greater in AUC would be of clinical importance as we observed in our study of lopinavir/ritonavir and methadone and our study of zidovudine and methadone.36 A sample size of 10 was needed to detect a 40% change in ARV drug AUC or oral clearance between control and buprenorphine-treated subjects with a power of 0.8. The studies of NRTI effects on buprenorphine, on the other hand, were within-subject. Since within-subject coefficients of variation are smaller, a sample size of seven was adequate to detect a difference of 40% in AUC between buprenorphine alone and in combination with an ARV agent.37 Student's paired t-test was used to test the significance of the differences in pharmacokinetic parameters for buprenorphine (within-subject analyses). The Wilcoxon test was used for the within-subject comparison of the nonparametric values of Tmax. Differences in pharmacokinetic parameters for NRTIs in the control versus the buprenorphine-treated group (between-groups comparisons) were obtained by the Kruskal–Wallis test or the Mann–Whitney test (for Tmax). A difference was considered statistically significant if the p value was ≤ .05 (two-tailed). Comparisons of subject characteristics were made by single factor analysis of variance (ANOVA).
RESULTS
Study Participants
A total of 47 individuals participated in this study. Twenty-seven opioid-dependent participants receiving a stable, daily, sublingual dose of 4:1 buprenorphine/naloxone (buprenorphine range: 14–16 mg) who were otherwise physically healthy and without other current mental illness completed the study. Studies of ddI and TDF included control arms of 10 participants each who were matched for age, race, gender, and weight to opioid-dependent participants. Table 1 shows demographic and medical characteristics of participants, according to study group. There were no significant differences in age, race, weight, or gender. Opioid-dependent subjects received a stable dose of buprenorphine/naloxone for at least 2 weeks prior to study entry. Based on clinical assessment, most participants stabilized on a dose of buprenorphine/naloxone 16/4 mg daily, with stabilization defined as lack of opiate withdrawal symptoms and cessation of opiate craving and use (as determined by urine toxicology screen). Few adverse events occurred during the study period, and no medical intervention was required to address adverse events in any participant over the course of the study. Concomitant medication use in this sample was rare. The abuse of substances other than opioids was a common occurrence both in the buprenorphine/naloxone and control groups with cocaine abuse most prevalent, followed by cannabis abuse and alcohol abuse (no participants met diagnostic criteria for alcohol dependence). Cigarette smoking was common, but daily use reported by all subjects was less than one pack per day (range: 0.3–0.7). Among opioid-dependent participants, 20–29% were IDUs. Serological evidence for hepatitis C infection was found in 10–14% of the opioid-dependent participants.
TABLE 1.
Participant demographics and characteristics
| Lamivudine Bup/Nlx*N = 7 | Didanosine Bup/Nlx N = 10 | Didanosine control N = 10 | Tenofovir Bup/Nlx N = 10 | Tenofovir control N = 10 | |
|---|---|---|---|---|---|
| Age (years) | 39 (2.6)†* | 35 (2.5) | 41 (3.0) | 35 (2.4) | 37 (3.0) |
| Weight (kg) | 101.9 (4.9) | 93 (9.0) | 84.4 (4.8) | 100.1 (7.1) | 94.6 (7.4) |
| Buprenorphine (mg/day) | 14.0 (1.0) | 16 | N/A | 16 | N/A |
| Female | 2 [28.5%] | 3 [30%] | 4 [40%] | 2 [20%] | 6 [60%] |
| Race | |||||
| African-American | 7 | 9 | 7 | 10 | 8 |
| Caucasian | — | 1 | 3 | — | 2 |
| Hispanic | — | — | — | — | |
| Mixed | — | — | — | — | |
| Substance use disorders: | |||||
| Opioid dependence | 5 | 9 | 0 | 7 | — |
| Cocaine abuse | 1 | 3 | 4 | 3 | 2 |
| Cannabis abuse | — | 1 | 1 | 1 | — |
| Alcohol abuse | — | — | 2 | — | 1 |
| Injection drug use | 2 [28.5%] | 2 [20%] | 0 | 0 | 0 |
| Nicotine use | |||||
| Packs/day | 0.3 (0.1) | 0.7 (0.2) | 0.6 (0.2) | 0.4 (0.1) | 0.2 (0.1) |
| Hepatitis C positive | 1 [14%] | 1 [10%] | 0 | 1 [10%] | 0 |
| AST pre/post | 25.4 (4.8)/ | 20.1 (1.9)/ | 22.3 (1.9)/ | 23.5 (3.7)/ | 27.8 (2.4)/ |
| Normal range: 0-35 U/L | 25.1 (5.2) | 10.7 (2.3) | 18.6 (2.1) | 26.5 (4.2)‡ | 21.6 (1.8)§ |
| ALT pre/post | 23.3 (4.4)/ | 19.4 (3.1)/ | 21.7 (3.2)/ | 22.5 (4.7)∥ | 30.7 (4.3)/ |
| Normal range: 0-35 U/L | 24.6 (5.3) | 18.6 (2.9) | 20.0 (4.2) | 24.9 (4.9) | 23.1 (2.7) |
| Adverse symptoms | 2.0 (1.1)/ | 7.4 (2.7)/ | 1.5 (1.2)/ | 2.4 (0.9)/ | 1.9 (1.1)/ |
| Pre/post | 2.6 (1.2) | 5.1 (2.2) | 0.6 (0.4) | 3.2 (1.0) | 1.8 (1.3) |
Buprenorphine/naloxone maintenance treatment.
mean (SE).
