Abstract
This study was conducted to determine whether drug interactions of clinical importance occur between buprenorphine, an opioid partial agonist medication used in treatment of opioid dependence, and the nonnucleoside reverse transcriptase inhibitor (NNRTI) nevirapine. Opioid-dependent, buprenorphine/naloxone-maintained, HIV-negative volunteers (n=7) participated in 24-hour sessions to determine the pharmacokinetics of buprenorphine alone and of buprenorphine and nevirapine following administration of 200 mg nevirapine daily for 15 days. Opiate withdrawal symptoms, cognitive effects, and adverse events were determined prior to and following nevirapine administration. Modest decreases were observed for AUC for buprenorphine and its metabolites. There was a trend for more rapid clearance of both buprenorphine (p = .08) and buprenorphine-3-glucuronide (p = .08). While no single effect reached statistical significance, the joint probability that the consistent declines in all measures of exposure were due to chance was extremely low, indicating that nevirapine significantly reduces overall exposure to buprenorphine and buprenorphine metabolites. Clinically significant consequences of the interaction were not observed. Buprenorphine did not alter nevirapine pharmacokinetics. Dose adjustments of either buprenorphine or nevirapine are not likely to be necessary when these drugs are coadministered for the treatment of opiate dependence and HIV disease.
INTRODUCTION
Injection drug use (IDU) is a significant risk factor for the development of HIV/AIDS. In the United States, approximately 27% of HIV infections are attributed directly to IDU or high-risk sexual behavior in the context of IDU.1 UNAIDS (the joint United Nations program on HIV/AIDS) estimates that IDU accounts for more than 80% of all HIV infections in Eastern Europe and Central Asia and is a major factor in HIV epidemics in countries in the Middle East, North Africa, South, and South-East Asia, and Latin America.2 A large number of injection drug users are opioid-addicted and the treatment of choice for such individuals is opioid replacement therapy. At this point, the opioid therapies in use include methadone and buprenorphine. Methadone is much more widely used than buprenorphine. Methadone has been available for over 40 years as a maintenance therapy for opioid dependence and is well-established as a treatment modality in specialized narcotic treatment programs.3 As the rates of HIV disease increase, secondary to unsafe drug injection practices and other high risk behaviors associated with IDU, such as unprotected sexual relations, particularly in developing nations, increasing focus has been placed on methadone maintenance therapy to try to decrease the risk of HIV transmission.
Buprenorphine, a mu-opioid receptor partial agonist shown to be effective in the treatment of opioid dependence,4 is a relatively new medication and much less widely used to date than methadone. This is despite advantages in its use including reduced toxicity in overdose situations and that it can be administered on a less than daily basis. Less than daily dosing is of benefit to drug abuse treatment programs that administer addiction pharmacotherapies. Further, buprenorphine is available by prescription from office-based practices of qualified physicians, which has significantly increased access to treatment for opioid dependence.4
Nevirapine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) that is a part of many highly active antiretroviral therapy (HAART) regimens. This is especially true in developing nations of limited resources for provision of antiretroviral (ARV) regimens. Nevirapine is a potent NNRTI that is available as a generic medication at relatively low cost. Unfortunately, nevirapine has been demonstrated to have a significant adverse drug interaction with methadone in which methadone metabolism is increased leading to opiate withdrawal.5 Of concern is that methadone-maintained patients with HIV disease who experience opiate withdrawal with HAART will be non-adherent to their HIV medications.6 This can lead to the development of viral mutations and HIV that is resistant to the HAART components of the regimen that the patient is receiving. Furthermore, such patients may increase their use of illicit drugs and alcohol and increase high risk behaviors for HIV transmission.7
When another NNRTI, efavirenz, was administered in the context of treatment of HIV/AIDS, methadone metabolism was also shown to be induced, leading to significant opiate withdrawal.8 However, this was not observed when efavirenz and buprenorphine were given concomitantly9 leading to the question of whether the NNRTI, nevirapine, would alter buprenorphine responses. The current study was undertaken to determine if nevirapine has significant drug interactions with buprenorphine.
METHODS
Procedures
The general design of this study has been reported previously.10 Eligible individuals provided written, voluntary, informed consent following university Institutional Review Board-approved protocols. Opioid-dependent participants received buprenorphine/naloxone treatment for their opioid addiction at no charge and were offered monetary compensation for their participation in the pharmacokinetics studies. Seven individuals who were opioid dependent and stable for at least 2 weeks on a daily dose of sublingual buprenorphine/naloxone 16/4 mg participated in two 24-hour blood sampling studies, one to determine baseline buprenorphine pharmacokinetics. This was followed by oral administration of nevirapine 200 mg daily for 15 days and a second 24-hour pharmacokinetic study was undertaken in which blood samples for buprenorphine and nevirapine plasma concentrations were obtained. Blood samples were obtained prior to buprenorphine/naloxone and HIV medication administration, and at .5, 1, 1.5, 2, 4, 6, 8, 12, and 24 hours (h) after administration.
