Abstract
To investigate the role of glucagon and insulin receptor binding in the glucagon hypersensitivity and insulin resistance which characterize the glucose intolerance of uremia, liver plasma membranes were prepared from control rats (blood urea nitrogen [BUN] 15±1 mg/100 ml, creatinine 0.7±0.2 mg/100 ml), and from 70% nephrectomized rats (BUN 30±2 mg/100 ml, creatinine 2.2±0.2 mg/100 ml), and from 90% nephrectomized rats (BUN 46±3 mg/100 ml, creatinine 4.20±0.7 mg/100 ml), 4 wk after surgery. As compared to controls, the 90% nephrectomized rats had significantly higher levels of plasma glucose (95±4 vs. 125±11 mg/100 ml), plasma insulin (28±9 vs. 52±11 μU/ml), and plasma glucagon (28±5 vs. 215±18 pg/ml). Similar, but less marked, elevations were observed in the 70% nephrectomized animals.
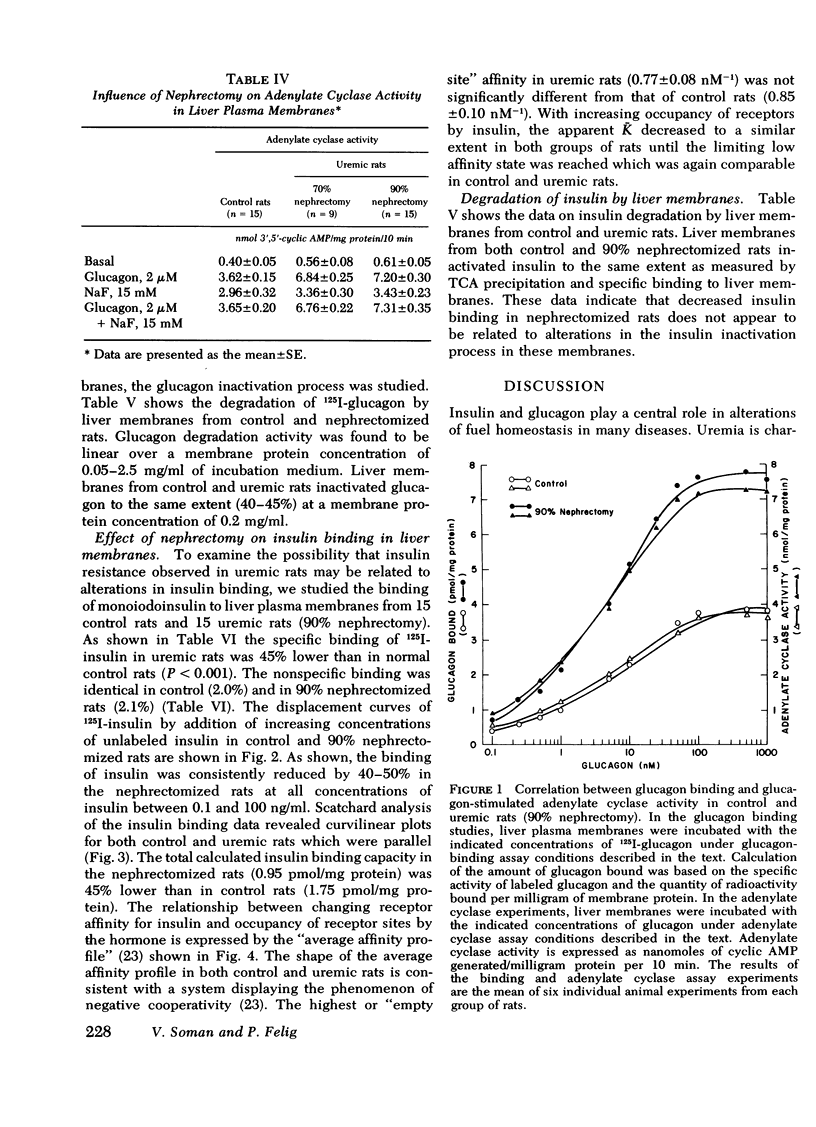
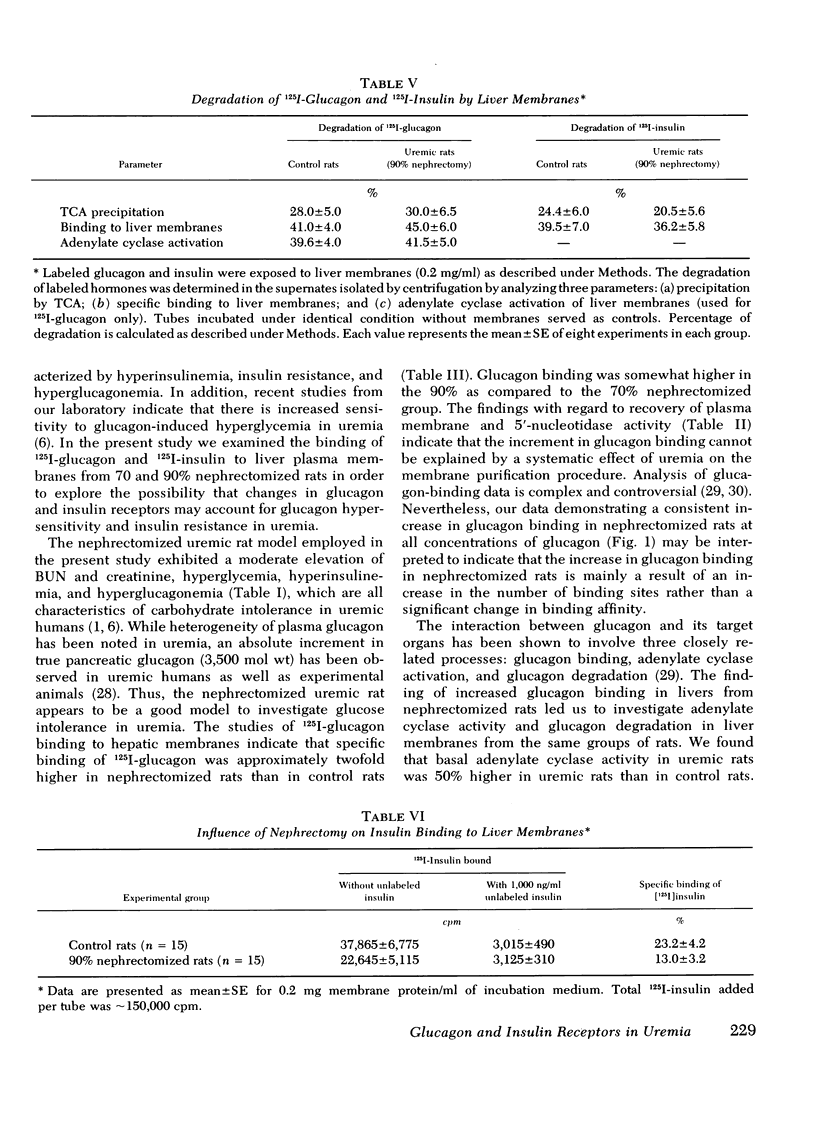
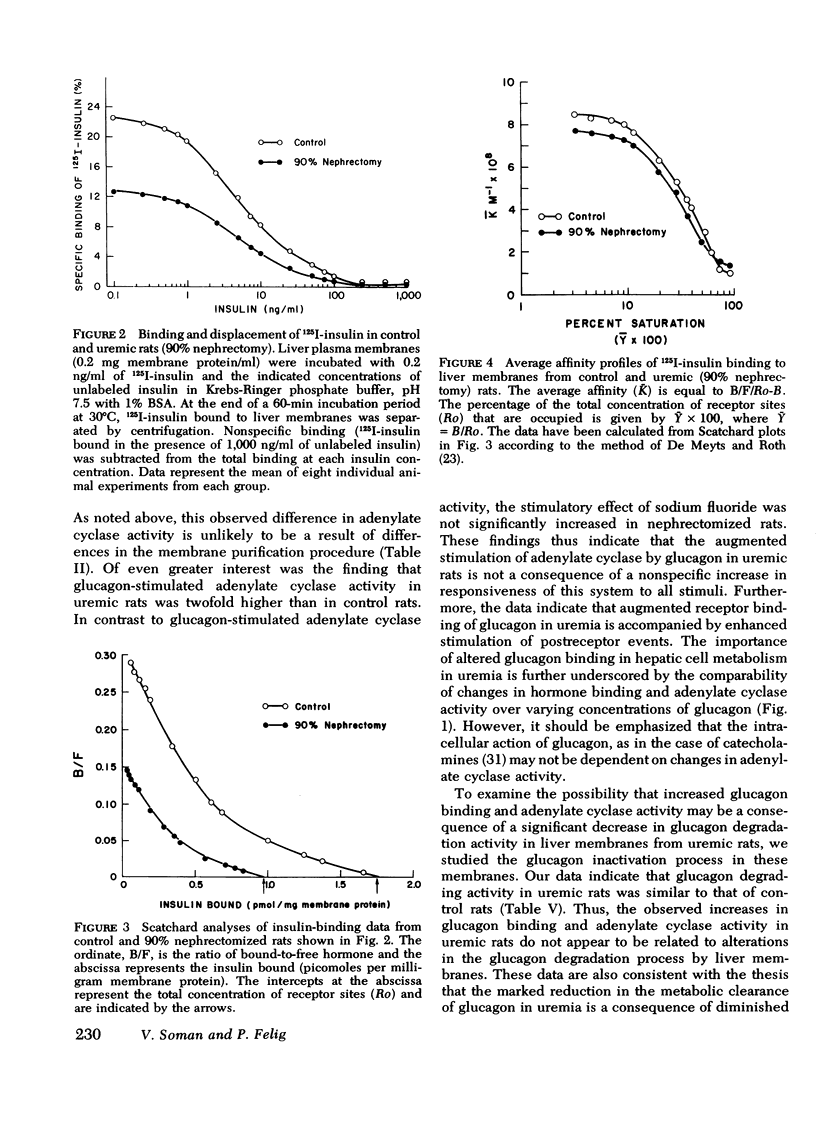
In liver plasma membranes from nephrectomized rats, specific binding of 125I-glucagon was increased by 80-120%. Furthermore, glucagon (2 μM)-stimulated adenylate cyclase activity in nephrectomized rats was twofold higher than in controls. In contrast, fluoridestimulated adenylate cyclase activity was similar in both groups of rats. In marked contrast to glucagon binding, specific binding of 125I-insulin to liver membranes from nephrectomized rats was reduced by 40-50% as compared to controls. Data analysis suggested that the changes in both glucagon and insulin binding are a consequence of alterations in binding capacity rather than changes in affinity. Liver plasma membranes from nephrectomized rats degraded 125I-glucagon and 125I-insulin to the same extent as control rats.
