Abstract
Introduction
Neurologic decompression sickness (NDCS) can affect high-altitude pilots, causing variable central nervous system symptoms. Five recent severe episodes prompted further investigation.
Methods
We report the hyperintense white matter (HWM) lesion imaging findings in 50 U-2 pilot volunteers, and compare 12 U-2 pilots who experienced clinical NDCS to 38 U-2 pilots who did not. The imaging data were collected using a 3T magnetic resonance imaging scanner and high-resolution (1-mm isotropic) three-dimensional fluid-attenuated inversion recovery sequence. Whole-brain and regional lesion volume and number were compared between groups.
Results
The NDCS group had significantly increased whole brain and insular volumes of HWM lesions. The intergroup difference in lesion numbers was not significant.
Conclusion
A clinical episode of NDCS was associated with a significant increase in HWM lesion volume, especially in the insula. We postulate this to be due to hypobaric exposure rather than hypoxia since all pilots were maintained on 100% oxygen throughout the flight. Further studies will be necessary to better understand the pathophysiology underlying these lesions.
Keywords: hyperintense white matter lesions, neurocognitive impairment, neurologic decompression sickness, high altitude, U-2 pilot
Neurologic decompression sickness (NDCS) is a common but underreported condition that affects high-altitude pilots ( 8 ). Neurologic symptoms associated with NDCS include syncope, nausea, disturbances of equilibrium and coordination, large sensory and motor tract dysfunction, amnesia, aphasia, hallucinations, tremor, and headaches (3). Variable degrees of neurological recovery may occur. The number of severe NDCS episodes in high-altitude U-2 pilots has increased in recent years, including five near-fatalities. This increase is potentially related to an increased operational tempo in these pilots, although this remains unproven (19). In all five cases, significant neurological symptoms that included neurocognitive impairments with confusion, aphasia, and memory loss and coordination impairment with ataxia and tremor were reported. Notably, no acute clinical symptoms or findings consistent with spinal cord involvement/injury were noted. This dichotomy in clinical signs suggests these pilots may have experienced brain injury as a consequence of the NDCS episode. Here, we hypothesize that pilots who suffered NDCS will demonstrate evidence for brain injury by having a higher number and volume of hyperintense white matter (HWM) cerebral lesions than those pilots who did not experience NDCS.
The number and volume of HWM regions are sensitive markers of cerebral health, commonly used to study the extent of the cerebral injury (9). Healthy cerebral white matter tracts are myelinated with compounds containing long-chain fatty acids with very short T2-relaxation time and thus appear dark on T2-weighted images. Local edema, often associated with degradation of the myelin sheath, results in localized accumulation of extracellular water, which leads to an increased signal intensity on a T2-weighted image. HWM lesions also form in normal aging, where they begin to occur during midadulthood (fourth-fifth decade of life). In both normal subjects and patients who suffered brain injury, the number and volume of HWM lesions are correlated with a decline in cerebral integrity (24), reduction in cerebral white matter and gray matter volumes (10,37), cerebral blood flow (26), and cerebral glucose metabolism (22). Increasing numbers and volumes of HWM regions have also been linked to cognitive declines, particularly in executive functioning ( 23 ), processing speed ( 35 ), and general cognitive status (14), and were correlated with the severity of neurocognitive deficits in neuropsychiatric and neurological disorders (31). The etiology of HWM is nonspecific and is commonly associated with cerebral ischemia and disruptions of cerebral circulation ( 30 ). Histopathological findings indicate there are two distinct types of HWM lesions: subcortical and ependymal. Subcortical HWM regions are more closely associated with ischemic factors (13). In contrast, periventricular ependymal HWM lesions are thought to be of nonischemic origin and potentially produced by pulse-wave encephalopathy (5,6,17). This condition refers to the microtears in the ependymal lining caused by the pulsa-tile movements of ventricular cerebrospinal fluid (CSF) (6,28,29).
Here we aimed to better understand the prevalence, the potential contributing factors, and the clinical implications of HWM lesions in a voluntary study performed in 50 United States Air Force (USAF) U-2 pilots, 12 of whom suffered clinical NDCS. In addition to our primary hypothesis, we further hypothesized that clinical NDCS will be associated with higher prevalence and volume of subcortical, rather than ependymal, lesions because of the differences in the pathogenic mechanism between these two types of lesions. This hypothesis was tested by analyzing the location of the lesions by both their type (subcortical and ependy-mal) and their number and volume within cerebral lobes.