The 200 mg dose of nevirapine administered in this study was one-half of the usual clinical dose. This was done to add an additional margin of safety for study participants because the standard clinical dose had been associated with a variety of adverse events. Because nevirapine is known to be a potent inducer of cytochrome P450 (CYP) 3A4, it was thought that the direction and clinical significance of any pharmacokinetic interactions could be detected using this lower dose.11 A published study has shown the induction of CYP 3A4 is equivalent to using either nevirapine 200 mg or nevirapine 400 mg daily dosing.12 The duration of nevirapine dosing of 15 days was based on maximum induction of CYP 3A4 by another NNRTI, efavirenz, after 11 days13 and 4 days required for maximum symptoms of opiate withdrawal related to reductions in buprenorphine concentrations were that to occur.14
The study reported here was designed to have 11 subjects, but was terminated after only seven subjects were enrolled due to reports of hepatotoxicity in some individuals being treated therapeutically with nevirapine.15,16 Although no adverse events had been observed in this study, a decision was made in conjunction with FDA reviewers and the Institutional Review Board at the University of California, San Francisco, that the risks to participants outweighed the benefits of study continuation. Pharmacokinetic studies in control individuals taking nevirapine alone were not undertaken because of the decision to stop the study. Instead, data on maximum plasma concentration (Cmax), clearance, time of Cmax (Tmax), and elimination half-life available in published literature11 were used in determining the effect of buprenorphine on nevirapine disposition.
Study procedures included standardized and validated measures of opioid withdrawal by clinician rating (Objective Opioid Withdrawal Scale [OOWS], scores ≥ 3 indicate moderate withdrawal symptoms)17 and cognitive impairment using the Mini-Mental State Examination18 for opioid-dependent participants (maximum score = 30; scores of < 27 indicate cognitive impairment). Adverse symptoms were recorded for all participants using an Adverse Symptoms Checklist that queried for a wide range of adverse experiences including changes in energy, gastrointestinal symptoms, central nervous system effects, genitourinary symptoms, and other somatic complaints scored for severity on an ordinal scale (0–3, with 0 = not present, 1 = mild, 2 = moderate, and 3 = severe, maximum possible score = 87). Ratings were administered at baseline (prior to buprenorphine/naloxone induction), following stabilization on buprenorphine (prior to antiretroviral administration), and at completion of the NNRTI dosing period. Ratings were completed in the morning just prior to daily buprenorphine/naloxone administration.
Drug Assays
Buprenorphine Assay
Buprenorphine and metabolite concentrations were determined using a liquid chromatography-tandem mass spectrometry method. In brief, buprenorphine-d4, and norbuprenorphine-d3 were added to samples as the internal standards. The pH of the matrix was adjusted to 9.3 with ammonium carbonate buffer and samples were extracted using C18 solid-phase extraction columns. The eluate was evaporated and reconstituted with .1% formic acid in water and analyzed by LC-MS/MS using electrospray ionization.19
Nevirapine Assay
Nevirapine plasma concentrations from human plasma were measured with a high-performance liquid chromatography (HPLC) method using a Waters Atlantic dC18 column, detected at a wavelength of 282 nm with a photodiode array detector. This method was calibrated within a linear range of 200–10,000 ng/ml as previously reported.20
Pharmacokinetics Analysis
Buprenorphine and metabolite pharmacokinetics were calculated following sublingual administration of buprenorphine/naloxone alone and again following administration of nevirapine. Area under the concentration-time curve (AUC), minimum plasma concentration (Cmin), Cmax, Tmax, and sublingual (buprenorphine), or oral (nevirapine) clearance (CL/F) were determined using the noncompartmental analysis module of WinNonLin Professional Version 3.2 (Pharsight Corp., Mountain View, CA21). For metabolites, CL/F was calculated based on the administered dose of parent compound. The F term thereby represents the fraction of parent drug ultimately converted to the metabolite. Cmax, Cmin, and Tmax were estimated by inspection of the raw data. For purposes of noncompartmental analysis, drug concentrations that were less than the limit of quantitation were expressed as one-half of the lower limit.