[ ] percent of sample affected.
p = .057.
p = .005.
p = .054.
Interaction between Buprenorphine and Nucleos(t)ide Reverse Transcriptase Inhibitors
1. Effect of didanosine on buprenorphine
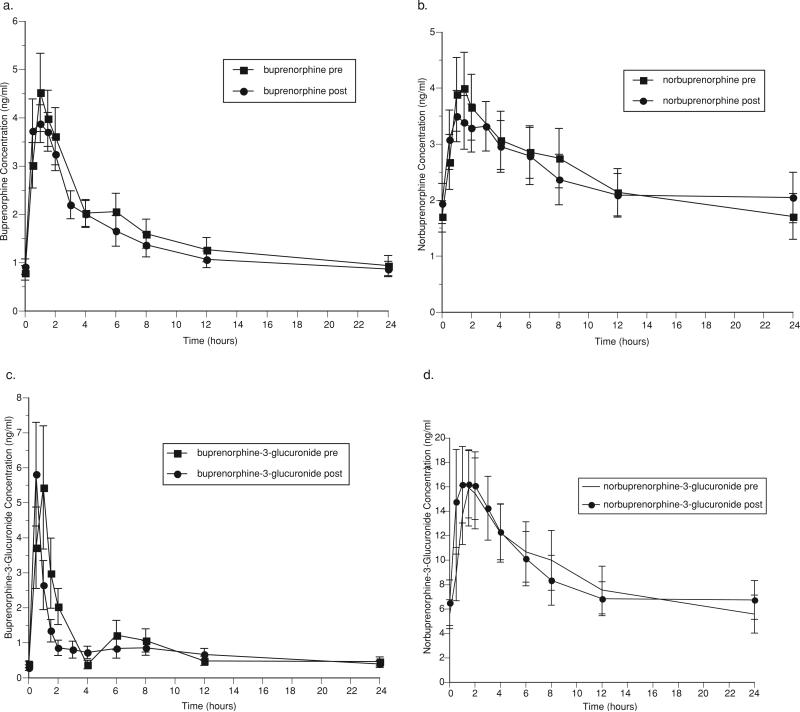
Pharmacokinetic parameters for buprenorphine and metabolites before and after ddI administration are shown in Table 2. Figure 1 graphically represents the mean concentrations of buprenorphine (1a), the active metabolite, norbuprenorphine (1b), and the inactive glucuronides of buprenorphine (1c) and norbuprenorphine (1d) over a 24-hour dosing interval. The co-administration of ddI with buprenorphine had essentially no significant effects on the pharmacokinetics of buprenorphine and metabolites. A decrease in Tmax for buprenorphine-3-glucuronide following ddI administration was statistically significant, but clinically inconsequential (pre-ddI: 2 h, post-ddI: 1 h (p = .02)) (Table 2).
TABLE 2.
Effect of didanosine on buprenorphine and buprenorphine metabolites in plasma
| Pharmacokinetic parameter | Pre-didanosine | Post-didanosine | p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0–24 (ng h/ml) | 39.2 (6.6) | 35.2 (4.8) | 0.20 |
| Cl/F (L/h) | 507 (72) | 538 (73) | 0.50 |
| Cmax (ng/ml) | 4.89 (0.73) | 4.70 (0.45) | 0.76 |
| Tmax (h) | 1.0 (0.5–1.5) | 1.0 (0.5–1.5) | NS |
| Cmin (ng/ml) | 0.94 (0.21) | 0.87 (0.16) | 0.65 |
| Norbuprenorphine | |||
| AUC0–24 (ng h/ml) | 57.7 (10.9) | 57.2 (10.1) | 0.94 |
| Cl/F (L/h) | 343 (46) | 383 (74) | 0.34 |
| Cmax (ng/ml) | 4.18 (0.65) | 3.82 (0.45) | 0.41 |
| Tmax (h) | 1.5 (0.5–4) | 1.0 (0.5–6) | NS |
| Cmin (ng/ml) | 1.71 (0.41) | 2.05 (0.45) | 0.40 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24 (ng h/ml) | 21.8 (6.0) | 19.5 (4.1) | 0.54 |
| Cl/F (L/h) | 1414 (379) | 1471 (388) | 0.48 |
| Cmax (ng/ml) | 5.94 (1.67) | 6.25 (1.39) | 0.85 |
| Tmax (h) | 0.5 (0.5–1.0) | 0.5 (0.5–1.5) | NS |
| Cmin (ng/ml) | 0.47 (0.13) | 0.40 (0.10) | 0.56 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24 (ng h/ml) | 210 (48) | 210 (43) | 0.98 |
| Cl/F (L/h) | 107 (17) | 111 (22) | 0.76 |
| Cmax (ng/ml) | 16.8 (3.0) | 19.8 (3.9) | 0.24 |
| Tmax (h) | 2.0 (1.0–4) | 1.0 (0.5–2) | 0.02 |
| Cmin (ng/ml) | 5.59 (1.56) | 6.74 (1.58) | 0.30 |
FIGURE 1.
