Abstract
High density lipoprotein (HDL) inhibited the binding (trypsin-releasable radioactivity), internalization (cell-associated radioactivity after trypsinization), and degradation (TCA-soluble non-iodide radioactivity) of 125I-low density lipoprotein (125I-LDL) by cultured normal human fibroblasts. At HDL:LDL molar ratios of 25:1 (protein ratios about 5:1), these parameters were reduced by about 25%. Unlabeled LDL was about 25 times more effective in reducing 125I-LDL binding, implying that if HDL and LDL bind at common sites the affinity of HDL for these sites is very low or that the interaction is on some other basis. The fractional reduction in 125I-LDL binding at a given HDL: 125I-LDL ratio was independent of 125I-LDL concentration and occurred equally with fibroblasts from a subject with homozygous familial hypercholesterolemia. Reciprocally, the binding, internalization, and degradation of 125I-HDL were reduced by LDL. Preincubation of fibroblasts with HDL (or LDL) reduced the subsequent binding of 125I-LDL (or 125I-HDL) during a second incubation. In other studies HDL reduced the net increase in cell cholesterol content induced by incubation with LDL. HDL alone had no net effect on cell cholesterol content.
These findings suggest that HDL reduces both the high affinity and the low affinity binding of LDL to human fibroblasts and that this in turn reduces the internalization and degradation of LDL. The effect of HDL on the LDL-induced changes in cell cholesterol content could be in part on this basis and in part on the basis of an HDL-stimulated release of cholesterol from the cells. These effects of HDL in