Statistical Analysis
Student’s paired t-test was used to test the significance of the differences in pharmacokinetic parameters for buprenorphine alone and in combination with nevirapine (within-subject analyses). The Wilcoxon test was used for the within-subject comparison of the values of Tmax. A difference was considered statistically significant if the p value was ≤ 0.05 (two-tailed) and a trend for statistical significance was designated as a p value < 0.1 but > 0.05 (two-tailed). Buprenorphine effects on nevirapine were assessed using the unpaired t-test in a between-subjects comparison versus historical controls. Comparisons of subject characteristics were made by single factor ANOVA.
RESULTS
Study Participants
Seven opioid-dependent individuals participated in this study. All were receiving a stable, daily, sublingual dose of buprenorphine/naloxone (16/4 mg). The participants were otherwise physically healthy and without current mental illness. Participants received no other concomitant medications over the course of study participation. Demographic characteristics for study participants are listed in Table 1. The sample consisted of seven African-Americans whose preferred route of heroin administration was nasal (five of seven). Two participants had a history of IDU. No participants were positive for antibody to Hepatitis C. Participants received a stable dose of buprenorphine/naloxone for at least 2 weeks prior to study entry. Based on clinical assessment, participants were stabilized on a dose of buprenorphine/naloxone 16/4 mg daily. (Stabilization was defined as lack of opiate withdrawal symptoms and cessation of opiate craving and use, as determined by clinical examination and urine toxicology screening, respectively.) Few adverse events occurred during the study period and no significant differences in adverse events were reported for any symptom before and after nevirapine administration. Further, no medical intervention was required to address adverse events in any participant over the course of the study. Cigarette smoking was common, but daily use reported by all subjects was less than one pack per day (.3 (.6)) (mean (SE)). Physiological changes associated with nevirapine administration were also modest and nonsignificant (Table 2). Concern for hepatotoxicity was emphasized in this study, but liver enzyme values remained within normal limits. Similarly, no significant effects of nevirapine on cardiac PR or QT interval were observed.
TABLE 1.
Participant characteristics
| N = 7 | |
|---|---|
| Age (years) | 36 (2.07)* |
| Weight (kg) | 107.8 (7.3) |
| Buprenorphine/Naloxone dose (mg/day) | 16.0 (.0)/4 (.0) |
| Female | 1 |
| Race: | |
| African-American | 7 |
| Caucasian | 0 |
| Substance use disorders: | |
| Opioid dependence | 7 |
| Cocaine abuse | 1 |
| Cannabis abuse | - |
| Alcohol abuse | - |
| IDU | 2 |
| Nicotine use (packs/day) | .3 (.1) |
| Hepatitis C positive | 0 |
Mean (SE).
TABLE 2.
Physiological and cognitive responses prior to and following nevirapine administration
| N = 7 | |
|---|---|
| AST pre/post (U/L) | 20.9 (2.5)/23.1 (3.9) |
| ALT pre/post (U/L) | 21.7 (3.6)/22.0 (4.2) |
| Total bilirubin pre/post (mg/dl) | .3 (.0)/.3 (.0) |
| Direct bilirubin pre/post (mg/dl) | .09 (.01)/.04 (.02) |
| Cholesterol pre/post (mg/dl) | 144.6 (11.0)/151.1 (14.3) |
| Triglyceride pre/post (mg/dl) | 104.7 (26.9)/131.0 (43.9) |
| PR interval pre/post (ms) | 193.4 (9.6)/192.9 (8.4) |
| QTc interval pre/post (ms) | 399.7 (5.4)/404.4 (3.7) |
| OOWS pre/post | .0 (.0)/.0 (.0) |
| MMSE pre/post | 29.3 (.2)/29.1 (.3) |
| Adverse symptoms total score pre/post | 2.4 (1.0)/2.9 (1.2) |
“Pre” samples were collected at screening for laboratory values; for OOWS, MMSE, and Adverse Symptoms instruments data were collected following buprenorphine/naloxone stabilization and in the morning on the day of the first dose of nevirapine 200 mg prior to dosing of either buprenorphine/naloxone and nevirapine.
“Post” samples and instruments were collected on the morning of the start of the second pharmacokinetics study just prior to buprenorphine/naloxone and nevirapine (dose 15) dosing.
