Abstract
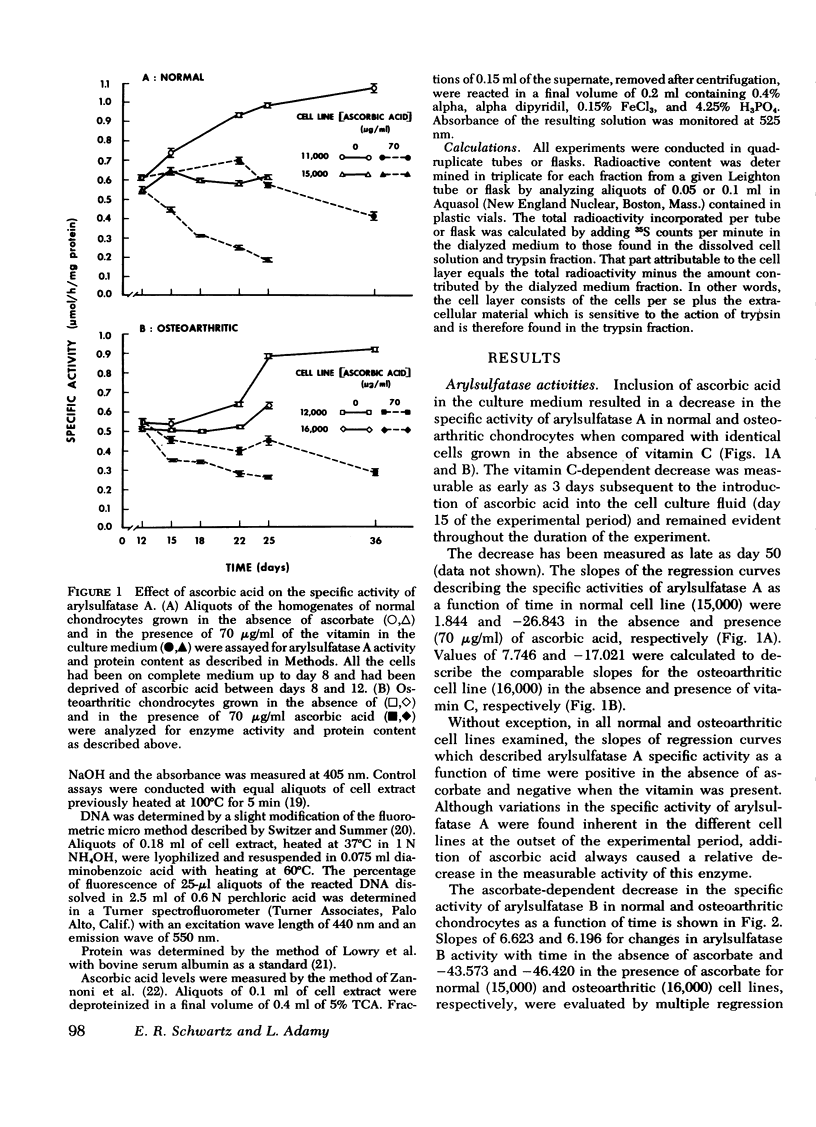
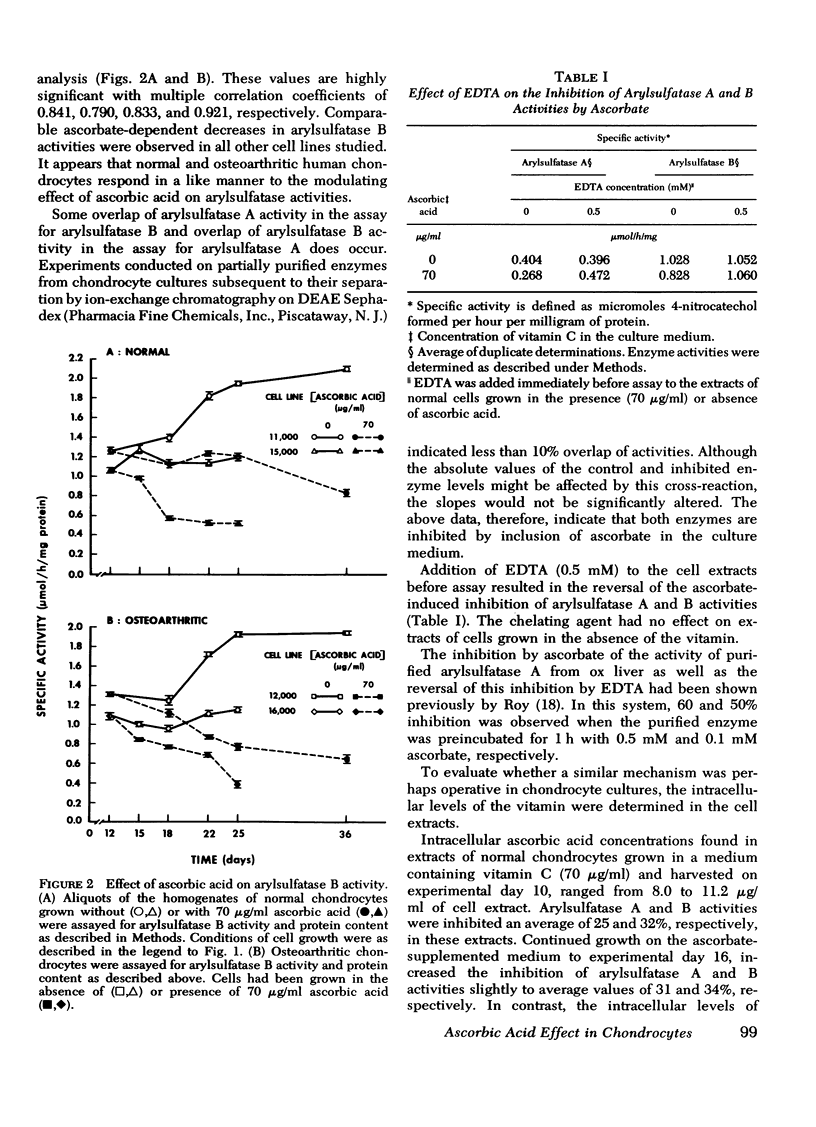
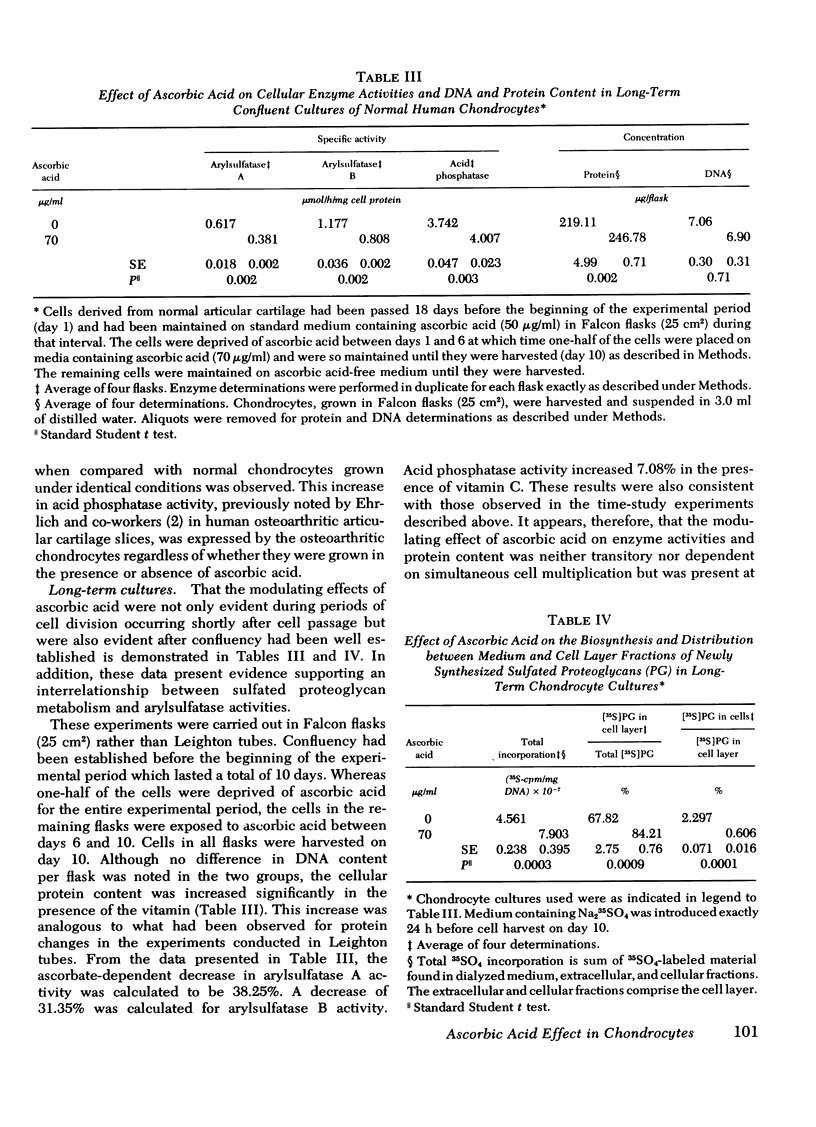
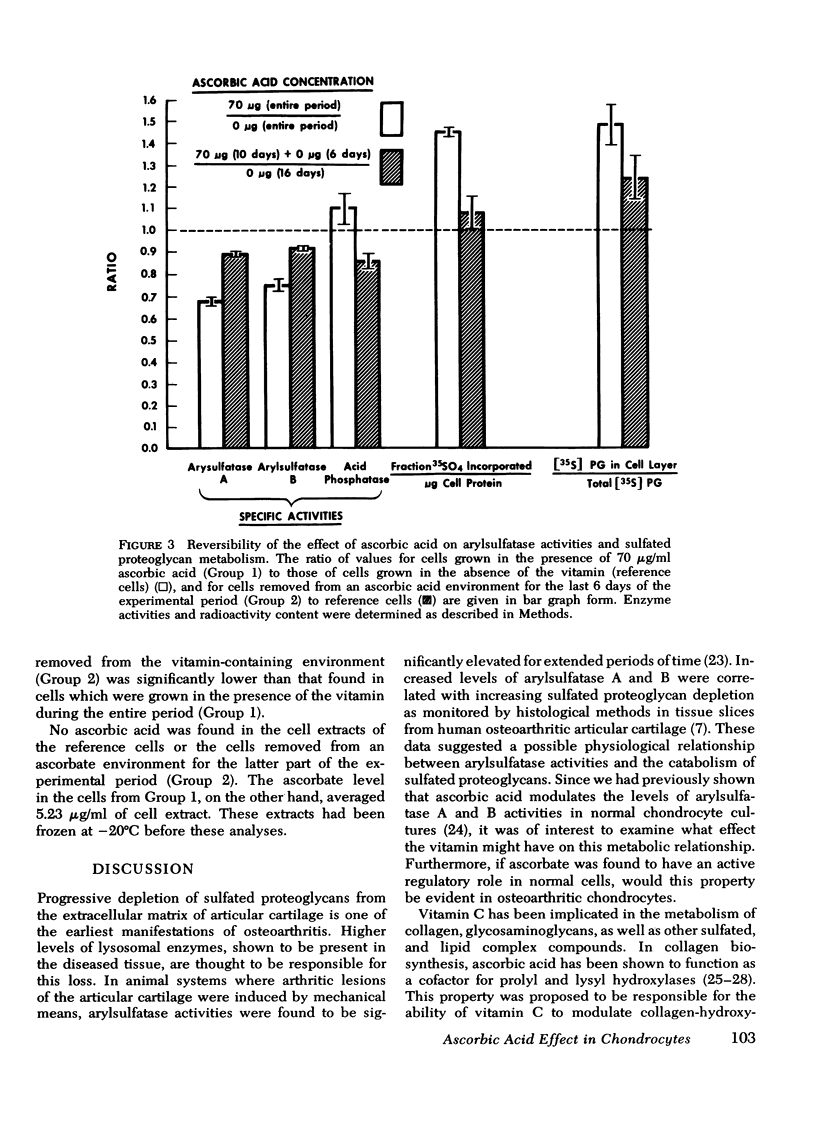
A correlation between increased arylsulfatase activities and decreased sulfated proteoglycan content in human osteoarthritic articular cartilage suggested a possible interrelationship between these parameters. Since we had previously shown that ascorbate caused a decrease in levels of arylsulfatase A and B activities in normal chondrocyte cultures, the validity of the above relationship was examined by measuring the effect of vitamin C on the biosynthesis and distribution of 35S-labeled proteoglycans and arylsulfatase A and B activities in cell extracts of chondrocytes derived from normal and osteoarthritic tissue. Arylsulfatase A and B activities were found to be reduced in the presence of ascorbic acid in all normal and osteoarthritic cell lines examined when measured 3, 6, 10, and 13 days after the introduction of the vitamin in the culture medium. Acid phosphatase activity, on the other hand, was found to be elevated in the presence of ascorbate. The inhibitory effect by ascorbic acid on arylsulfatase activities could be reversed by withdrawing the vitamin from the nutrient medium. Addition of EDTA to the cell extracts before assay also reversed the inhibiton. Sulfated proteoglycan biosynthesis as reflected in 35S-sulfate uptake per milligram of DNA was significantly increased in the presence of ascorbic acid. The distribution of the newly synthesized molecules between the cell layer and medium fractions was altered. In the presence of ascorbate, more deposition into the cell layer of newly synthesized macromolecules occurred. These data suggest an inverse relationship between arylsulfatase activities and the stability of the newly synthesized sulfated proteoglycans in the extracellular matrix.