vitro may be relevant to the negative correlations reported from in vivo studies between plasma HDL concentration and both body cholesterol pool size and the prevalence of clinically manifest atherosclerosis but further studies will be needed to establish this.
Full text
PDF


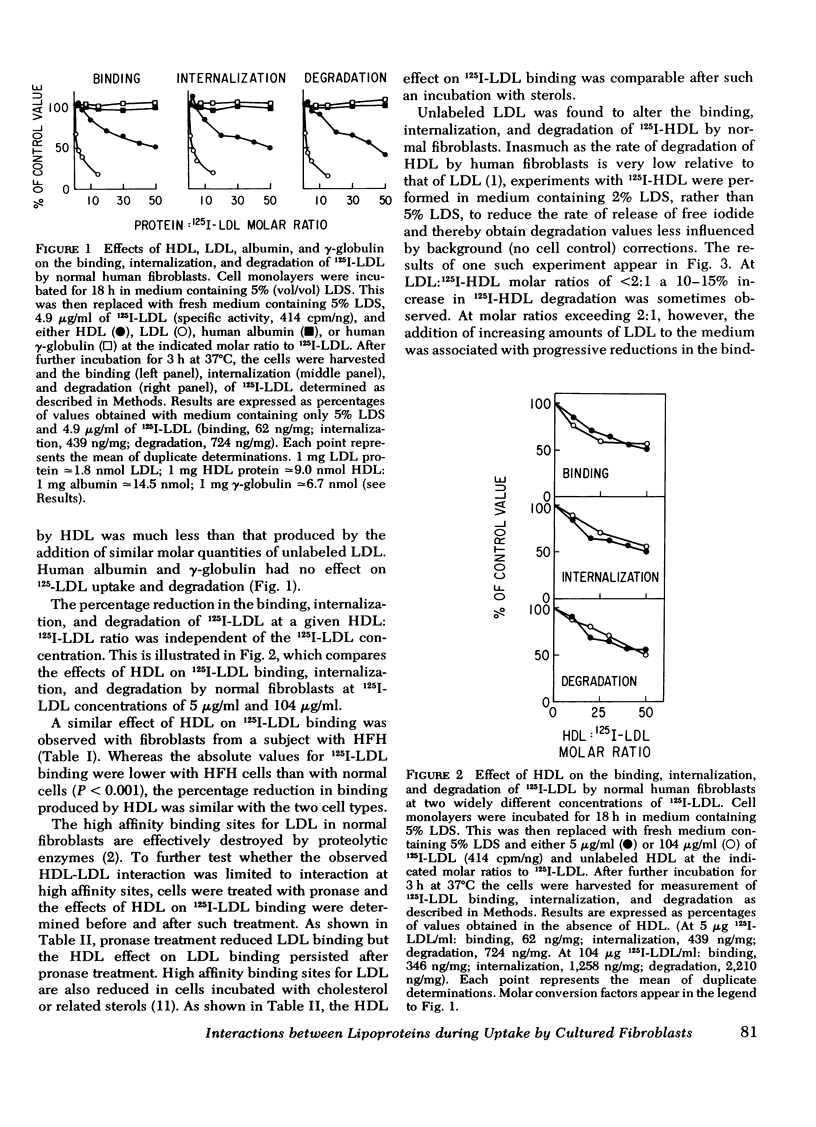
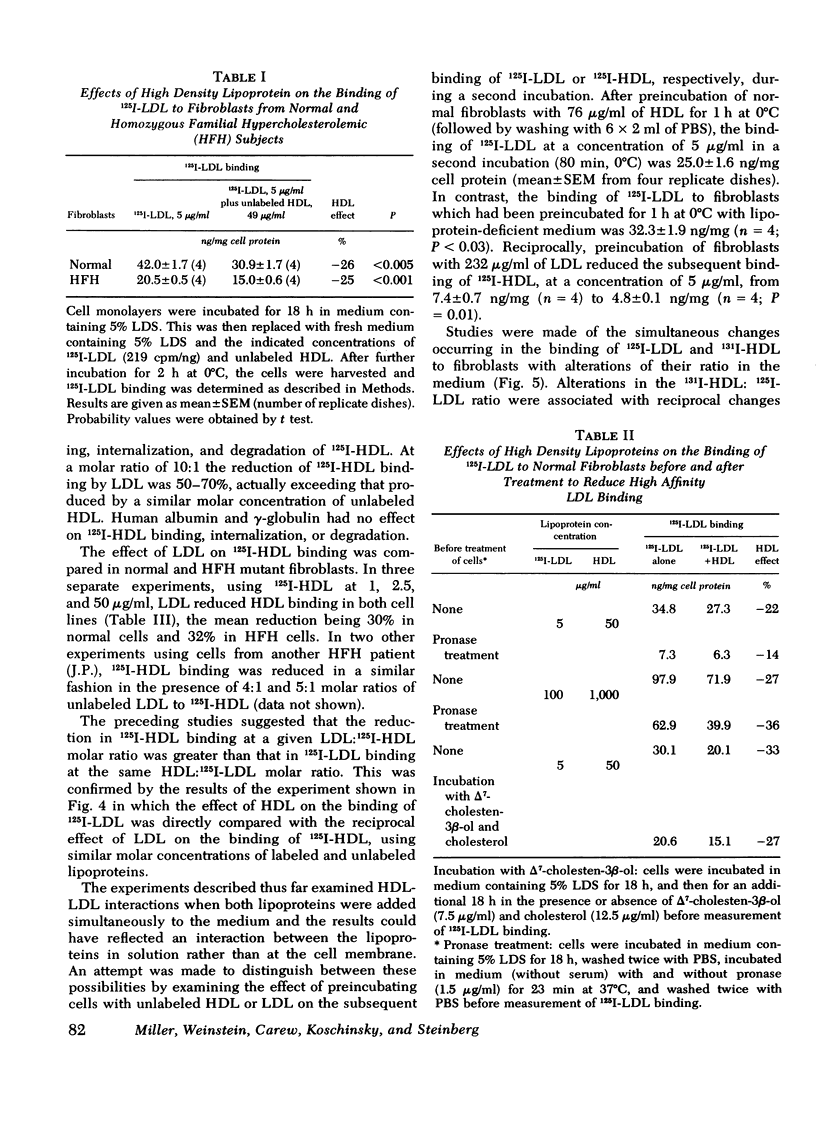
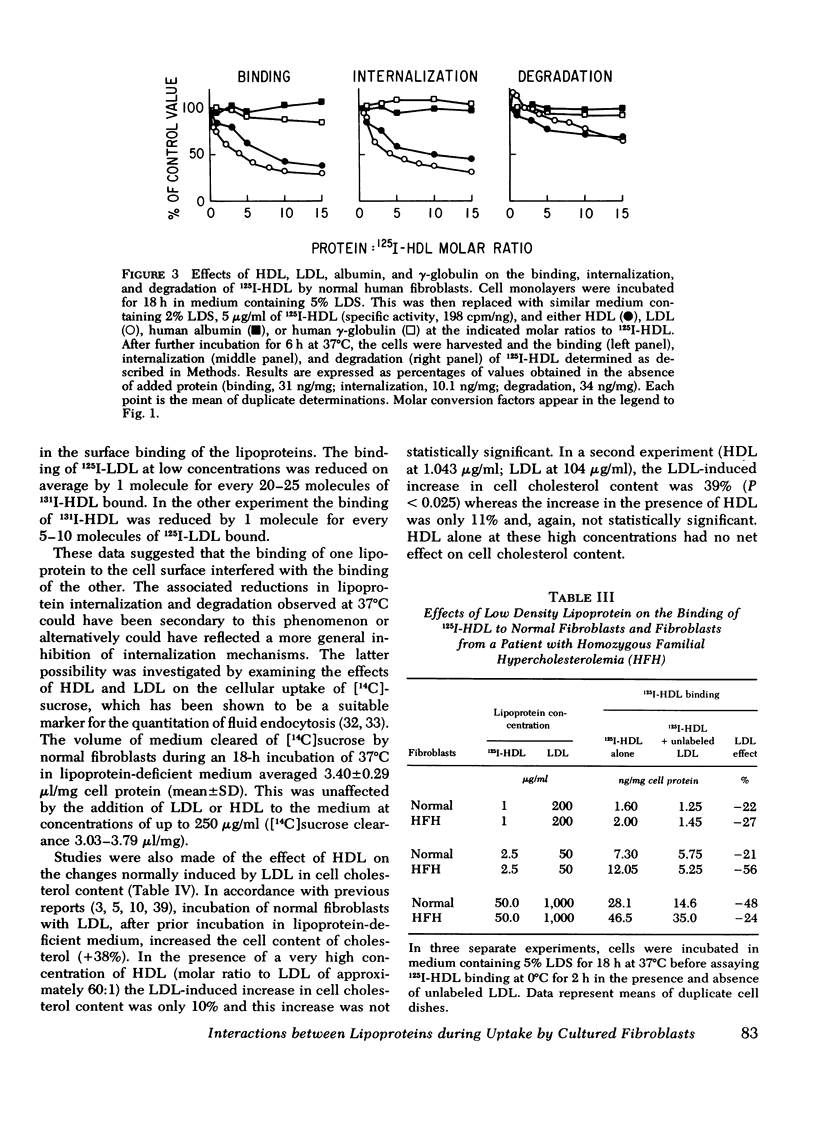
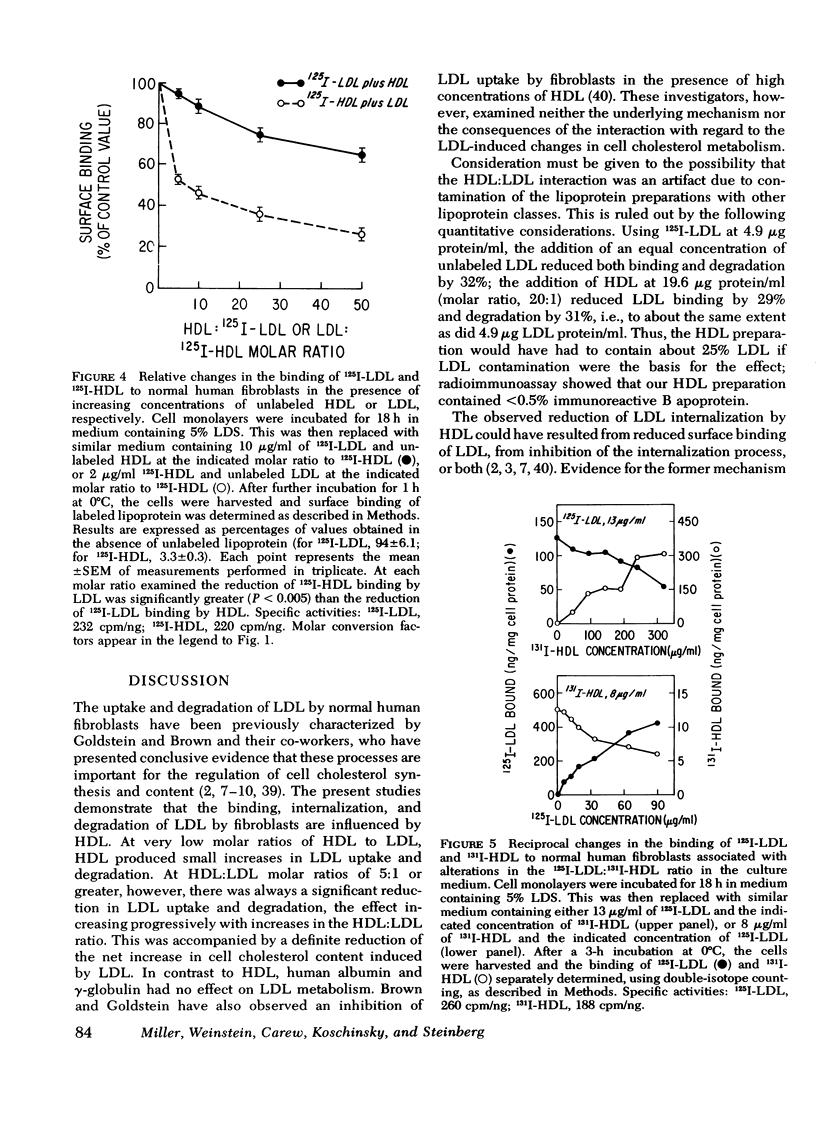




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAGDON J. H., HAVEL R. J., BOYLE E. Human serum lipoproteins. I. Chemical composition of four fractions. J Lab Clin Med. 1956 Jul;48(1):36–42. [PubMed] [Google Scholar]
- Becker G., Ashwood-Smith M. J. Endocytosis in Chinese hamster fibroblasts. Inhibition by glucose. Exp Cell Res. 1973 Dec;82(2):310–314. doi: 10.1016/0014-4827(73)90346-7. [DOI] [PubMed] [Google Scholar]
- Bersot T. P., Mahley R. W., Brown M. S., Goldstein J. L. Interaction of swine lipoproteins with the low density lipoprotein receptor in human fibroblasts. J Biol Chem. 1976 Apr 25;251(8):2395–2398. [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Receptor-dependent hydrolysis of cholesteryl esters contained in plasma low density lipoprotein. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2925–2929. doi: 10.1073/pnas.72.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- Carew T. E., Koschinsky T., Hayes S. B., Steinberg D. A mechanism by which high-density lipoproteins may slow the atherogenic process. Lancet. 1976 Jun 19;1(7973):1315–1317. doi: 10.1016/s0140-6736(76)92650-7. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Fröberg S. O. Concentration of cholesterol and triglycerides in skeletal muscle of healthy men and myocardial infarction patients. Acta Med Scand. 1973 Dec;194(6):553–558. doi: 10.1111/j.0954-6820.1973.tb19491.x. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. Physiological role of lecithin-cholesterol acyltransferase. Am J Clin Nutr. 1970 Aug;23(8):1129–1136. doi: 10.1093/ajcn/23.8.1129. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T. T., MacGee J., Morrison J. A., Glueck C. J. Quantitative analysis of cholesterol in 5 to 20 microliter of plasma. J Lipid Res. 1974 May;15(3):286–291. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRIS B., COURTICE F. C. Lipid exchange between plasma and lymph in experimental lipaemia. Q J Exp Physiol Cogn Med Sci. 1955 Apr;40(2):149–160. doi: 10.1113/expphysiol.1955.sp001106. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Miller N. E., Nestel P. J., Clifton-Bligh P. Relationships between plasma lipoprotein cholesterol concentrations and the pool size and metabolism of cholesterol in man. Atherosclerosis. 1976 May-Jun;23(3):535–547. doi: 10.1016/0021-9150(76)90013-7. [DOI] [PubMed] [Google Scholar]
- Moutafis C. D., Myant N. B. The metabolism of cholesterol in two hypercholesterolaemic patients treated with cholestyramine. Clin Sci. 1969 Oct;37(2):443–454. [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. V. Human serum lipoproteins and their interrelationships. Adv Biol Med Phys. 1967;11:109–158. doi: 10.1016/b978-1-4832-3107-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Rhoads G. G., Gulbrandsen C. L., Kagan A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med. 1976 Feb 5;294(6):293–298. doi: 10.1056/NEJM197602052940601. [DOI] [PubMed] [Google Scholar]