METHODS
Subjects
This study did not constitute human research as determined by the Air Force Research Laboratory Institutional Review Board (4/2011) and the University of Texas Health Sciences Center San Antonio Institutional Review Board (5/2011). Pilots were recruited with strict adherence to Department of Defense Instruction 3216.02 guidelines. All high-altitude U-2 pilots currently on active duty in the USAF were invited to participate. Pilot participation was voluntary without commander involvement or knowledge. The pilots provided informed consent prior to participation. All pilots were healthy at the time of testing, meeting USAF Flying Class II standards (36). An indepth medical and flight history was obtained from each pilot including identification of any episodes that might constitute type I (mild) decompression sickness or type II (severe or neurological) decompression sickness. Each pilot underwent 2 h of neurocognitive testing; in some pilots comparison to identical neurocognitive testing performed at time of entry into undergraduate pilot training was possible. Finally, each pilot underwent a 1-h-long magnetic resonance imaging (MRI) examination.
Imaging
Structural MRI data were collected at the Research Imaging Institute using a Siemens 3T Tim Trio scanner equipped with multichannel phase array coil. T2-weighted, three-dimensional (3D), high-resolution (isotropic 1-mm), fluid-attenuated inversion recovery (FLAIR) data were collected using a turbo-spin-echo sequence with the following parameters: TR/TE/TI/Flip angle/ETL = 5 s/353 ms/1.8 s/180°/221. This 3D FLAIR protocol was specifically designed to overcome the limitations of a two-dimensional, thick-slice (5- to 10-mm) clinical imaging protocol and to permit increased detection of smaller lesions and accurate tracing of lesion boundaries (20,24). This 3D FLAIR sequence uses a nonselective inversion radio frequency (RF) pulse to suppress CSF pulsation artifacts to reduce false-negative hyperintense artifacts that can be seen near CSF-containing structures in the two-dimensional FLAIR sequences (2).
In addition to FLAIR data, high-resolution (isotropic 0.8-mm, voxel size = 0.5 mm 3) T1-weighted data were collected with the following sequence parameters: TE/ TR/TI = 3.04/2100/785 ms, flip angle = 11°. A retrospective motion-correction technique (21) was used to reduce subject motion-related artifacts.
Image Analyses
Measurement of the number and volume of the HWM lesions from FLAIR images is described elsewhere (25). Briefly, FLAIR images were preprocessed by removal of nonbrain tissue using FSL BET (brain extraction tool), freely available from the Oxford Centre for Functional MRI of the Brain (FMRIB) (33) (Fig. 1A). Next, FLAIR images for individual subjects were registered to their corresponding T1-weighted images using FSL FLIRT (FMRIB ’s linear image registration tool) (34) (Fig. 1B). The T1-weighted images were then registered to a common, Talairach-atlas-based stereotactic frame using FSL FLIRT (34) and nine-parameter (three each for rotation, translation, and scaling) global normalization transformation. The purpose of this step is to reduce interindi-vidual anatomical variance in the global brain size, shape, and orientation and to permit the use of automated labeling approaches by using a digital brain atlas (27) (Fig. 1C). Next, all images were corrected for radio frequency (RF) inhomogeneity artifact using the FSL BET method with default parameters. RF inhomogeneity artifact manifests itself as a low-frequency variation of MRI image intensity that impedes intensity-based image analysis unless corrected. HWM regions were then manually delineated in 3D-space using in-house software (http://ric.uthscsa.edu/mango) by an experienced neuroanatomist with high intrarater (r 2 >0.9) test-retest reproducibility who was blind to subjects’ age and diagnosis status. Interrater correlation was also high (rho = 0.924). During the labeling, HWM regions were coded as ependymal regions, contiguous with CSF structures, and as subcortical regions as previously described (17). Finally, the volume and location of HWM lesions were analyzed using the boundaries for five cerebral lobes and insula extracted from the digital Talairach atlas (Fig. 1D).
Fig. 1.
