Abstract
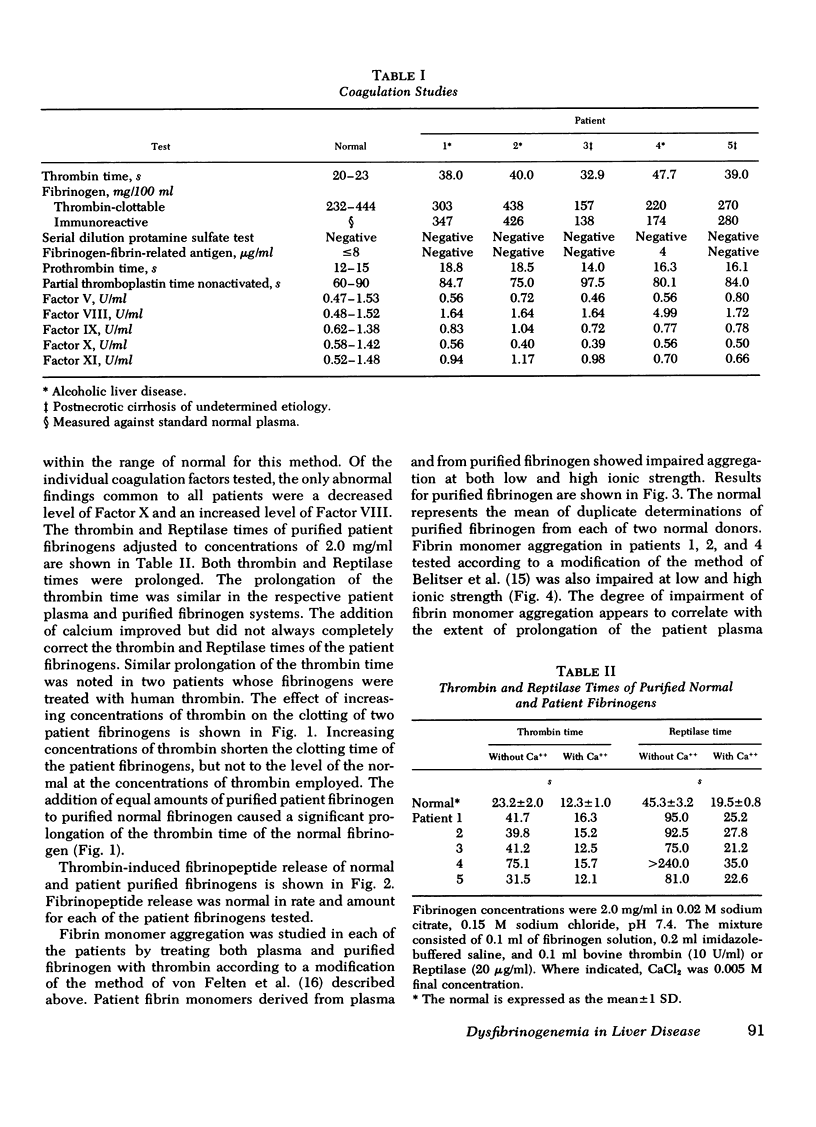
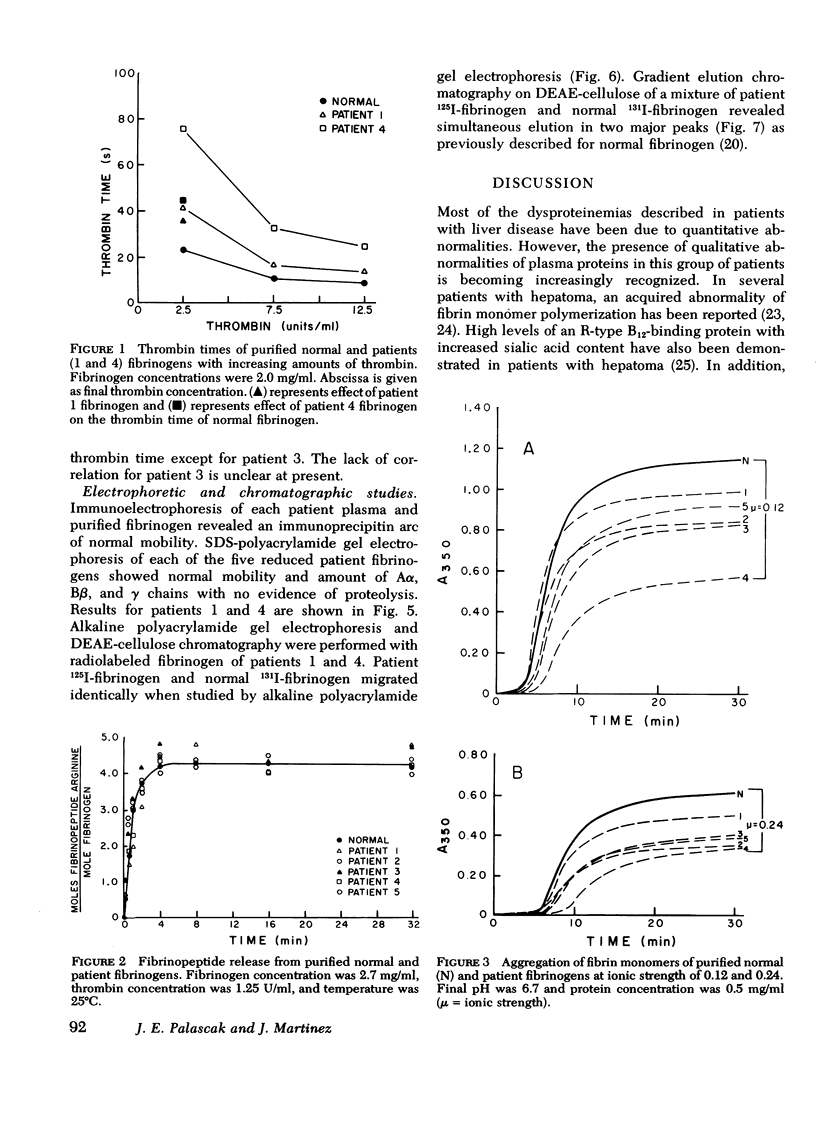
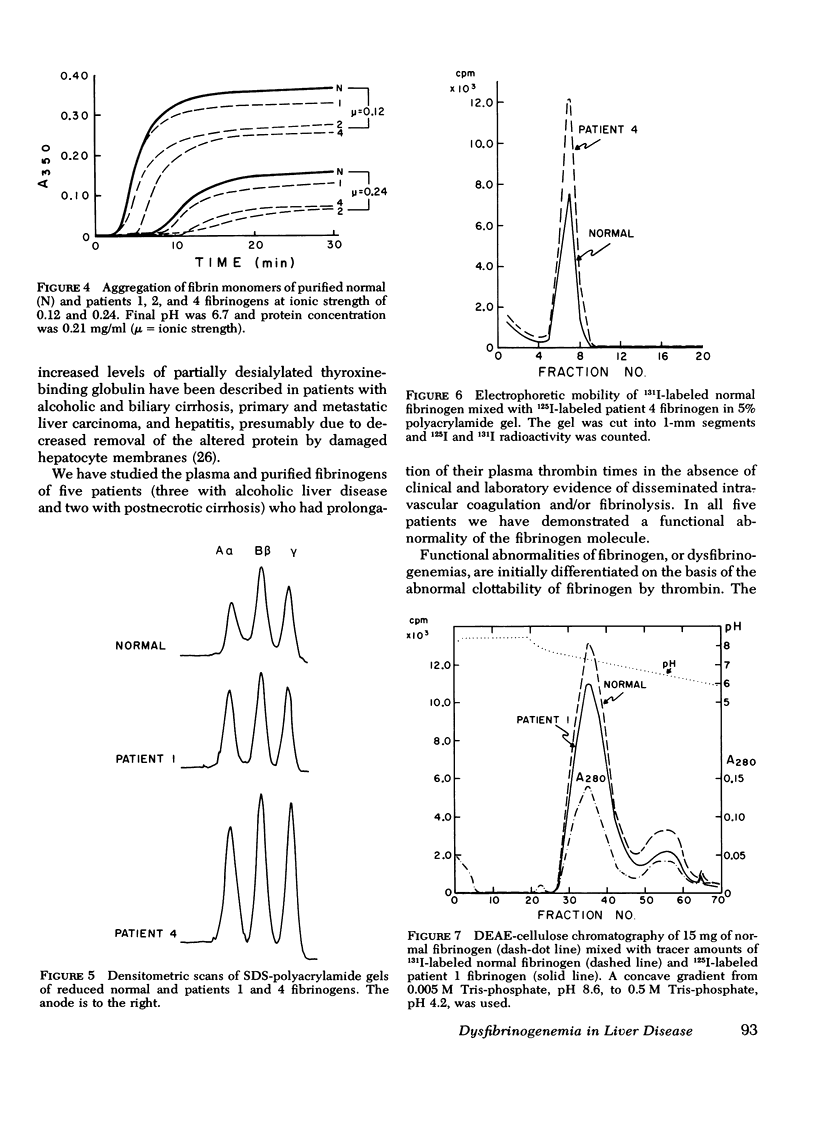
To test the possibility that a functionally abnormal fibrinogen may exist in some patients with liver disease, we studied the plasma and purified fibrinogens of five patients whose plasma thrombin times were prolonged at least 40% over normal controls. In no patient was there evidence of disseminated intravascular coagulation and/or fibrinolysis. No abnormalities were detected by immunoelectrophoresis of plasmas or purified fibrinogens. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of reduced patient fibrinogens showed normal mobility and amount of Aα, Bβ, and γ chains. Alkaline polyacrylamide gel electrophoresis and gradient elution, DEAE-cellulose chromatography of admixtures of radio-iodinated patient 125I-fibrinogen and normal 131I-fibrinogen showed identical mobility in the gel and simultaneous elution from the column, respectively. Thrombin and Reptilase (Abbott Scientific Products Div., Abbott Laboratories, South Pasadena, Calif.) times of purified patient fibrinogens were prolonged, and calcium ions improved but did not completely correct these defects. Increasing amounts of thrombin progressively shortened the clotting times of patient fibrinogens but not to the level of normal. Addition of equal amounts of patient fibrinogen to normal fibrinogen resulted in a prolongation of the thrombin time of the normal protein. Thrombin-induced fibrinopeptide release was normal. Fibrin monomers prepared from patient plasmas and purified fibrinogens demonstrated impaired aggregation at low (0.12) and high (0.24) ionic strength. These studies demonstrate that some patients with liver disease and prolonged plasma thrombin times have a dysfibrinogenemia functionally characterized by an abnormality of fibrin monomer polymerization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom A. L. Intravascular coagulation and the liver. Br J Haematol. 