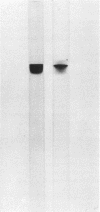
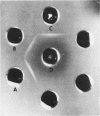
Abstract
Diabetes stimulates the functional activity of the intestinal brush border membrane with enhancement of both hydrolytic enzyme activity and membrane transport systems. To determine the mechanism of this effect, we studied the effects of streptozotocin diabetes on the metabolism of one membrane protein, sucrase-isomaltase, which increases its activity in diabetes. The protein was purified and an antiserum prepared. Sucrase-isomaltase from control and diabetic rats was immunologically identical as shown by Ouchterlony double-diffusion analysis of papain-solubilized mucosal proteins. The increase in sucrase enzyme activity in diabetic animals (31.0±1.4 U SEM 5 days after streptozotocin vs. 13.1±1.0 in controls) was the consequence of increased enzyme protein and not an alteration in catalytic efficiency as demonstrated by quantitative immunoprecipitin reactions.
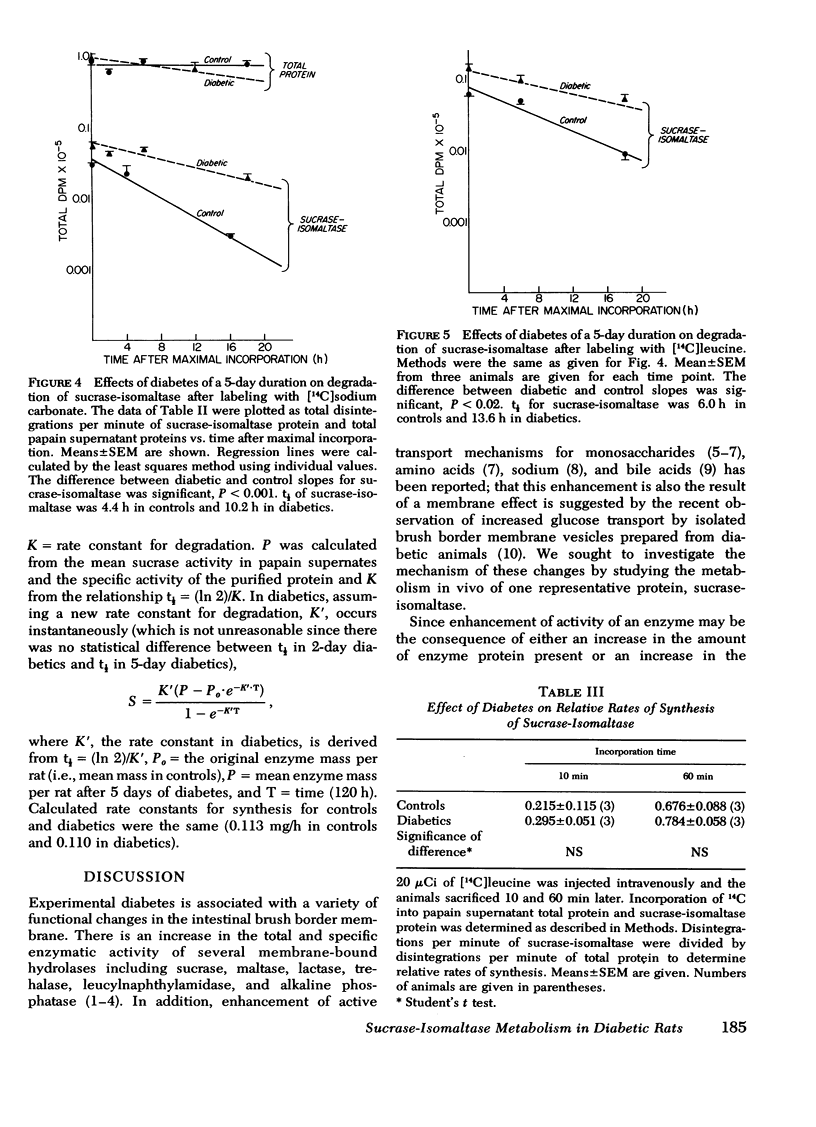
To account for increased sucrase-isomaltase protein in diabetes we studied papain-solubilized mucosal proteins labeled by injection of [14C]carbonate and [14C]leucine and analyzed incorporation into sucrase-isomaltase protein (anti-serum precipitable) and total protein (trichloroacetic acid precipitable). We found that diabetes did not affect the decay of labeled total protein, but prolonged the decay of labeled sucrase-isomaltase. t½ of sucrase-isomaltase was 4.4 h in control animals after [14C]carbonate injection and 8.8 and 10.2 h, respectively, 2 and 5 days after induction of streptozotocin diabetes. We obtained similar results in experiments with [14C]leucine with diabetes increasing t½ from 6 to 13.6 h. Diabetes did not appear to increase the rate of addition of sucrase-isomaltase to the brush border membrane, since it did not affect the 10- and 60-min incorporations of isotope into sucrase-isomaltase protein relative to incorporation into total protein and did not alter rate constants for synthesis calculated from the t½ and the change in enzyme mass over time.