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E., Roy A. B. The sulphatase of ox liver. XI. The isoelectric focussing of a purified preparation of sulphatase B. Biochim Biophys Acta. 1968 Oct 21;168(2):243–251. doi: 10.1016/0005-2795(68)90147-5. [DOI] [PubMed] [Google Scholar]
- Altman R. D., Pita J. C., Howell D. S. Degradation of proteoglycans in human osteoarthritic cartilage. Arthritis Rheum. 1973 Mar-Apr;16(2):179–185. doi: 10.1002/art.1780160207. [DOI] [PubMed] [Google Scholar]
- Baker E. M., 3rd, Hammer D. C., March S. C., Tolbert B. M., Canham J. E. Ascorbate sulfate: a urinary metabolite of ascorbic acid in man. Science. 1971 Aug 27;173(3999):826–827. doi: 10.1126/science.173.3999.826. [DOI] [PubMed] [Google Scholar]
- Bates C. J., Prynne C. J., Levene C. I. The synthesis of underhydroxylated collagen by 3 T6 mouse fibroblasts in culture. Biochim Biophys Acta. 1972 Apr 15;263(2):397–405. doi: 10.1016/0005-2795(72)90091-8. [DOI] [PubMed] [Google Scholar]
- Bollet A. J. Connective tissue polysaccharide metabolism and the pathogenesis of osteoarthritis. Adv Intern Med. 1967;13:33–60. [PubMed] [Google Scholar]
- DiPietro D. L., Zengerle F. S. Separation and properties of three acid phosphatases from human placenta. J Biol Chem. 1967 Jul 25;242(14):3391–3395. [PubMed] [Google Scholar]
- Ehrlich M. G., Mankin H. J., Treadwell B. V. Acid hydrolase activity in osteoarthritic and normal human cartilage. J Bone Joint Surg Am. 1973 Jul;55(5):1068–1076. [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Fung D., Peak S., Kihara H. Uridine diphospho-N-acetylgalactosamine-4-sulfate sulfohydrolase activity of human arylsulfatase B and its deficiency in the Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1975 Jan 2;64(3):955–962. doi: 10.1016/0006-291x(75)90140-0. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Miller R. T., Kihara H. Sulfoglycerogalactolipid from rat testis: a substrate for pure human arylsulfatase A. Biochem Biophys Res Commun. 1974 Nov 6;61(1):348–354. doi: 10.1016/0006-291x(74)90573-7. [DOI] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Sanders D. L., Kihara H. Arylsulfatase B deficiency in Maroteaux-Lamy syndrome cultured fibroblasts. Biochem Biophys Res Commun. 1974 Jul 24;59(2):455–461. doi: 10.1016/s0006-291x(74)80001-x. [DOI] [PubMed] [Google Scholar]
- Ginter E. Cholesterol: vitamin C controls its transformation to bile acids. Science. 1973 Feb 16;179(4074):702–704. doi: 10.1126/science.179.4074.702. [DOI] [PubMed] [Google Scholar]
- Ginter E. Letter: Vitamin C and plasma lipids. N Engl J Med. 1976 Mar 4;294(10):559–560. doi: 10.1056/NEJM197603042941025. [DOI] [PubMed] [Google Scholar]
- Gold E. W., Gussler D., Schwartz E. R. Enzymes from human articular cartilage: isolation of arylsulfatase B and its comparison with arylsulfatase A. Connect Tissue Res. 1976;4(4):237–245. doi: 10.3109/03008207609152226. [DOI] [PubMed] [Google Scholar]
- Hausmann E. Cofactor requirements for the enzymatic hydroxylation of lysine in a polypeptide precursor of collagen. Biochim Biophys Acta. 1967 Apr 11;133(3):591–593. doi: 10.1016/0005-2795(67)90566-1. [DOI] [PubMed] [Google Scholar]