- Samuel P., Perl W. Long-term decay of serum cholesterol radioactivity: body cholesterol metabolism in normals and in patients with hyperlipoproteinemia and atherosclerosis. J Clin Invest. 1970 Feb;49(2):346–357. doi: 10.1172/JCI106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A., Granda J. L. Effects of ultracentrifugation on the human serum high-density (1.063 less than p less than 1.21 g/ml) lipoprotein. Biochemistry. 1966 Feb;5(2):446–455. doi: 10.1021/bi00866a008. [DOI] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Sniderman A. D., Carew T. E., Chandler J. G., Steinberg D. Paradoxical increase in rate of catabolism of low-density lipoproteins after hepatectomy. Science. 1974 Feb 8;183(4124):526–528. doi: 10.1126/science.183.4124.526. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Comparative uptake of rat and human serum low-density and high-density lipoproteins by rat aortic smooth muscle cells in culture. Circ Res. 1975 Mar;36(3):436–443. doi: 10.1161/01.res.36.3.436. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Stein O., Weinstein D. B., Stein Y., Steinberg D. Binding, internalization, and degradation of low density lipoprotein by normal human fibroblasts and by fibroblasts from a case of homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1976 Jan;73(1):14–18. doi: 10.1073/pnas.73.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Y., Glangeaud M. C., Fainaru M., Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975 Jan 24;380(1):106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]