Thus, enhanced sucrase activity in the diabetic animal is the consequence of an increase in sucrase-isomaltase protein which develops because of a decrease in its rate of degradation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Kinzie J. L. Regulation of small intestinal protein metabolism. Gastroenterology. 1973 Mar;64(3):471–496. [PubMed] [Google Scholar]
- Alpers D. H. Protein synthesis in intestinal mucosa: the effect of route of administration of precursor amino acids. J Clin Invest. 1972 Jan;51(1):167–173. doi: 10.1172/JCI106788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Tedesco F. J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim Biophys Acta. 1975 Aug 5;401(1):28–40. doi: 10.1016/0005-2736(75)90338-7. [DOI] [PubMed] [Google Scholar]
- Alpers D. H. The relation of size to the relative rates of degradation of intestinal brush border proteins. J Clin Invest. 1972 Oct;51(10):2621–2630. doi: 10.1172/JCI107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amherdt M., Harris V., Renold A. E., Orci L., Unger R. H. Hepatic autography in uncontrolled experimental diabetes and its relationships to insulin and glucagon. J Clin Invest. 1974 Jul;54(1):188–193. doi: 10.1172/JCI107742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis C., Olsen W. A. Intestinal mucosal disaccharidases in chronic pancreatitis. Am J Dig Dis. 1974 May;19(5):417–421. doi: 10.1007/BF01255605. [DOI] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- Caspary W. F. Effect of insulin and experimental diabetes mellitus on the digestive-absorptive function of the small intestine. Digestion. 1973 Oct;9(3):248–263. doi: 10.1159/000197452. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Rhein A. M., Creutzfeldt W. Increase of intestinal brush border hydrolases in mucosa of streptozotocin-diabetic rats. Diabetologia. 1972 Dec;8(6):412–414. doi: 10.1007/BF01212169. [DOI] [PubMed] [Google Scholar]
- Cox R. P., Elson N. A., Tu S. H., Griffin M. J. Hormonal induction of alkaline phosphatase activity by an increase in catalytic efficiency of the enzyme. J Mol Biol. 1971 May 28;58(1):197–215. doi: 10.1016/0022-2836(71)90241-5. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genel M., London D., Holtzapple P. G., Segal S. Uptake of alpha-methylglucoside by normal and diabetic human jejunal mucosa. J Lab Clin Med. 1971 May;77(5):743–750. [PubMed] [Google Scholar]
- Hopfer U. Diabetes mellitus: changes in the transport properties of isolated intestinal microvillous membranes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2027–2031. doi: 10.1073/pnas.72.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- Kelly J. J., Alpers D. H. Blood group antigenicity of purified human intestinal disaccharidases. J Biol Chem. 1973 Dec 10;248(23):8216–8221. [PubMed] [Google Scholar]
- Kolínská J., Kraml J. Separation and characterization of sucrose-isomaltase and of glucoamylase of rat intestine. Biochim Biophys Acta. 1972 Sep 19;284(1):235–247. doi: 10.1016/0005-2744(72)90062-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millward D. J. Protein turnover in skeletal muscle. I. The measurement of rates of synthesis and catabolism of skeletal muscle protein using (14C)Na2CO3 to label protein. Clin Sci. 1970 Nov;39(5):577–590. doi: 10.1042/cs0390577. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Olsen W. A., Korsmo H. Enhancement of intestinal sucrase activity in experimental diabetes: the role of intraluminal factors. J Lab Clin Med. 1975 May;85(5):832–837. [PubMed] [Google Scholar]
- Olsen W. A., Rogers L. Jejunal sucrase activity in diabetic rats. J Lab Clin Med. 1971 May;77(5):838–842. [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Rudo N. D., Rosenberg I. R., Wissler R. W. The effect of partial starvation and glucagon treatment on intestinal villus morphology and cell migration. Proc Soc Exp Biol Med. 1976 Jun;152(2):277–280. doi: 10.3181/00379727-152-39378. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- Spiro R. G. Biochemistry of the renal glomerular basement membrane and its alterations in diabetes mellitus. N Engl J Med. 1973 Jun 21;288(25):1337–1342. doi: 10.1056/NEJM197306212882506. [DOI] [PubMed] [Google Scholar]
- Tandon R. K., Srivastava L. M., Pandey S. C. Increased disaccharidase activity in human diabetics. Am J Clin Nutr. 1975 Jun;28(6):621–625. doi: 10.1093/ajcn/28.6.621. [DOI] [PubMed] [Google Scholar]
- Younoszai M. K., Schedl H. P. Effect of diabetes on intestinal disaccharidase activities. J Lab Clin Med. 1972 Apr;79(4):579–586. [PubMed] [Google Scholar]