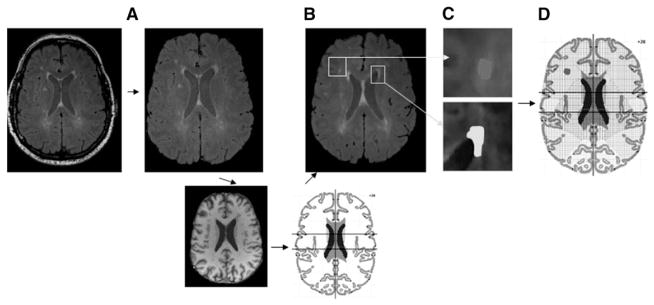
HWM lesions data processing pipeline included removal of nonbrain tissue (A), global spatial normalization to the corresponding T1-weighted image that was registered to Talairach reference frame and correction for RF homogeneity (B), delineation of subcortical (yellow) and epen-dymal (green) lesions (C), Talairach-atlas-based analysis of regional distribution of HWM lesions by cerebral lobe (D).
Statistical Analysis
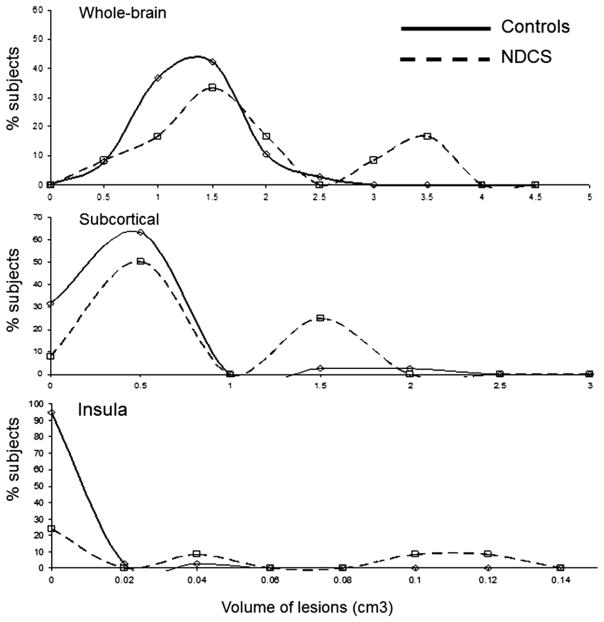
Group-wise differences in age and flight hours were assessed using a two-tailed t-test. The same method could not be used to compare differences in the number and volume of the HWM lesions because these data for NDCS subjects were not normally distributed (Fig. 2). A single-tailed Mann-Whitney U-test (also known as the Mann-Whitney-Wilcoxon or Wilcoxon rank-sum test) was therefore used to perform the intergroup comparisons on HWM measurements. This is a nonparametric test that assumes no specific distribution of the data and estimates the probability that one sample is stochastically greater than the other. We considered a P-value ≤ 0.05 statistically significant, demonstrating an association between a clinical NDCS event and an increase in HWM lesion volume and number. A P-value of 0.05 < P ≤ 0.10 suggests a potentially significant finding that is limited by our small sample size.
Fig. 2.
Histogram of the volume of the HWM lesions in NDCS and normal controls.
RESULTS
No significant differences were observed between the pilots who had experienced an episode of NDCS and those who had not in age (37.4 ± 5.2 yr vs. 38.9 ± 6.1, P = 0.46), total high-altitude flight hours (774 ± 499 h vs. 743 ± 408, P = 0.84), or average high-flight hours per month over their U-2 career (18.1 ± 6.4 h/mo vs. 16.5 ± 7.2, P = 0.56). No difference was noted in the presence of mild hypertension or mild hyperlipidemia. No pilot had a history of significant head injury, significant scuba diving history, episode of decompression illness associated with diving, or high-altitude exposure other than that associated with USAF flight duties.
HWM lesions were clearly visualized on 3D FLAIR MRI ( Fig. 3 and 4 ). The average and lobar-based HWM lesion number and volume (total, subcortical, ependymal) are provided in Table I. The NDCS pilots demonstrated a significantly higher total HWM lesion volume (P = 0.026) compared to the non-NDCS pilots but not a significant increase in total lesion count (P = 0.120). Analysis of the lesion by type (subcortical vs. ependy-mal) did not demonstrate a significant difference between NDCS pilots and non-NDCS pilots (P = 0.059). Examination of regional measurements revealed pilots who experienced NDCS had significantly higher number and volume of insular subcortical lesions (P = 0.020 and P = 0.018, respectively).
Fig. 3.