1975 May;30(1):1–7. doi: 10.1111/j.1365-2141.1975.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Boyer M. H., Shainoff J. R., Ratnoff O. D. Acceleration of fibrin polymerization by calcium ions. Blood. 1972 Mar;39(3):382–387. [PubMed] [Google Scholar]
- Burger R. L., Waxman S., Gilbert H. S., Mehlman C. S., Allen R. H. Isolation and characterization of a novel vitamin B12-binding protein associated with hepatocellular carcinoma. J Clin Invest. 1975 Nov;56(5):1262–1270. doi: 10.1172/JCI108202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DENSON K. W. The specific assay of Prower-Stuart factor and factor VII. Acta Haematol. 1961 Feb;25:105–120. doi: 10.1159/000206523. [DOI] [PubMed] [Google Scholar]
- Deykin D., Cochios F., DeCamp G., Lopez A. Hepatic removal of activated factor X by the perfused rabbit liver. Am J Physiol. 1968 Feb;214(2):414–419. doi: 10.1152/ajplegacy.1968.214.2.414. [DOI] [PubMed] [Google Scholar]
- Deykin D. The role of the liver in serum-induced hypercoagulability. J Clin Invest. 1966 Feb;45(2):256–263. doi: 10.1172/JCI105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS B. C., STRANSKY A. A quick and accurate method for the determination of fibronogen in plasma. J Lab Clin Med. 1961 Sep;58:477–488. [PubMed] [Google Scholar]
- FLETCHER A. P., BIEDERMAN O., MOORE D., ALKJAERSIG N., SHERRY S. ABNORMAL PLASMINOGEN-PLASMIN SYSTEM ACTIVITY (FIBRINOLYSIS) IN PATIENTS WITH HEPATIC CIRRHOSIS: ITS CAUSE AND CONSEQUENCES. J Clin Invest. 1964 Apr;43:681–695. doi: 10.1172/JCI104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Givelber H. M., Shainoff J. R., Finlayson J. S. Fibrinogen Bethesda: a congenital dysfibrinogenemia with delayed fibrinopeptide release. J Clin Invest. 1971 Sep;50(9):1819–1830. doi: 10.1172/JCI106673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDISTY R. M., MACPHERSON J. C. A one-stage factor VIII (antihaemophilic globulin) assay and its use on venous and capillary plasma. Thromb Diath Haemorrh. 1962 May 15;7:215–228. [PubMed] [Google Scholar]