Interaction between Buprenorphine and Nevirapine
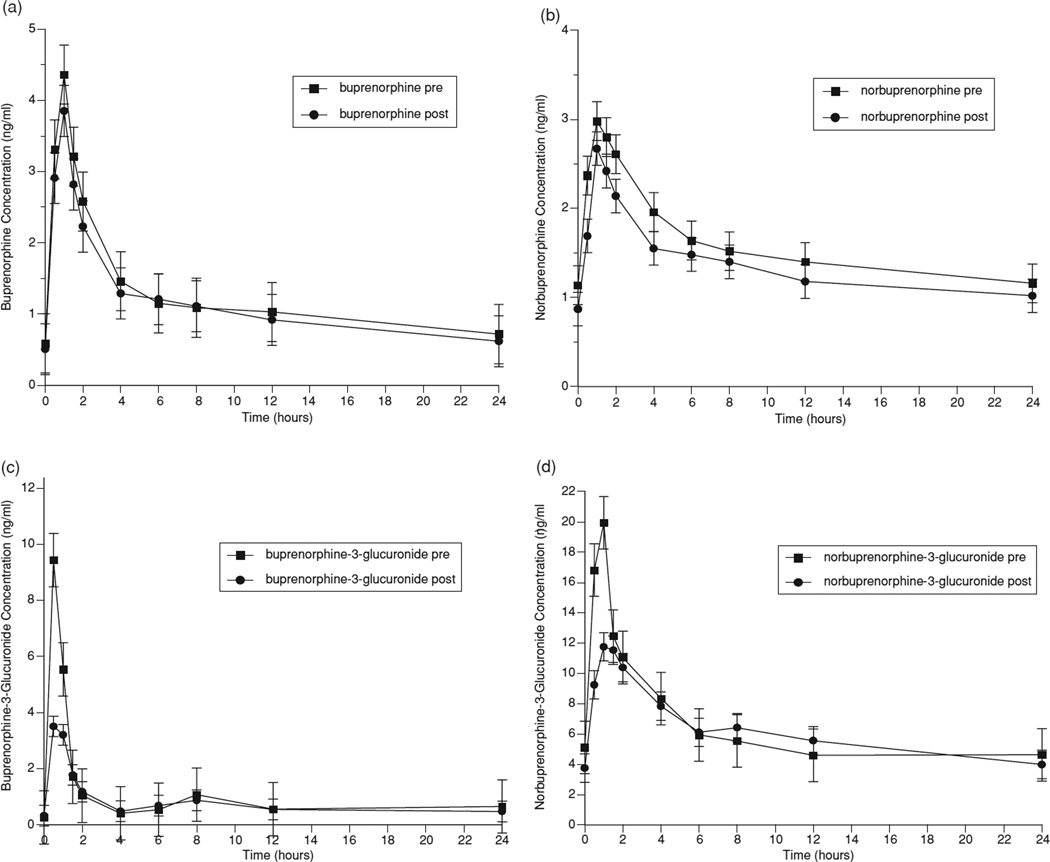
Pharmacokinetic parameters for buprenorphine and metabolites before and after nevirapine administration are shown in Table 3. Figure 1 graphically represents the mean concentrations of buprenorphine (1a), the active metabolite, norbuprenorphine (1b), and the inactive glucuronides of buprenorphine (1c), and norbuprenorphine (1d) over a 24-hour dosing interval. The coadministration of nevirapine with buprenorphine modestly decreased buprenorphine AUC (Table 3). Similarly, norbuprenorphine AUC0–24 was reduced but neither change was statistically significant. Clearance of buprenorphine and buprenorphine-3-glucuronide increased, approaching statistical significance (p = .08 for each). Values for AUC, Cmax, and C24 for buprenorphine and all of its metabolites were decreased following nevirapine administration, although, again, with no single alteration reaching statistical significance. Since participants served as their own controls, the consistent pattern of reductions in multiple measures is not likely to be the chance result of random effects. For example, the likelihood that the AUC findings would have occurred by chance can be calculated as the product of the probabilities for each event. The likelihood that all four AUCs would have been decreased due to chance would be the product of the individual p values or .35 × .15 × .13 × .87 = .006. These findings indicate that nevirapine decreases exposure to buprenorphine and its metabolites with chronic dosing.
TABLE 3.