Effect of didanosine on (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide, and (d) norbuprenorphine-3-glucuronide.
No subjects showed evidence of opiate withdrawal symptoms (OOWS pre-ddI administration: 0.1 (0.1), post-ddI administration: 0 (0) (n.s.)), nor were cognitive deficits detected (MMSE pre-ddI administration: 28.7 (0.5), post-ddI administration: 29.1 (0.4); total score possible on the MMSE: 30). Administration of ddI had no significant effect on hepatic enzyme activity (AST, ALT). The most frequently reported adverse symptoms in buprenorphine/naloxone-maintained individuals administered ddI were constipation, frequent urination, headache, poor memory, increased appetite, waking during the night or too early, decreased libido, problems with sexual arousal and delayed/absent orgasm, all of which declined with ddI administration, except for constipation and decreased libido. The only symptoms that increased with ddI administration were poor coordination (pre-ddI: 1 participant reported “mild” incoordination; post-ddI: 2 participants reported “mild” poor coordination) and difficulty urinating (pre-ddI: 1 participant “mild” difficulty with urination; post-ddI: 2 participants “mild” difficulty with urination). There were no statistically significant changes in adverse symptoms pre- and post-ddI administration in this sample.
2. Effect of lamivudine on buprenorphine
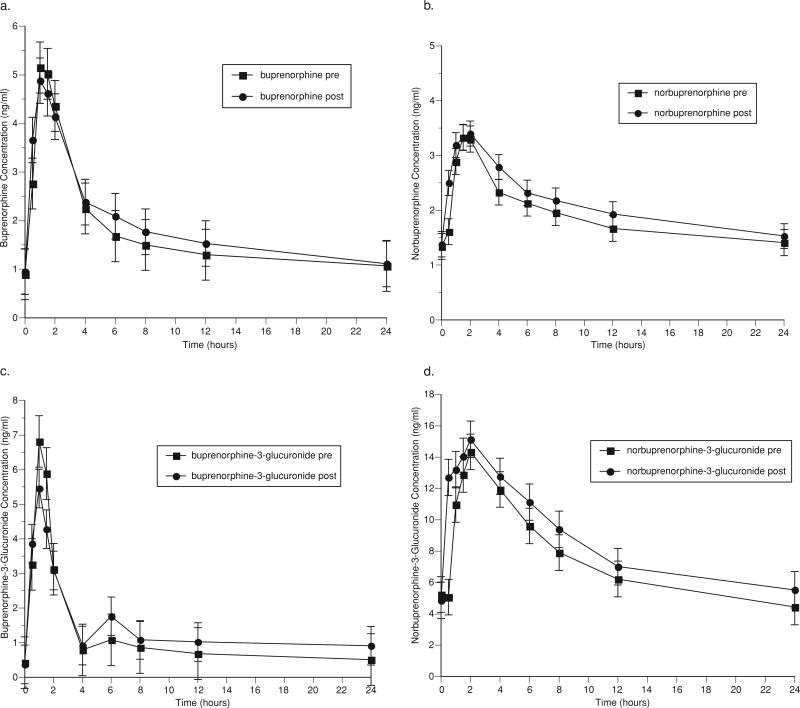
Pharmacokinetic parameters for buprenorphine and metabolites before and after 3TC administration are shown in Table 3. Figure 2 graphically represents the mean concentrations of buprenorphine (2a), the active metabolite, norbuprenorphine (2b), and the inactive glucuronides of buprenorphine (2c) and norbuprenorphine (2d) over a 24-hour (h) dosing interval. The co-administration of 3TC with buprenorphine had few effects on the pharmacokinetics of buprenorphine and its metabolites. The AUC0–24 of buprenorphine, norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide were modestly increased following 3TC administration, but only the increase in norbuprenorphine-3-glucuronide AUC0–24 was statistically significant (pre-3TC: 177 ng h/ml, post-3TC: 205 ng h/ml (p = .02)). Cmax for norbuprenorphine-3-glucuronide was also significantly increased following 3TC administration (pre-3TC: 14.8 ng/ml, post-3TC: 18.8 ng/ml (p = .04)) (Table 3).
TABLE 3.