- Houston J. B., Levy G. Modification of drug biotransformation by vitamin C in man. Nature. 1975 May 1;255(5503):78–79. doi: 10.1038/255078a0. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavietes B. B. Kinetics of matrix synthesis in cartilage cell cultures. Exp Cell Res. 1971 Sep;68(1):43–48. doi: 10.1016/0014-4827(71)90584-2. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Matalon R., Arbogast B., Dorfman A. Deficiency of chondroitin sulfate N-acetylgalactosamine 4-sulfate sulfatase in Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1450–1457. doi: 10.1016/s0006-291x(74)80446-8. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Cerebroside 3-sulfate as a physiological substrate of arylsulfatase A. Biochim Biophys Acta. 1968 Mar 25;151(3):619–627. doi: 10.1016/0005-2744(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Mumma R. O., Verlangieri A. J. Isolation of ascorbic acid 2-sulfate from selected rat organs. Biochim Biophys Acta. 1972 Jul 19;273(2):249–253. doi: 10.1016/0304-4165(72)90214-0. [DOI] [PubMed] [Google Scholar]
- Nathans A. H., Kitabchi A. E. Effect of ascorbic acid on ACTH-induced cyclic AMP formation and steroidogenesis in isolated adrenal cells of vitamin E-deficient rats. Biochim Biophys Acta. 1975 Aug 13;399(2):244–253. doi: 10.1016/0304-4165(75)90255-x. [DOI] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. ENZYMATIC HYDROXYLATION OF PROLINE IN MICROSOMAL POLYPEPTIDE LEADING TO FORMATION OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Feb;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B. Regulation of collagen secretion by ascorbic acid in 3T3 and chick embryo fibroblasts. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1343–1350. doi: 10.1016/0006-291x(72)90614-6. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. II. The purification and properties of sulphatase A. Biochem J. 1953 Nov;55(4):653–661. doi: 10.1042/bj0550653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. B. L-ascorbic acid 2-sulphate. A substrate for mammalian arylsulphatases. Biochim Biophys Acta. 1975 Feb 19;377(2):356–363. doi: 10.1016/0005-2744(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Roy A. B. The sulphatase of ox liver. 