Effect of nevirapine on buprenorphine and buprenorphine metabolites in plasma
| Pharmacokinetic parameter |
Prene- virapine |
Postne- virapine |
p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0–24(ng*h/ml) | 29.9 (3.8) | 27.1 (6.9) | .35 |
| Cl/F (L/h) | 591 (77) | 707 (118) | .08 |
| Cmax (ng/ml) | 4.93 (1.07) | 3.88 (.69) | .16 |
| Tmax (h) | 1.0 (.5–1.5) | 1.0 (.5–1.0) | n.s. |
| C24 | .72 (0.13) | .62 (.11) | .24 |
| Norbuprenorphine | |||
| AUC0–24 (ng*h/ml) | 37.5 (6.1) | 32.1 (7.2) | .15 |
| Cl/F (L/h) | 490 (68) | 665 (146) | .16 |
| Cmax (ng/ml) | 3.41 (.60) | 2.74 (.45) | 0.09 |
| Tmax (h) | 1.5 (.5–1.5) | 1.0 (1.0–1.5) | n.s. |
| C24 | 1.16 (.26) | 1.02 (.29) | .16 |
| Buprenorphine-3-glucuronide | |||
| AUC0–24 (ng*h/ml) | 19.8 (5.3) | 14.6 (5.5) | .13 |
| Cl/F (L/h) | 1,301 (348) | 2,225 (576) | .08 |
| Cmax (ng/ml) | 10.82 (3.59) | 4.94 (2.01) | .17 |
| Tmax (h) | .5 (.5–1.5) | 1.0 (.5–1.0) | n.s. |
| C24 | .40 (.21) | .30 (.17) | .31 |
| Norbuprenorphine-3-glucuronide | |||
| AUC0–24 (ng*h/ml) | 150 (23) | 146 (32) | .87 |
| Cl/F (L/h) | 123 (18) | 137 (23) | .32 |
| Cmax (ng/ml) | 21.0 (5.7) | 12.7 (2.65) | .23 |
| Tmax (h) | 1.0 (.5–4.0) | 1.0 (.5–1.5) | n.s. |
| C24 | 4.6 (1.3) | 4.0 (1.1) | .53 |
Note: Values are the mean (standard error of the mean) for seven subjects, except that Tmax is given as median (range). Student’s paired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
FIGURE 1.
Effect of nevirapine on (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide, and (d) norbuprenorphine-3-glucuronide.
Despite declines in exposure to buprenorphine and norbuprenorphine with nevirapine coadministration, no subjects showed evidence of opiate withdrawal symptoms (OOWS prenevirapine administration: 0 (0), postnevirapine administration: .0 (.0)) nor were cognitive deficits detected (MMSE prenevirapine administration: 29.3 (.2), postnevirapine administration: 29.1 (.3); total score possible on the MMSE: 30).
Adverse symptoms were infrequently reported by study participants receiving nevirapine. One of the seven participants reported a slight increase in constipation, one participant reported moderate sedation, one participant reported nightmares that were rated “moderate” in severity, while one participant reported a slight improvement in the symptom “Delayed or absent orgasm,” and one individual reported a slight improvement for the symptom “Increased appetite.” No other adverse symptoms were reported and no differences were statistically significant.
Nevirapine concentrations were measured over a 24-hour dosing interval in buprenorphine/naloxone-maintained individuals and pharmacokinetics calculated and compared to those of historical controls who received a single dose of nevirapine 200 mg orally11 (Table 4). These historical controls were a subset of a sample of 21 subjects (three women) with ages ranging from 19–46 years. All were HIV-infected and six had AIDS. CD4 counts ranged from 0–379 (mean (SD): 191 (124)). Twelve were homosexual, nine were injection drug users, two were both homosexual, and injection drug users. Three participants were Latino and one was African-American with the remaining participants being Caucasian. Three of these participants received a 200 mg nevirapine dose, but the paper does not specify which participants received what nevirapine dose.11
TABLE 4.
Nevirapine pharmacokinetics during buprenorphine coadministration
| Nevirapine parameter |
Nevirapine (historical controls n = 3) |
Nevirapine- buprenorphine (n = 7) |
p |
|---|---|---|---|
| AUCtau (h* µg/ml) | 136 (24) | 199.0 (28) | .13 |
| Clss/F (L/h) | 2.1 (.7) | 2.8 (.2) | .30 |
| Cmax (µg/ml) | 2.1 (.3) | 3.9 (.2) | .01 |
| Tmax (h) | 1.5 (.5–4.0) | 1.0 (1.0–4.0) | .77 |
| T1/2 (h) | 43 (14) | 37 (5) | .71 |
Note: Values are the mean (standard error of the mean) for seven subjects who participated in the study, except that the discontinuous variable, Tmax, is given as median (range).
Historical controls: Three subjects from a sample of 21 HIV-infected individuals received nevirapine 200 mg (single dose). See Results section “Interaction between Buprenorphine and Nevirapine” for more detail on the composition of the historical control sample.