Effect of lamivudine on buprenorphine and buprenorphine metabolites in plasma
| Pharmacokinetic parameter | Pre-lamivudine | Post-lamivudine | p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0–24 (ng h/ml) | 41.3 (5.5) | 45.2 (6.9) | 0.19 |
| Cl/F (L/h) | 435 (65) | 409 (66) | 0.30 |
| Cmax (ng/ml) | 5.60 (0.90) | 5.72 (0.91) | 0.88 |
| Tmax (h) | 1.0 (1.0–2.0) | 1.0 (0.5–1.5) | NS |
| Cmin (ng/ml) | 1.07 (0.17) | 1.11 (0.22) | 0.67 |
| Norbuprenorphine | |||
| AUC0–24 (ng h/ml) | 45.0 (7.5) | 50.3 (8.2) | 0.13 |
| Cl/F (L/h) | 415 (61) | 371 (57) | 0.13 |
| Cmax (ng/ml) | 3.53 (0.68) | 3.75 (0.59) | 0.93 |
| Tmax (h) | 2.0 (1.5–2.0) | 2.0 (0.5–4.0) | NS |
| Cmin (ng/ml) | 1.41 (0.20) | 1.53 (0.32) | 0.44 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24 (ng h/ml) | 26.8 (9.4) | 33.0 (12.4) | 0.06 |
| Cl/F (L/h) | 1499 (515) | 1371 (515) | 0.13 |
| Cmax (ng/ml) | 7.45 (2.76) | 7.38 (2.27) | 0.59 |
| Tmax (h) | 1.0 (1.0–2.0) | 0.5 (0.5–1.5) | NS |
| Cmin (ng/ml) | 0.51 (0.19) | 0.91 (0.42) | 0.44 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24 (ngh/ml) | 177 (45) | 205 (48) | 0.02 |
| Cl/F (L/h) | 127 (27) | 104 (20) | 0.13 |
| Cmax (ng/ml) | 14.8 (4.5) | 18.8 (4.5) | 0.04 |
| Tmax (h) | 2.0 (1.5–4.0) | 2.0 (0.5–4) | NS |
| Cmin (ng/ml) | 4.4 (0.8) | 5.5 (1.5) | 0.17 |
FIGURE 2.
Effect of lamivudine on (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide, and (d) norbuprenorphine-3-glucuronide.
No subjects displayed evidence of opiate withdrawal symptoms (OOWS pre-3TC administration: 0 (0), post-3TC administration: 0 (0) (n.s.)), nor were cognitive deficits detected (MMSE pre-3TC administration: 29.0 (0.3), post-3TC administration: 29.3 (0.2); total score possible on the MMSE: 30). Administration of 3TC had no significant effect on hepatic enzymes (AST, ALT). The most frequently reported adverse symptoms in buprenorphine/naloxone-maintained individuals administered 3TC were constipation and increased appetite, both of which remained unchanged with 3TC administration. There were no statistically significant changes in adverse symptoms pre- and post-3TC administration in this sample.
3. Effect of tenofovir on buprenorphine
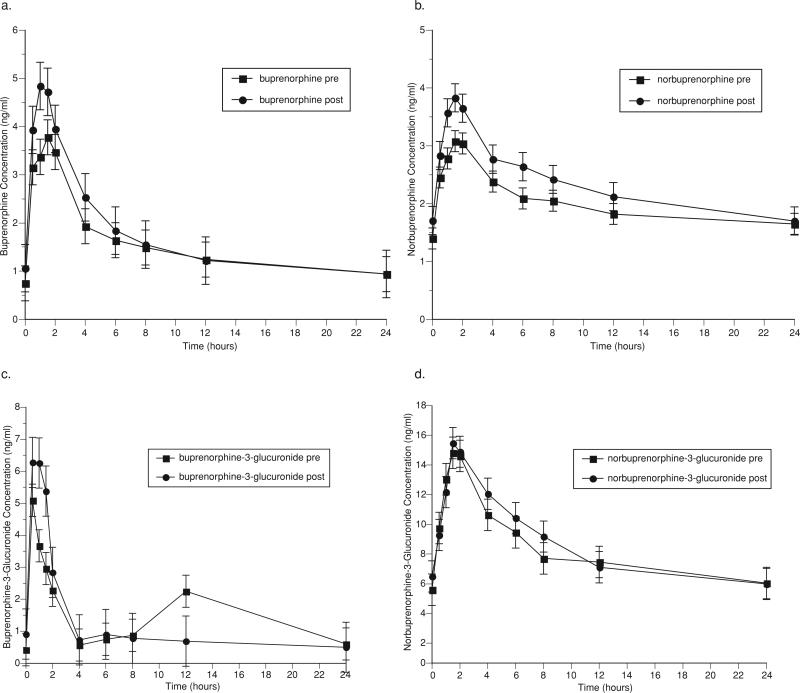
Pharmacokinetic parameters for buprenorphine and metabolites before and after TDF administration are shown in Table 4. Figure 3 graphically represents the mean concentrations of buprenorphine (3a), the active metabolite, norbuprenorphine (3b), and the inactive glucuronides of buprenorphine (3c) and norbuprenorphine (3d) over a 24-hour dosing interval. TDF administration to buprenorphine/naloxone-maintained study participants had no statistically significant effects on the pharmacokinetic parameters of buprenorphine and its metabolites. The clearance of norbuprenorphine was modestly decreased (pre-TDF: 425 L/h, post-TDF: 351 L/h (p = 0.19)) resulting in a slight increase in the AUC0–24 (pre-TDF: 47.9 ng h/ml, post-3TC: 55.3 ng h/ml (p = 0.29)) (Fig. 3b, Table 4). These changes did not reach statistical significance and were not clinically important.