- HOROWITZ H. I., WILCOX W. P., FUJIMOTO M. M. Assay of plasma thromboplastin antecedent (PTA) with artificially depleted normal plasma. Blood. 1963 Jul;22:35–43. [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Green A. M., Pensky J., Williams S., Zinn A., Carlson D. M. Measurement of circulating desialylated glycoproteins and correlation with hepatocellular damage. J Clin Invest. 1974 Sep;54(3):555–562. doi: 10.1172/JCI107792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Holburn R. R., Shapiro S. S., Erslev A. J. Fibrinogen Philadelphia. A hereditary hypodysfibrinogenemia characterized by fibrinogen hypercatabolism. J Clin Invest. 1974 Feb;53(2):600–611. doi: 10.1172/JCI107595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Palascak J., Peters C. Functional and metabolic properties of human asialofibrinogen. J Lab Clin Med. 1977 Feb;89(2):367–377. [PubMed] [Google Scholar]
- Melliger E. J. Detection of fibrinogen degradation products by use of antibody coated latex particles. The possibilities and limits of the method. Thromb Diath Haemorrh. 1970 May 31;23(2):211–227. [PubMed] [Google Scholar]
- Merskey C., Kleiner G. J., Johnson A. J. Quantitative estimation of split products of fibrinogen in human serum, relation to diagnosis and treatment. Blood. 1966 Jul;28(1):1–18. [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A., Galanakis D. Human fibrinogen heterogeneities. I. Structural and related studies of plasma fibrinogens which are high solubility catabolic intermediates. J Biol Chem. 1972 Aug 25;247(16):5210–5219. [PubMed] [Google Scholar]