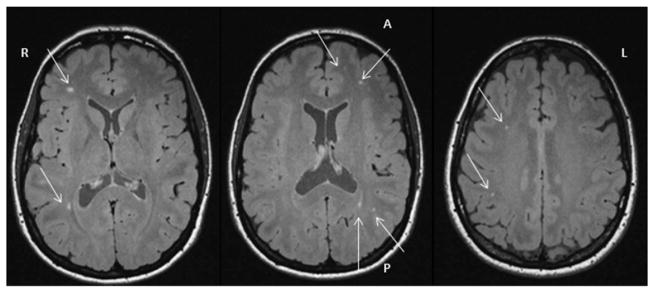
Multiple HWM lesions in pilot 1.
Fig. 4.
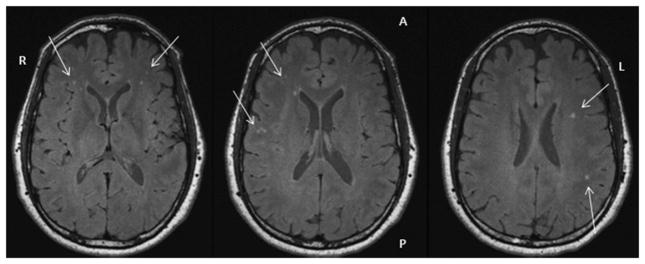
Multiple HWM lesions in pilot 2.
TABLE I.
WHOLE-BRAIN AND REGIONAL MEASUREMENTS FOR THE NUMBER AND VOLUME OF HWM LESIONS.
| WHM Lesions | All Pilots (N = 50)
|
Pilots with NDCS (N = 12)
|
Pilots without NDCS (N = 38)
|
Z (p) (One-Tailed Mann-Whitney) |
|---|---|---|---|---|
| Average ± 1 SD | Average ± 1 SD | Average ± 1 SD | ||
| N whole-brain lesions | 8.20 ± 7.33 | 12.25 ± 12.23 | 6.92 ± 4.09 | −1.176 (0.120) |
| V whole-brain lesions (cm 3) | 1.05 ± 0.54 | 1.37 ± 0.79 | 0.95 ± 0.37 | −1.954 (0.026) |
| By Lesion Type | ||||
| Nsubcortical | 3.76 ± 6.10 | 7.25 ± 10.66 | 2.66 ± 2.85 | −1.041 (0.149) |
| Vsubcortical(cm 3) | 0.07 ± 0.12 | 0.13 ± 0.14 | 0.054 ± 0.11 | −1.612 (0.059) |
| Nependymal | 4.44 ± 2.48 | 5.00 ± 2.55 | 4.26 ± 2.42 | −0.997 (0.160) |
| Vependymal(cm 3) | 0.98 ± 0.54 | 1.24 ± 0.84 | 0.89 ± 0.37 | −1.204 (0.115) |
| By Region | ||||
| Nsubcorticalfrontal lobe | 2.28 ± 3.72 | 4.50 ± 6.32 | 1.58 ± 1.89 | −1.108 (0.134) |
| Vsubcortical frontal lobe (cm 3) | 0.03 ± 0.06 | 0.07 ± 0.10 | 0.02 ± 0.03 | −1.306 (0.096) |
| Nsubcortical parietal lobe | 0.52 ± 0.17 | 1.25 ± 3.06 | 0.29 ± 0.65 | −0.487 (0.313) |
| Vsubcortical parietal lobe (cm 3) | 0.005 ± 0.018 | 0.014 ± 0.034 | 0.002 ± 0.005 | −0.580 (0.281) |
| Nsubcortical temporal lobe | 0.30 ± 0.70 | 0.58 ± 1.12 | 0.21 ± 0.47 | −1.134 (0.129) |
| Vsubcortical temporal lobe (cm 3) | 0.004 ± 0.009 | 0.008 ± 0.014 | 0.003 ± 0.006 | −1.332 (0.092) |
| Nsubcortical occipital lobe | 0.06 ± 0.31 | 0.17 ± 0.55 | 0.03 ± 0.16 | −0.903 (0.183) |
| Vsubcortical occipital lobe (cm 3) | 0.001 ± 0.004 | 0.003 ± 0.009 | 0.0001 ± 0.0005 | −0.903 (0.183) |
| Nsubcortical limbic lobe | 0.22 ± 0.51 | 0.17 ± 0.37 | 0.24 ± 0.53 | −0.204 (0.419) |
| Vsubcortical limbic lobe (cm 3) | 0.008 ± 0.031 | 0.003 ± 0.007 | 0.009 ± 0.036 | −0.136 (0.446) |
| Nsubcortical insula | 0.16 ± 0.54 | 0.50 ± 0.96 | 0.05 ± 0.22 | −2.051 (0.020) |
| Vsubcortical insula (cm 3) | 0.006 ± 0.021 | 0.020 ± 0.038 | 0.001 ± 0.005 | −2.094 (0.018) |
DISCUSSION
Our study demonstrated an elevation in whole-brain volume (P = 0.026) but not whole-brain number of HWM lesions in pilots who experienced clinical NDCS when compared to a matching group of pilots who had not. In agreement with our initial hypothesis, pilots who experienced NDCS had a higher number and volume of subcortical HWM lesions in the insular white matter regions (P = 0.020; P = 0.018). Histopathological findings suggest subcortical HWM lesions are produced by ischemic and/or neuroinflamatory etiologies (4,6,7,13). Therefore, elevation of subcortical HWM lesions in NDCS might be associated with a barometric-pressure-related gas microemboli leading to loss of permeability/occlusion of small cerebral vessels and subsequent immune-system-mediated gliosis. If indeed this is the mechanism, this might explain the delayed onset and/or the clinical relapse in the first 2 wk observed in some pilots following initial successful hyperbaric treatment.