Clearance, elimination half-life, Tmax, or AUCtau were not significantly different in either group. The apparently higher Cmax in buprenorphine/naloxone-treated individuals compared to that of the historical controls, is likely to be largely due to the historical controls being in a single-dose study with no drug present at time zero, whereas our studies were done at steady state, with a substantial nevirapine trough concentration present at time zero (2.2 (.2) µg/ml). The mean difference between Cmax and C0 for the current sample was 1.6 (.2) µg/ml, while that for historical controls was 2.1 (.7) µg/ml. This difference is not significant, (p = .23).
DISCUSSION
This study sought to examine whether drug interactions of clinical significance occur when buprenorphine/naloxone and nevirapine are administered concomitantly. Trends for increases in buprenorphine and buprenorphine-3-glucuronide clearance were observed and all buprenorphine and metabolite concentrations were modestly, but consistently decreased. Joint probability determinations indicated that the observed effects are unlikely to be due to random events. Buprenorphine had no significant effect on nevirapine pharmacokinetics.
Although we were only able to complete measurements in seven subjects, the consistency of the findings is compelling. Moreover, it is unlikely that a larger study will be subsequently carried out. Safety concerns led us to use a dose of only 200 mg of nevirapine, rather than the usual clinical dose of 400 mg daily. It remains possible that more significant effects on buprenorphine might be seen with a 400 mg daily dose. However, Arroyo et al. have shown that 200 mg of nevirapine produced the same induction of methadone metabolism as was seen with a 400 mg dose.12 Thus, it is also possible that the 200 mg dose produced a maximum effect on buprenorphine pharmacokinetics.
Nevirapine is a potent NNRTI that is used in many HAART regimens. Specifically, because nevirapine is off-patent and less expensive than many newer ARV medications, it continues to be widely used, particularly in resource-limited settings. Many countries, including developing nations, have major public health problems with opioid addiction and IDU. These conditions have led to large numbers who suffer with both HIV/AIDS and heroin addiction. Methadone maintenance therapy has been increasingly accepted as a treatment for this population. Methadone has been shown to help to initiate abstinence from heroin abuse, to be associated with increases in heath, lower rates of criminal activity, and increase employment.3 Methadone is also advantageous in its low cost per dose. However, methadone has been shown to have several clinically significant adverse events associated with concomitant use of certain antiretroviral medications, including nevirapine.22
Nevirapine has been shown to be a potent inducer of both CYP 3A4 and 2B6,23 which are the primary metabolic enzymes in methadone clearance.24,25 Further, nevirapine treatment has been associated with opiate withdrawal symptoms in methadone-treated patients.26 It can be difficult to stabilize patients with HIV disease prescribed nevirapine on methadone maintenance. Such patients often require higher than usual doses of methadone and some physicians unfamiliar with drug interactions related to opiate withdrawal resulting from nevirapine-induced metabolism of methadone are reluctant to prescribe adequate doses of methadone. These providers may also have concerns about the rate and amount of methadone dose increase and the fear of opiate overdose. It can take several weeks to stabilize such patients on an adequate dose of methadone.8 Once stabilization occurs, there may also be difficulty with tapering methadone dose to avoid opiate toxicity should nevirapine no longer be effective for treatment of the patient’s HIV disease. The process of tapering requires good communication with all providers as well as a relatively rapid methadone taper once nevirapine is stopped. This prospect may generate anxiety both in the providers and in the patient who fears the onset of opiate withdrawal with rapid taper.
Because of the difficulties associated with coadministration of nevirapine and methadone and because nevirapine is a frequently prescribed HIV therapeutic, it was important to determine if similar difficulties might be encountered when buprenorphine was used as a treatment for opioid dependence in those with HIV disease who might require treatment with nevirapine. Although, the sample size was smaller than intended, we were able to show modest decreases in buprenorphine and buprenorphine metabolite exposure after nevirapine administration over a 15-day study period. Importantly, however, no participant complained of symptoms that could be attributed to opiate withdrawal.
There may be several reasons that the withdrawal observed with methadone in the presence of nevirapine does not occur with buprenorphine/naloxone treatment. Nevirapine only produces relatively small decreases in exposure to buprenorphine and its metabolites. Most of the effects are on the peak concentrations, whereas trough concentrations are nearly identical. In contrast to buprenorphine, nevirapine dramatically reduces methadone AUC and concentrations at all time points to about half of pretreatment values.12,27,28 While methadone metabolism has generally been ascribed primarily to CYP 3A4,24 recent data suggest that CYP 2B6 may be principally responsible for its metabolism in humans.25 Nevirapine induces both of these enzymes.29,30 Buprenorphine, on the other hand, is metabolized principally by CYP 3A4 and CYP 2C8.31 The difference in the extent of decrease in exposure for methadone versus buprenorphine in the presence of nevirapine may explain the marked difference between the two opioids in terms of development of opiate withdrawal symptoms.