- Niewiarowski S., Gurewich V. Laboratory identification of intravascular coagulation. The serial dilution protamine sulfate test for the detection of fibrin monomer and fibrin degradation products. J Lab Clin Med. 1971 Apr;77(4):665–676. [PubMed] [Google Scholar]
- Ratnoff O. D., Forman W. B. Criteria for the differentiation of dysfibrinogenemic states. Semin Hematol. 1976 Apr;13(2):141–157. [PubMed] [Google Scholar]
- Roberts H. R., Cederbaum A. I. The liver and blood coagulation: physiology and pathology. Gastroenterology. 1972 Aug;63(2):297–320. [PubMed] [Google Scholar]
- Tytgat G. N., Collen D., Verstraete M. Metabolism of fibrinogen in cirrhosis of the liver. J Clin Invest. 1971 Aug;50(8):1690–1701. doi: 10.1172/JCI106658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghe R., van Damme B., Molla A., Vermylen J. Dysfibrinogenaemia associated with primary hepatoma. Scand J Haematol. 1972;9(5):451–458. doi: 10.1111/j.1600-0609.1972.tb00968.x. [DOI] [PubMed] [Google Scholar]
- WOLF P. A modification for routine laboratory use of Stefanini's method of estimating factor V activity in human oxalated plasma. J Clin Pathol. 1953 Feb;6(1):34–38. doi: 10.1136/jcp.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls W. D., Losowsky M. S. The hemostatic defect of liver disease. Gastroenterology. 1971 Jan;60(1):108–119. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- von Felten A., Frick P. G., Straub P. W. Studies on fibrin monomer aggregation in congenital dysfibrinogenaemia (fibrinogen "Zürich"): separation of a pathological from a normal fibrin fraction. Br J Haematol. 1969 Apr;16(4):353–361. doi: 10.1111/j.1365-2141.1969.tb00412.x. [DOI] [PubMed] [Google Scholar]
- von Felten A., Straub P. W., Frick P. G. Dysfibrinogenemia in a patient with primary hepatoma. First observation of an acquired abnormality of fibrin monomer aggregation. N Engl J Med. 1969 Feb 20;280(8):405–409. doi: 10.1056/NEJM196902202800802. [DOI] [PubMed] [Google Scholar]