TABLE 4.
Effect of tenofovir on buprenorphine and buprenorphine metabolites in plasma
| Pharmacokinetic parameter | Pre-tenofovir | Post-tenofovir | p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0–24 (ng h/ml) | 36.8 (5.4) | 40.7 (6.2) | 0.42 |
| Cl/F (L/h) | 527 (77) | 467 (61) | 0.26 |
| Cmax (ng/ml) | 4.72 (1.03) | 5.64 (1.18) | 0.21 |
| Tmax (h) | 1.5 (0.5–2) | 1.25 (0.5–4) | NS |
| Cmin (ng/ml) | 0.94 (0.17) | 0.94 (0.17) | 0.99 |
| Norbuprenorphine | |||
| AUC0–24 (ng h/ml) | 47.9 (8.4) | 55.3 (9.4) | 0.29 |
| Cl/F (L/h) | 425 (61) | 351 (45) | 0.19 |
| Cmax (ng/ml) | 3.54 (0.70) | 3.95 (0.68) | 0.41 |
| Tmax (h) | 1.75 (1–6) | 1.5 (1–4) | NS |
| Cmin (ng/ml) | 1.65 (0.32) | 1.70 (0.28) | 0.85 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24 (ng h/ml) | 35.7 (14.0) | 26.7 (5.6) | 0.49 |
| Cl/F (L/h) | 1,106 (311) | 1,100 (339) | 0.96 |
| Cmax (ng/ml) | 7.32 (1.93) | 8.82 (1.74) | 0.29 |
| Tmax (h) | 0.75 (0.5–12) | 0.5 (0.5–1.5) | NS |
| Cmin (ng/ml) | 0.60 (0.23) | 0.50 (0.12) | 0.54 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24 (ng h/ml) | 198 (26) | 204 (37) | 0.83 |
| Cl/F (L/h) | 97 (15) | 93 (11) | 0.82 |
| Cmax (ng/ml) | 17.0 (2.6) | 16.0 (3.2) | 0.68 |
| Tmax (h) | 2.0 (1.5–6) | 1.75 (1–6) | NS |
| Cmin (ng/ml) | 6.04 (0.92) | 5.99 (1.14) | 0.97 |
Note: For pharmacokinetics analyses of the effect of NRTIs on buprenorphine: values are the mean (standard error of the mean) for 10 subjects who participated in both sessions, except that Tmax is given as median (range). Student's paired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
FIGURE 3.
Effect of tenofovir on (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide, and (d) norbuprenorphine-3-glucuronide.
TDF administration had no clinically significant effect on hepatic enzyme activity (AST, ALT) in buprenorphine/naloxone-maintained participants, but there was a trend toward an increase in AST following TDF administration (pre-TDF: 23.5 (3.7), post-TDF: 26.5 (4.2), p = 0.057), again not clinically significant as values remained in the normal range. Control subjects showed a statistically significant, but clinically unimportant, decrease in AST (pre-TDF: 27.8 (2.4), post-TDF: 21.6 (1.8), p = .005) and ALT (pre-TDF: 30.7 (4.3), post-TDF: 23.1 (2.7), p = .054, all values within normal range for these indices of hepatic injury) (Table 1). The most frequently reported adverse symptoms in buprenorphine/naloxone-maintained individuals administered TDF were constipation, increased appetite, problems with sexual arousal, and delayed/absent orgasm. The only symptom that increased with TDF administration was constipation (pre-TDF: 3 participants reported; post-TDF: 6 participants reported). There were no statistically significant changes in adverse symptoms pre- and post-TDF administration in this sample.
4. Effect of buprenorphine on didanosine, lamivudine or tenofovir
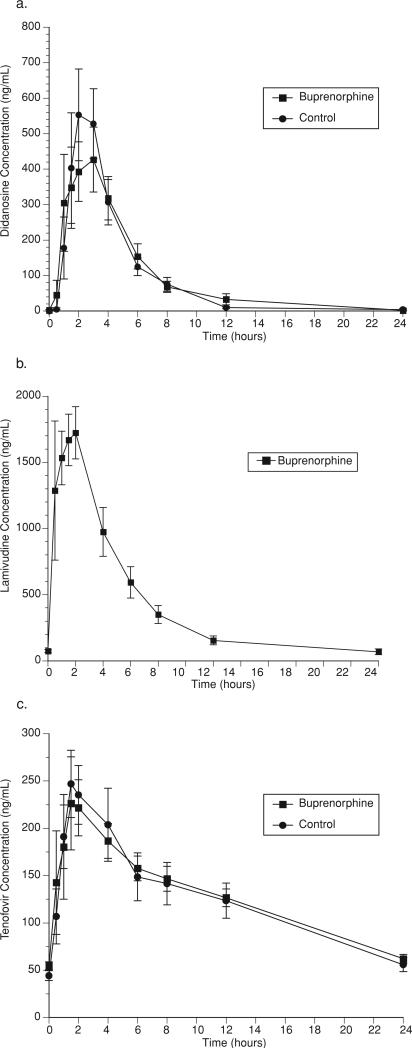
Buprenorphine/naloxone administration had no effect on the disposition of ddI except for a modest decrease in Cmax that was not statistically significant (ddI in buprenorphine-maintained: 612 ng/ml; ddI alone: 719 ng/ml (p = NS)) (Fig. 4a, Table 5). Concentrations of ddI remained in the therapeutic range during buprenorphine/naloxone treatment.