Other pharmacodynamic reasons as to why no opiate withdrawal was observed in study participants in the face of decrements in buprenorphine and norbuprenorphine plasma concentrations may also contribute to these findings. Buprenorphine is an opioid partial agonist. It exhibits a ceiling effect at which increasing doses are not associated with further mu-opioid agonist effects.32 Therefore, the level of opioid dependence may be less than that with a full mu agonist such as methadone resulting in less opiate withdrawal in the presence of a medication that induces buprenorphine metabolism. Another possible explanation is the described high affinity of buprenorphine for the mu-opioid receptor and its slow dissociation from that receptor33 such that plasma concentrations may not be reflective of receptor occupancy levels in the brain. Finally, while results of this study show that nevirapine administration reduces concentrations of both buprenorphine and norbuprenorphine (an active metabolite of buprenorphine with mu-opioid agonist effects34), the combined mu-opioid agonist effects of the two opioids may have been sufficient to prevent opiate withdrawal symptoms. This is in contrast to methadone where its metabolism produces an inactive metabolite.35 Therefore, as methadone concentrations decline in the presence of nevirapine which induces its metabolism, patients receiving both medications are likely to experience opiate withdrawal.
Given the very distinct differences between reported effects of nevirapine on methadone including significant opiate withdrawal5 and observations of the lack of clinically significant drug interactions between nevirapine and buprenorphine, it would appear that buprenorphine confers significant advantages in the treatment of HIV disease in opiate-addicted patients needing this medication. Although buprenorphine is currently more expensive than methadone in a direct comparison of medication alone costs; there are other costs associated with methadone that should be considered in selecting opioid therapy to treat opiate addiction in those with HIV/AIDS. Methadone must be administered in the context of a highly regulated narcotic treatment program. Meeting the costs of regulatory requirements and nursing requirements for methadone maintenance therapy are substantial and, to a large degree, eliminate the cost difference for these two treatments.36 Further, the cost in physician and clinician time in monitoring for opiate withdrawal and rapidly addressing this problem should it arise, can be quite burdensome to providers and costly in terms of medical treatment time needed to address the clinical care issues presented. While there will always be patients with opiate addiction and HIV/AIDS who either prefer methadone or who would benefit from the structure of a narcotic treatment program, the alternative treatment of opioid dependence with buprenorphine in this population may provide substantial clinical benefit both in terms of treatment of HIV disease as well as treatment of opioid dependence.
In summary, the present study shows that chronic nevirapine treatment reduced buprenorphine exposure, but did not lead to opiate withdrawal in any study subject. These findings are in marked contrast to the effects of nevirapine on methadone, where concentrations were approximately halved, and withdrawal effects were seen in eight of nine subjects.26 Buprenorphine treatment should be considered in opioid-dependent patients with HIV/AIDS who may receive treatment with nevirapine.
Acknowledgments
This study was supported by grants RO1 DA 13004 (Dr. McCance-Katz), KO2 DA00478 (Dr.McCance-Katz), K24 DA 023359 (Dr. McCance-Katz), and RO1 DA 10100 (Dr. Moody) from the National Institute on Drug Abuse, Bethesda, Md; and by grant M01RR00065 from the National Center for Research Resources, Bethesda, Md to the General Clinical Research Center at Virginia Commonwealth University.
The authors thank Lauren Kelly, M.S.W. and Justine Arenander, B.A. for technical assistance with this project. The analytical contributions of Jill Lapham and Jill Hochreiter in the Translational Pharmacology Research Core laboratory in the NYS Center of Excellence in Bioinformatics and Life Sciences are appreciated.
Footnotes
Declaration of Interest
Dr. Moody has received research funding and consultation fees from Reckitt Benckiser, the manufacturer of buprenorphine. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Department of Health and Human Services Centers for Disease Control and Prevention. [Accessed May 31, 2009]; Available at: http://www.cdc.gov/hiv/topics/surveillance/united_states.htm.
- 2. [Accessed June 4. 2009]; Available at: http://www.unaids.org/en/PolicyAndPractice/Key Populations/InjectDrugUsers/.