Several previous studies reported imaging findings associated with NDCS in high-altitude mountain climbing (1,12) and deep sea divers (11,15). High-altitude mountain climbers have been reported as having an increased preponderance of subcortical HWM lesions and enlarged Virchow-Robin spaces relative to normal controls. Elevated numbers of frontal and parietal HWM lesions were also reported in deep sea divers compared to age-matched controls, though the mechanism of this pathology remains speculative. Previous reports suggested increased number and volume of lesions were potentially related to extreme changes in barometric pressure, although hypoxia was also named among potential contributing factors. The pilots in our study were maintained on 100% oxygen with aircraft cabin pressures in the range of 8534–8839 m (28,000–29,000 ft). Previous studies in altitude chambers demonstrated a 100% oxygen inhaled concentration maintains normal arterial oxygenation to altitudes up to 10,060 m (33,000 ft) (16), making hypoxia an unlikely contributing factor in this study.
The clinical significance of these lesions remains unknown. The association between executive function and episodic memory with the frontal volume of HWM lesions has previously been reported by this and other groups (23,32,35). One U-2 pilot developed mild permanent executive processing cognitive impairment, presumably due to multiple large frontal and parietal HWM lesions, assumed to be secondary to NDCS (18). The pilots enrolled in this study reported improvement of their cognitive functioning back to baseline within 1–12 mo following the clinical NDCS episode. Additional studies examining the relationship between HWM lesions and cognitive findings are ongoing in this sample.
While this study demonstrates a significant increase in HWM lesion volume following an episode of clinical NDCS, still unknown is whether or not there is an increase in HWM lesions in all high-altitude pilots exposed to severe hypobaric conditions when compared to a matched control group; an ongoing companion study will attempt to answer this question. Also unknown is the effect of severe hypobaric conditions on more sensitive measurements of cerebral integrity derived from the MRS and diffusion tensor imaging data; analysis of these data is in progress and will be subsequently reported. This study demonstrates a correlation between subcortical HWM lesions and clinical NDCS but does not provide a causative explanation. We speculate there may be an inflammatory component induced by exposure to extreme hypobaric conditions, but why this should have a predilection for the insular regions remains unclear.
CONCLUSION
Clinical neurologic decompression sickness following extreme altitude exposure is associated with increased hyperintense white matter lesion volume with a predilection surprisingly for insular regions. This pattern of HWM lesion distribution appears unique and related to clinical NDCS. This pattern is distinctively different from that in U-2 pilots who have not experienced clinical NDCS. Still unknown are the exact precipitating factors, the mechanism of injury, the micropathological changes, and the clinical implications both now and in the future. Current ongoing studies are addressing these issues.
Acknowledgments
The authors would like to thank Mr. Jared Haynes for statistical analysis and Ms. Elaine “Sandy” Kawano for editorial assistance.