FIGURE 4.
Effect of buprenorphine on (a) didanosine, (b) lamivudine, and (c) tenofovir.
TABLE 5.
Effect of buprenorphine on didanosine in plasma
| Pharmacokinetic parameter | Control | Buprenorphine | p value |
|---|---|---|---|
| AUC0–12 (h ng/ml) | 2,278 (320) | 2,336 (322) | NS |
| Cl/F (L/h) | 298 (126) | 210 (32) | NS |
| Cmax (ng/ml) | 719 (130) | 612 (120) | NS |
| Tmax (h) | 2.5 (1.5–4.0) | 2.5 (1.0–12.0) | NS |
Over a 24-hour dosing interval, 3TC concentrations were measured in buprenorphine/naloxone-maintained individuals (Fig. 4b). 3TC concentrations did not differ significantly from values observed in healthy, nonopioid-dependent, historical controls individuals29 (AUC: (buprenorphine-maintained) 10.4 ± 2.4 h μg/ml, (historical controls): 11.8 (range:10.4–13.3 h μg/ml) (data for historical controls is expressed as range); Cmax: (buprenorphine-maintained) 2.2 ± 0.8 μg/ml, (historical controls) 2.6 (range: 2.1–3.4) μg/ml).
Buprenorphine/naloxone administration had no effect on the disposition of TDF (Fig. 4c, Table 6). Concentrations of TDF remained in the therapeutic range during buprenorphine/naloxone treatment.
TABLE 6.
Effect of buprenorphine on tenofovir in plasma
| Pharmacokinetic parameter | Control | Buprenorphine | p value |
|---|---|---|---|
| AUC0–12 (h ng/ml) | 3,039 (385) | 3,080 (290) | NS |
| Cl/F (L/h) | 119 (21) | 104 (8) | NS |
| Cmax (ng/ml) | 321 (33) | 265 (44) | NS |
| Tmax (h) | 1.7 (0.5–4.0) | 2.0 (0.5–4.0) | NS |
Note: For pharmacokinetics analyses of the effect of buprenorphine on either didanosine or tenofovir: values are the mean (standard error of the mean) for 10 subjects who participated in both sessions, except that the discontinuous variable, Tmax, is given as median (range). Student's unpaired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
DISCUSSION
The findings from this study indicate that the standard doses of the NRTI medications ddI, 3TC, and TDF that are regularly used in clinical care of HIV and/or chronic HBV disease (for 3TC and TDF) may be given to individuals maintained with standard clinical doses of buprenorphine/naloxone without the occurrence of clinically significant drug interactions. No pharmacodynamic effects were observed related to any of the drugs tested in combination.
We should note that the previous study showing a decrease in ddI AUC when taken concomitantly with methadone was performed with an older ddI, immediate-release formulation.16 Today ddI is available as an encapsulated enteric-coated, bead formulation that better avoids gastric degradation. The enteric-coated ddI formulation was used in the current study and has also been shown to achieve therapeutic concentrations in methadone-maintained patients.23
The findings of this study are of added importance because, in addition to their use in the treatment of HIV disease, the NRTIs are routinely prescribed for the treatment of HBV. These two infections often occur concomitantly in the same patient. For opioid-dependent patients who present with one or both of these viral infections, treating their opioid dependence is usually essential for enhancing adherence to antiretroviral medications. In current use in the United States, there are two FDA-approved, currently available opioid therapies for the treatment of opioid dependence, methadone and buprenorphine. Methadone has exhibited several significant interactions with NRTIs, including ddI, stavudine, and zidovudine.16,37
To date, interaction studies with buprenorphine given concomitantly with HIV antiretroviral medications have not demonstrated a decrease in buprenorphine plasma concentration that has produced opiate withdrawal.38,39 Conversely, several studies have demonstrated opioid withdrawal when subjects receiving methadone are concomitantly given antiretroviral medications that increase methadone clearance.13,14,40,41 Untreated or inadequately treated opiate withdrawal symptoms are a primary reason why patients drop out of an opioid treatment program, which may lead to increased illicit drug use and suboptimal HIV treatment that can in turn lead to the development of viral resistance to current HIV medications.4 Therefore, some methadone-maintained patients will require significant increases in methadone dose when receiving HIV medications that may induce methadone metabolism. Consideration of careful taper of methadone doses that may have been increased in some patients concomitantly receiving certain HIV medications known to be associated with opiate withdrawal should be undertaken if the HIV medication is discontinued. A methadone dose significantly higher than that on which the patient had been stabilized and which is not lowered after discontinuation of an inducing medication may put the patient at risk for toxicities, including cardiac arrhythmias (torsades de pointes).42 Thus far, buprenorphine has not had such adverse events identified making it preferable to methadone for those opioid-addicted patients requiring treatment with an antiretroviral medication for HIV or hepatitis.