- 3.Ball J, Corty E, Bond H, et al. The reduction of intravenous heroin use non-opiate abuse, and crime during methadone maintenance treatment: Further findings. NIDA Res Monogr. 1988;81:224–230. [PubMed] [Google Scholar]
- 4.McCance-Katz EF. Office based treatment of opioid dependence with buprenorphine. Harv Rev Psychiatry. 2004;12:321–338. doi: 10.1080/10673220490905688. [DOI] [PubMed] [Google Scholar]
- 5.Back D, Gibbons S, Khoo S. Pharmacokinetic drug interactions with nevirapine. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S8–S14. doi: 10.1097/00126334-200309011-00003. [DOI] [PubMed] [Google Scholar]
- 6.Samet JH, Libman H, Steger KA, et al. Compliance with zidovudine therapy in patients infected with human immunodeficiency virus, type 1: A cross-sectional study in a municipal hospital clinic. Am J Med. 1992;92:495–502. doi: 10.1016/0002-9343(92)90746-x. [DOI] [PubMed] [Google Scholar]
- 7.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. AIDS. 2002;16:2175–2182. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCance-Katz EF, Gourevitch MN, Arnsten J, et al. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 9.McCance-Katz EF. Treatment of opioid dependence and HIV/HCV co-infection in opioid dependent patients: The importance of drug interactions between opioids and antiretroviral medications. Clin Infect Dis. 2005;41:S89–S95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- 10.McCance-Katz EF, Moody DE, Morse G, et al. Interactions between buprenorphine and antiretrovirals I: Non-nucleoside reverse transcriptase inhibitors I: Efavirenz and delavirdine. Clin Infect Dis. 2006;43(Suppl 4):S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 11.Cheeseman SH, Hattox SE, McLaughlin MM, et al. Pharmacokinetics of nevirapine: Initial single-rising-dose study in humans. Antimicrob Agents Chemother. 1993;37:178–182. doi: 10.1128/aac.37.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo E, Valenzuela B, Portilla J, et al. Pharmacokinetics of methadone in human-immunodeficiency-virus-infected patients receiving nevirapine once daily. Eur J Clin Pharmacol. 2007;63:669–675. doi: 10.1007/s00228-007-0299-z. [DOI] [PubMed] [Google Scholar]
- 13.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlman JJ, Jr, Levine B, Johnson RE, et al. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–559. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanne I, Mommeja-Marin H, Hinkle J, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 16.de Maat MM, Beijnen JH, Schellens JH, et al. Chronic hepatotoxicity after long-term antiretroviral treatment including nevirapine. J Infect. 2005;50(3):262–264. doi: 10.1016/j.jinf.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Handlesman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opioid withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR, et al. “Mini-Mental State”:A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 20.Rezk NL, Tidwell RR, Kashuba AD. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 21.WinNonlin Professional [computer program]. Version 3.2. Mountain View, CA: [Google Scholar]
- 22.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: A review. Am J Addict. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Physicians’ Desk Reference: Viramune (nevirapine) 2007;61:873–878. [Google Scholar]
- 24.Iribarne C, Berthou F, Baird S, et al. Involvement of cytochrome P450 3A4 enzyme in the n-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–373. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 25.Totah RA, Sheffels P, Roberts T, et al. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 26.Barry M, Mulcahy F, Merry C, et al. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Stocker H, Kruse G, Kreckel P, et al. Nevirapine significantly reduces the levels of racemic methadone and (R)-methadone in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:4148–4153. doi: 10.1128/AAC.48.11.4148-4153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke SM, Mulcahy FM, Tjia J, et al. Pharmacokinetic interactions of nevirapine and methadone and guidelines for use of nevirapine to treat injection drug users. Clin Infect Dis. 2001;33:1595–1597. doi: 10.1086/322519. [DOI] [PubMed] [Google Scholar]
- 29.Faucette SR, Zhang TC, Moore R, et al. Relative activation of human pregnane × receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riska P, Lamson M, MacGregor T, et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos. 1999;27:895–901. [PubMed] [Google Scholar]
- 31.Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos. 2006;34:440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 32.Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 33.Cowan A, Lewis JW. Buprenorphine: Combatting Drug Abuse with a Unique Opioid. New York, NY: Wiley-Liss; 1995. [Google Scholar]
- 34.Cone E, Gorodetzky C, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12:577–581. [PubMed] [Google Scholar]
- 35.Ferrari A, Coccia CP, Bertolini A, et al. Methadone–metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–559. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Jones ES, Moore BA, Sindelar JL, O’Connor PG, et al. Cost analysis of clinic and office-based treatment of opioid dependence: Results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend. 2009;99:132–140. doi: 10.1016/j.drugalcdep.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]