8. The action of carbonyl reagents on sulphatase A. Biochim Biophys Acta. 1970 Jan 14;198(1):76–81. doi: 10.1016/0005-2744(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Schafer I. A., Sullivan J. C., Svejcar J., Kofoed J., Robertson W. van B. Vitamin C-induced increase of dermatan sulfate in cultured Hurler's fibroblasts. Science. 1966 Aug 26;153(3739):1008–1010. doi: 10.1126/science.153.3739.1008. [DOI] [PubMed] [Google Scholar]
- Schartz E. R., Ogle R. C., Thompson R. C. Aryl sulfatase activities in normal and pathologic human articular cartilage. Arthritis Rheum. 1974 Jul-Aug;17(4):455–467. doi: 10.1002/art.1780170417. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Adamy L. Effect of ascorbic acid on arylsulfatase A and B activities in human chondrocyte cultures. Connect Tissue Res. 1976;4(4):211–218. doi: 10.3109/03008207609152223. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Kirkpatrick P. R., Thompson R. C. Sulfate metabolism in human chondrocyte cultures. J Clin Invest. 1974 Nov;54(5):1056–1063. doi: 10.1172/JCI107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. R., Kirkpatrick P. R., Thompson R. C. The effect of environmental pH on glycosaminoglycan metabolism by normal human chondrocytes. J Lab Clin Med. 1976 Feb;87(2):198–205. [PubMed] [Google Scholar]
- Sledge C. B. Biochemical events in the epiphyseal plate and their physiologic control. Clin Orthop Relat Res. 1968 Nov-Dec;61:37–47. [PubMed] [Google Scholar]