Another advantage of buprenorphine when compared to methadone is the ability of qualified primary care and infectious disease physicians to prescribe buprenorphine to opioid-dependent patients for whom they are also providing care for other medical illnesses, such as HIV/AIDS and Hepatitis B. There is a ceiling to the opioid agonist effects of buprenorphine, which may decrease the potential for abuse when compared to full opioid agonists and which also decreases the risk of opioid toxicity. There is some evidence that the addition of naloxone to buprenorphine diminishes risk of injected abuse of the medication. These properties of the buprenorphine/naloxone product that help to deter abuse potential along with the Drug Addiction Treatment Act of 200043 allow treatment of opioid addiction to expand beyond the setting of specialized narcotic treatment programs. It must also be noted, however, that abuse of buprenorphine/naloxone by injection has been recently reported.44 Buprenorphine/naloxone has less been found to produce less positive subjective effects than buprenorphine alone; but a recent study has shown that both buprenorphine formulations may be abused.45
By co-prescribing buprenorphine, qualified physicians treating infectious diseases such as HIV and/or HBV have the ability to assist with improved adherence to prescribed antiretroviral medications among opioid-dependent patients who would otherwise be at high risk of poor compliance if their opioid dependence was not addressed. Others have shown that co-location of healthcare services for those with co-occurring conditions improves clinical outcomes.46 Taken together, these findings underscore the potential benefits of buprenorphine/naloxone treatment in patients with co-occurring illnesses such as HIV disease, HBV, and opioid dependence.
It should be noted that there are limitations to this study. We opted not to recruit a control sample for the lamivudine component of the study. The reason for this is that 3TC is largely excreted unchanged in the urine and this medication is not metabolized by the CYP 450 system.29 While the findings for 3TC pharmacokinetics in buprenorphine participants did not differ significantly from that in historical controls; it remains a potential limitation of the study.
This study provides additional findings that indicate buprenorphine/naloxone for the treatment of opioid dependence in patients with HIV and/or HBV disease(s) is associated with fewer adverse drug interactions when compared to methadone treatment. The lack of adverse drug interactions between buprenorphine and the antiretroviral medications in the current study may simplify and improve safety while providing effective treatment for co-existing medical conditions. These are important results for clinicians providing care for these challenging diseases.
Acknowledgments
This study was supported by grants RO1 DA 13004 (Dr. McCance-Katz), KO2 DA00478 (Dr. McCance-Katz), and RO1 DA 10100 (Dr. Moody) from the National Institute on Drug Abuse, Bethesda, Md; and by grant M01RR00065 from the National Center for Research Resources, Bethesda, Md to the General Clinical Research Center at Virginia Commonwealth University.
The authors thank Lauren Kelly, BA, MSW and Justine Arenander, BA for expert technical assistance on this study. The analytical contributions of Jill Lapham and Jill Hochreiter in the Translational Pharmacology Research Core laboratory in the NYS Center of Excellence in Bioinformatics and Life Sciences is appreciated.
Footnotes
Declaration of Interest
Dr. Moody has received research funding from Reckitt Benckiser, the manufacturer of buprenorphine. All other authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.UNAIDS [April 15, 2009]; Available at: http://www.unaids.org/en/knowledgecentre/hivdata/epidemiology/epislides.asp.
- 2.Peters MG. Diagnosis and management of hepatitis B virus and HIV coinfection. Top HIV Med. 2007;15:163–166. [PubMed] [Google Scholar]
- 3.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 6.Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665–1670. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Friedland G. Adherence, compliance, and HAART. AIDS Clin Care. 1997;9:51–54. 58. [PubMed] [Google Scholar]
- 8.Backmund M, Meyer K, Schuetz C, et al. Factors associated with exposure to hepatitis B virus in injection drug users. Drug Alcohol Depend. 2006;84:154–159. doi: 10.1016/j.drugalcdep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 9.McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: The importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005;41(Suppl 1):S89–S95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- 10.Kharasch ED, Hoffer C, Whittington D, et al. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Gourevitch MN, Friedland GH. Interactions between methadone and medications used to treat HIV infection: A review. Mt Sinai J Med. 2000;67:429–436. [PubMed] [Google Scholar]
- 12.Altice FL, Friedland GH, Cooney EL. Nevirapine induced opiate withdrawal among injection drug users with HIV infection receiving methadone. AIDS. 1999;13:957–962. doi: 10.1097/00002030-199905280-00012. [DOI] [PubMed] [Google Scholar]
- 13.Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213–217. doi: 10.1046/j.1365-2125.2001.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCance-Katz EF, Rainey PM, Friedland G, et al. The pro-tease inhibitor lopinavir-ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- 15.McCance-Katz EF, Rainey PM, Smith P, et al. Drug interactions between opioids and antiretroviral medications: Interaction between methadone, LAAM, and delavirdine. Am J Addict. 2006;15:23–34. doi: 10.1080/10550490500419029. [DOI] [PubMed] [Google Scholar]