The authors would like to thank Gregory L. Hundemer, M.D., Kihyeok Lee, M.D., and Julie M. Foreman, BSOE, 9th Medical Group; Lance M. Nussbaum, M.D., 12th Reconnaissance Squadron; and Andrew D. Woodrow, M.Sc., 9th Physiological Support Squadron, Beale AFB, CA, for their assistance in facilitating the study for the U-2 pilot volunteers.
This research was supported by a United States Air Force Surgeon General grant (Log I-11-44) to S.A.M. and a National Institute of Biomedical Imaging and Bioengineering grant (K01 EB006395) to P.V.K.
Contributor Information
Stephen A. McGuire, U.S. Air Force School of Aerospace Medicine, Aerospace Medicine Consultation Division, Wright-Patterson AFB, OH; Department of Neurology, University of Texas Health Sciences Center, San Antonio, TX
Paul M. Sherman, Department of Neuroradiology, 59th Medical Wing, Lackland AFB, TX
Anthony C. Brown, Department of Neurology, University of Texas Health Sciences Center, San Antonio, TX
Andrew Y. Robinson, Department of Neurology, 59th Medical Wing, Lackland AFB, TX
David F. Tate, Research Imaging Institute, University of Texas Health Sciences Center, San Antonio, TX
Peter T. Fox, Research Imaging Institute, University of Texas Health Sciences Center, San Antonio, TX
Peter V. Kochunov, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore, MD
References
- 1.Anooshiravani M, Dumont L, Mardirosoff C, Soto-Debeuf G, Delavelle J. Brain magnetic resonance imaging (MRI) and neurological changes after a single high altitude climb. Med Sci Sports Exerc. 1999;31:969–72. doi: 10.1097/00005768-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi R, Caruthers SD, Janardhan V, Wasay M. Intraventricular CSF pulsation artifact on fast fluid-attenuated inversion-recovery MR images: analysis of 100 consecutive normal studies. AJNR Am J Neuroradiol. 2000;21:503–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Balldin UI, Pilmanis AA, Webb JT. Central nervous system decompression sickness and venous gas emboli in hypobaric conditions. Aviat Space Environ Med. 2004;75:969–72. [PubMed] [Google Scholar]
- 4.Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann N Y Acad Sci. 2004;1012:224–36. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- 5.Bateman GA. Pulse-wave encephalopathy: a comparative study of the hydrodynamics of leukoaraiosis and normal-pressure hydrocephalus. Neuroradiology. 2002;44:740–8. doi: 10.1007/s00234-002-0812-0. [DOI] [PubMed] [Google Scholar]
- 6.Bateman GA. Pulse wave encephalopathy: a spectrum hypothesis incorporating Alzheimer ’ s disease, vascular dementia and normal pressure hydrocephalus. Med Hypotheses. 2004;62:182–7. doi: 10.1016/S0306-9877(03)00330-X. [DOI] [PubMed] [Google Scholar]
- 7.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 8.Bendrick GA, Ainscough MJ, Pilmanis AA, Bisson RU. Prevalence of decompression sickness among U-2 pilots. Aviat Space Environ Med. 1996;67:199–206. [PubMed] [Google Scholar]
- 9.Ding K, Marquez de la Plata C, Wang JY, Mumphrey M, Moore C, et al. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J Neuro-trauma. 2008;25:1433–40. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du AT, Schuff N, Chao LL, Kornak J, Ezekiel F, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26:553–9. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Erdem I, Yildiz S, Uzun G, Sonmez G, Senol MG, et al. Cerebral white-matter lesions in asymptomatic military divers. Aviat Space Environ Med. 2009;80:2–4. doi: 10.3357/asem.2234.2009. [DOI] [PubMed] [Google Scholar]
- 12.Fayed N, Modrego PJ, Morales H. Evidence of brain damage after high-altitude climbing by means of magnetic resonance imaging. Am J Med. 2006;119:168.e1–6. doi: 10.1016/j.amjmed.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 13.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci. 2008;273:3–9. doi: 10.1016/j.jns.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Gempp E, Sbardella F, Stephant E, Constantin P, De Maistre S, et al. Brain MRI signal abnormalities and right-to-left shunting in asymptomatic military divers. Aviat Space Environ Med. 2010;81:1008–12. doi: 10.3357/asem.2786.2010. [DOI] [PubMed] [Google Scholar]