These results demonstrate that: (a) the 70 and 90% nephrectomized rats simulate the hyperglycemia, hyperinsulinemia, and hyperglucagonemia observed in clinical uremia; (b) in these animals specific binding of glucagon to liver membranes is increased and is accompanied by higher glucagon-stimulated adenylate cyclase activity; and (c) specific binding of insulin is markedly decreased. These findings thus provide evidence of oppositely directed, simultaneous changes in glucagon and insulin receptor binding in partially nephrectomized rats. Such changes may account for the hypersensitivity to glucagon and may contribute to resistance to insulin observed in the glucose intolerance of uremia.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Kaumann A. J. Receptors and acceptors: a necessary distinction in hormone binding studies. Adv Cyclic Nucleotide Res. 1974;4(0):239–281. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L. Relation of glucagon-specific binding sites to glucagon-dependent stimulation of adenylyl cyclase activity in plasma membranes of rat liver. J Biol Chem. 1973 Mar 25;248(6):2056–2061. [PubMed] [Google Scholar]
- Cerletty J. M., Engbring N. H. Azotemia and glucose intolerance. Ann Intern Med. 1967 Jun;66(6):1097–1108. doi: 10.7326/0003-4819-66-6-1097. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Kaplan S. A. Increased insulin binding by hepatic plasma membranes from diabetic rats: normalization by insulin therapy. J Clin Invest. 1977 Jan;59(1):22–30. doi: 10.1172/JCI108618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Andres R., Edgar P., Walker W. G. Carbohydrate metabolism in uremia: a review. Medicine (Baltimore) 1973 Sep;52(5):469–481. doi: 10.1097/00005792-197309000-00009. [DOI] [PubMed] [Google Scholar]
- Emmanouel D. S., Jaspan J. B., Kuku S. F., Rubenstein A. H., Katz A. I., Huen A. H. Pathogenesis and characterization of hyperglucagonemia in the uremic rat. J Clin Invest. 1976 Nov;58(5):1266–1272. doi: 10.1172/JCI108581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchereau-Peron M., Rançon F., Freychet P., Rosselin G. Effect of feeding and fasting on the early steps of glucagon action in isolated rat liver cells. Endocrinology. 1976 Mar;98(3):755–760. doi: 10.1210/endo-98-3-755. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Blecher M., Binder R., Perrino P. V., Recant L. Hormone receptors, 5. Binding of glucagon and insulin to human circulating mononuclear cells in diabetes mellitus. Endocr Res Commun. 1975;2(4-5):367–376. doi: 10.1080/07435807509089009. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Martin F. I., Melick R. A. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J Clin Invest. 1976 Dec;58(6):1435–1441. doi: 10.1172/JCI108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kaufman J. M., DiMeola H. J., Siegel N. J., Lytton B., Kashgarian M., Hayslett J. P. Compensatory adaptation of structure and function following progressive renal ablation. Kidney Int. 1974 Jul;6(1):10–17. doi: 10.1038/ki.1974.72. [DOI] [PubMed] [Google Scholar]
- Kaufman J. M., Siegel N. J., Hayslett J. P. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res. 1975 Feb;36(2):286–293. doi: 10.1161/01.res.36.2.286. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Protein interactions with small molecules. Relationships between stoichiometric binding constants, site binding constants, and empirical binding parameters. J Biol Chem. 1975 Apr 25;250(8):3001–3009. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Milgrom E., Thi L., Atger M., Baulieu E. E. Mechanisms regulating the concentration and the conformation of progesterone receptor(s) in the uterus. J Biol Chem. 1973 Sep 25;248(18):6366–6374. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Effects of fasting on insulin binding, glucose transport, and glucose oxidation in isolated rat adipocytes: relationships between insulin receptors and insulin action. J Clin Invest. 1976 Dec;58(6):1450–1460. doi: 10.1172/JCI108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Birnbaumer L., Rodbell M. Inactivation of glucagon by plasma membranes of rat liver. J Biol Chem. 1972 Apr 25;247(8):2295–2301. [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Prolactin receptors in rat liver: possible induction by prolactin. Science. 1975 Apr 4;188(4183):57–59. doi: 10.1126/science.163493. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soli A. H., Kahn C. R., Neville D. M., Jr, Roth J. Insulin receptor deficiency in genetic and acquired obesity. J Clin Invest. 1975 Oct;56(4):769–780. doi: 10.1172/JCI108155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz I. M., Rubenstein A. H., Bersohn I., Abrahams C., Lowy C. Carbohydrate metabolism in renal disease. Q J Med. 1970 Apr;39(154):201–226. [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Westervelt F. B., Jr Uremia and insulin response. Arch Intern Med. 1970 Nov;126(5):865–869. [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