- 16.Rainey PM, Friedland G, Cance-Katz EF, et al. Interaction of methadone with didanosine and stavudine. J Acquir Immune Defic Syndr. 2000;24:241–248. doi: 10.1097/00126334-200007010-00007. [DOI] [PubMed] [Google Scholar]
- 17.McCance-Katz EF. Office-based buprenorphine treatment for opioid-dependent patients. Harv Rev Psychiatry. 2004;12:321–338. doi: 10.1080/10673220490905688. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos. 2006;34:440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 19.Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12:577–581. [PubMed] [Google Scholar]
- 20.Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 21.Hammer SM, Eron JJ, Jr., Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 22.DHHS guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Washington, DC: 2008. 2005. [February 20, 2009]. Available at: http://aidsinfo.nih.gov/contentfiles/adultsandadolescentsGL.pdf. [Google Scholar]
- 23.Friedland G, Rainey P, Jatlow P, et al. Pharmacokinetics (pK) of didanosine (ddI) from encapsulated enteric coated bead formulation (EC) vs chewable tablet formulation in patients (pts) on chronic methadone therapy.. 14th International AIDS Conference; Barcelona, Spain. July 2002; Abstract number: TuPeB4496. 2002. [Google Scholar]
- 24.Ayoub WS, Keeffe EB. Review article: Current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2008;28:167–177. doi: 10.1111/j.1365-2036.2008.03731.x. [DOI] [PubMed] [Google Scholar]
- 25.Leemans WF, Janssen HL, de Man RA. Future prospectives for the management of chronic hepatitis B. World J Gastroenterol. 2007;13:2554–2567. doi: 10.3748/wjg.v13.i18.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoulim F. Antiviral therapy of chronic hepatitis B. Antiviral Res. 2006;71:206–215. doi: 10.1016/j.antiviral.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Benhamou Y. Treatment algorithm for chronic hepatitis B in HIV-infected patients. J Hepatol. 2006;44(1 Suppl):S90–S94. doi: 10.1016/j.jhep.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Rainey PM, Friedland GH, Snidow JW, et al. The pharmacokinetics of methadone following co-administration with a lamivudine/zidovudine combination tablet in opiate-dependent subjects. Am J Addict. 2002;11:66–74. doi: 10.1080/10550490252801657. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MA, Moore KH, Yuen GJ, et al. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41–66. doi: 10.2165/00003088-199936010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Moody DE, Cance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 33.Knupp CA, Stancato FA, Papp EA, et al. Quantitation of didanosine in human plasma and urine by high-performance liquid chromatography. J Chromatogr. 1990;533:282–290. doi: 10.1016/s0378-4347(00)82215-x. [DOI] [PubMed] [Google Scholar]
- 34.Rezk NL, Tidwell RR, Kashuba AD. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 35.Delahunty T, Bushman L, Fletcher CV. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:6–12. doi: 10.1016/j.jchromb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 36.McCance-Katz EF, Rainey PM, Jatlow P, et al. Methadone effects on zidovudine disposition (AIDS Clinical Trials Group 262). J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:435–443. doi: 10.1097/00042560-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hove and London; Hillsdale, NJ: 1988. [Google Scholar]
- 38.MCance-Katz EF, Moody DE, Morse GD, et al. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43(Suppl 4):S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 39.McCance-Katz EF, Moody DE, Morse G, et al. Interactions between buprenorphine and antiretrovirals II: Protease inhibitors, nelfinavir, lopinavir/ritonavir, or ritonavir. Clin Infect Dis. 2006;43(Suppl 4):S235–246. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 40.Bart PA, Rizzardi PG, Gallant S, et al. Methadone blood concentrations are decreased by the administration of abacavir plus amprenavir. Ther Drug Monit. 2001;23:553–555. doi: 10.1097/00007691-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Stocker H, Kruse G, Kreckel P, et al. Nevirapine significantly reduces the levels of racemic methadone and (R)-methadone in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:4148–4153. doi: 10.1128/AAC.48.11.4148-4153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luthi B, Huttner A, Speck RF, et al. Methadone-induced torsade de pointes after stopping lopinavir-ritonavir. Eur J Clin Microbiol Infect Dis. 2007;26:367–369. doi: 10.1007/s10096-007-0293-5. [DOI] [PubMed] [Google Scholar]
- 43. [May 13, 2009];Drug Addiction Treatment Act of 2000. Public Law 106-310, title XXXV, sections 3501–3502. 2000 Availableat: http://buprenorphine.samhsa.gov/data.html.
- 44.Bruce RD, Govindasamy S, Sylla L, et al. Lack of reduction in buprenorphine injection after introduction of co-formulated buprenorphine/naloxone to the Malaysian market. Am J Drug Alcohol Abuse. 2009;35:68–72. doi: 10.1080/00952990802585406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samet JH, Friedmann P, Saitz R. Benefits of linking primary medical care and substance abuse services: Patient, provider, and societal perspectives. Arch Intern Med. 2001;161:85–91. doi: 10.1001/archinte.161.1.85. [DOI] [PubMed] [Google Scholar]