- 16.Harding RM. Pressure changes and hypoxia in aviation. In: Pandolf KB, Burr RE, editors. Medical aspects of harsh environments. Vol. 2. Washington, DC: Office of The Surgeon General, Department of the Army; 2002. pp. 984–1012. [Google Scholar]
- 17.Henry Feugeas MC, De Marco G, Idy Peretti I, Godon-Hardy S, Fredy D, Schouman Claeys E. Age-related cerebral white matter changes and pulse-wave encephalopathy: observations with three-dimensional MRI. Magn Reson Imaging. 2005;23:929–37. doi: 10.1016/j.mri.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Jersey SL, Baril RT, McCarty RD, Millhouse CM. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64–8. doi: 10.3357/asem.2303.2010. [DOI] [PubMed] [Google Scholar]
- 19.Jersey SL, Hundemer GL, Stuart RP, West KN, Michaelson RS, Pilmanis AA. Neurological altitude decompression sickness among U-2 pilots: 2002–2009. Aviat Space Environ Med. 2011;82:673–82. doi: 10.3357/asem.2851.2011. [DOI] [PubMed] [Google Scholar]
- 20.Kochunov P, Glahn D, Winkler A, Duggirala R, Olvera RL, et al. Analysis of genetic variability and whole genome linkage of whole-brain, subcortical, and ependymal hyperintense white matter volume. Stroke. 2009;40:3685–90. doi: 10.1161/STROKEAHA.109.565390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochunov P, Lancaster JL, Glahn DC, Purdy D, Laird AR, et al. Retrospective motion correction protocol for high-resolution anatomical MRI. Hum Brain Mapp. 2006;27:957–62. doi: 10.1002/hbm.20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochunov P, Ramage AE, Lancaster JL, Robin DA, Narayana S, et al. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. Neuroimage. 2009;45:17–28. doi: 10.1016/j.neuroimage.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochunov P, Robin DA, Royall DR, Coyle T, Lancaster J, et al. Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp. 2009;30:2581–94. doi: 10.1002/hbm.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochunov P, Thompson PM, Coyle TR, Lancaster JL, Kochunov V, et al. Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp. 2008;29:36–45. doi: 10.1002/hbm.20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–87. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Kraut MA, Beason-Held LL, Elkins WD, Resnick SM. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab. 2008;28:190–7. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
- 27.Mazziotta JC, Toga AW, Evans AC, Fox PT, Lancaster JL. Digital brain atlases. Trends Neurosci. 1995;18:210–1. doi: 10.1016/0166-2236(95)93904-c. [DOI] [PubMed] [Google Scholar]
- 28.Miura K, Soyama Y, Morikawa Y, Nishijo M, Nakanishi Y, et al. Comparison of four blood pressure indexes for the prediction of 10-year stroke risk in middle-aged and older Asians. Hypertension. 2004;44:715–20. doi: 10.1161/01.HYP.0000145108.23948.7b. [DOI] [PubMed] [Google Scholar]
- 29.Nair GV, Chaput LA, Vittinghoff E, Herrington DM. Pulse pressure and cardiovascular events in postmenopausal women with coronary heart disease. Chest. 2005;127:1498–506. doi: 10.1378/chest.127.5.1498. [DOI] [PubMed] [Google Scholar]
- 30.Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report: a review. Stroke. 1995;26:1293–301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 31.Sachdev P, Brodaty H. Quantitative study of signal hyperintensities on T2-weighted magnetic resonance imaging in late-onset schizophrenia. Am J Psychiatry. 1999;156:1958–67. doi: 10.1176/ajp.156.12.1958. [DOI] [PubMed] [Google Scholar]
- 32.Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–9. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Tate DF, Jefferson AL, Brickman AM, Hoth KF, Gunstad J, et al. Regional white matter signal abnormalities and cognitive correlates among geriatric patients with treated cardiovascular disease. Brain Imaging Behav. 2008;2:200–6. doi: 10.1007/s11682-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Air Force. Air Force Instruction. Washington, DC: Department of the Air Force; 2009. Sep 24, Medical examinations and standards; pp. 48–123. [Google Scholar]
- 37.Wen W, Sachdev PS, Chen X, Anstey K. Gray matter reduction is correlated with white matter hyperintensity volume: a voxel-based morphometric study in a large epidemiological sample. Neuroimage. 2006;29:1031–9. doi: 10.1016/j.neuroimage.2005.08.057. [DOI] [PubMed] [Google Scholar]