- Sokoloff L., Malemud C. J., Green W. T., Jr Sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum. 1970 Mar-Apr;13(2):118–124. doi: 10.1002/art.1780130203. [DOI] [PubMed] [Google Scholar]
- Srivastava V. M., Malemud C. J., Hough A. J., Bland J. H., Sokoloff L. Preliminary experience with cell culture of human articular chondrocytes. Arthritis Rheum. 1974 Mar-Apr;17(2):165–169. doi: 10.1002/art.1780170209. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., Udenfriend S. Activation of prolyl hydroxylase in L-929 fibroblasts by ascorbic acid. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1090–1093. doi: 10.1073/pnas.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. A., Austin J. H., Crocker A. C., LaFrance M. Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome). I. Sulfatase B deficiency in tissues. Am J Dis Child. 1973 Dec;126(6):747–755. doi: 10.1001/archpedi.1973.02110190597003. [DOI] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Clark I. Acid hydrolases in slices of articular cartilage and synovium from normal and abnormal joints. Proc Soc Exp Biol Med. 1970 Mar;133(3):1102–1108. doi: 10.3181/00379727-133-34633. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Jr An experimental study of surface injury to articular cartilage and enzyme responses within the joint. Clin Orthop Relat Res. 1975;(107):239–248. doi: 10.1097/00003086-197503000-00028. [DOI] [PubMed] [Google Scholar]
- WOLBACH S. B., MADDOCK C. L. Cortisone and matrix formation in experimental scorbutus and repair therefrom, with contributions to the pathology of experimental scorbutus. AMA Arch Pathol. 1952 Jan;53(1):54–69. [PubMed] [Google Scholar]
- Zannoni V., Lynch M., Goldstein S., Sato P. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974 Sep;11(1):41–48. doi: 10.1016/0006-2944(74)90093-3. [DOI] [PubMed] [Google Scholar]



