This work examines the early transcriptomic consequences of interspecific crosses between Arabidopsis thaliana and Arabidopsis arenosa, finding that the incompatibility response includes disruption of endosperm development and activation of defense pathways, but, surprisingly, no identifiable role for widespread transposon activation.
Abstract
Seed death resulting from hybridization between Arabidopsis thaliana and Arabidopsis arenosa has complex genetic determination and involves deregulation 5 to 8 d after pollination (DAP) of AGAMOUS-LIKE genes and retroelements. To identify causal mechanisms, we compared transcriptomes of compatible and incompatible hybrids and parents at 3 DAP. Hybrids misexpressed endosperm and seed coat regulators and hyperactivated genes encoding ribosomal, photosynthetic, stress-related, and immune response proteins. Regulatory disruption was more severe in Columbia-0 hybrids than in C24 hybrids, consistent with the degree of incompatibility. Maternal loss-of-function alleles for endosperm growth factor TRANSPARENT TESTA GLABRA2 and HAIKU1 and defense response regulators NON-EXPRESSOR OF PATHOGENESIS RELATED1 and SALICYLIC ACID INDUCTION-DEFICIENT2 increased hybrid seed survival. The activation of presumed POLYCOMB REPRESSIVE COMPLEX (PRC) targets, together with a 20-fold reduction in expression of FERTILIZATION INDEPENDENT SEED2, indicated a PRC role. Proximity to transposable elements affected natural variation for gene regulation, but transposon activation did not differ from controls. Collectively, this investigation provides candidates for multigenic orchestration of the incompatibility response through disruption of endosperm development, a novel role for communication between endosperm and maternal tissues and for pathways previously connected to immunity, but, surprisingly, does not identify a role for transposons.
INTRODUCTION
Parental combination as well as the genotype of each parent determines hybrid phenotype suggests that the outcome of hybridization depends on the balance of positive and negative interactions between parental genomes. In one outcome, heterosis, hybrids are more vigorous than either parent as exemplified by increased yield in maize intraspecific hybrids (reviewed in Springer and Stupar, 2007). Heterosis has been attributed to positive gene interactions (reviewed in Hochholdinger and Hoecker, 2007; Chen, 2010) ranging from genome-wide effects to single-locus effects (Lippman and Zamir, 2007; Krieger et al., 2010). Hybridization can also lead to reduced hybrid fitness, including seed failure, germination defects, hybrid necrosis, and sterility. Manifestation of these traits is thought to result from the interaction of diverged parental genes and result in hybrid incompatibly (Dobzhansky, 1936; Muller, 1942; Orr, 1995; reviewed in Bomblies et al., 2007; Rieseberg and Blackman, 2010; Maheshwari and Barbash, 2011). The molecular basis of hybrid incompatibility has been variously attributed to cis- or trans-regulatory changes, copy number changes, and amino acid changes (Krüger et al., 2002; Bomblies et al., 2007; Dilkes et al., 2008; Chou et al., 2010; Chou and Leu, 2010), and, thus far, there is little overlap between genes detected in one genus to another (reviewed in Rieseberg and Blackman, 2010). These interactions can affect different targets including pathogen responses (Bomblies et al., 2007; Jeuken et al., 2009; Yamamoto et al., 2010; Mizuno et al., 2011), suppression of transposable elements (TEs) (McClintock, 1984; Shaked et al., 2001; Kashkush et al., 2003; Madlung et al., 2005; Josefsson et al., 2006; Ungerer et al., 2006; Martienssen, 2010), small RNA pathways (Ha et al., 2009; Ng et al., 2012; Shivaprasad et al., 2012; Zhang et al., 2012), and developmental regulatory pathways, such as genomic imprinting in the seed (Josefsson et al., 2006). Importantly, although all these molecular mechanisms have been documented as affected by hybridization in at least one system, their relative contribution to hybrid incompatibility is unclear.
In Arabidopsis species, there is natural variation for interspecific hybrid incompatibility, and the molecular nature of seed death remains poorly understood (Burkart-Waco et al., 2012). When Arabidopsis thaliana is crossed to its close relative Arabidopsis arenosa, abnormalities are observed in later stages of seed development (Josefsson et al., 2006; Walia et al., 2009; Burkart-Waco et al., 2012). This cross can only be performed using A. thaliana as the seed parent (Comai et al., 2000; Bushell et al., 2003). The genetic basis of the differential response between highly incompatible A. thaliana Columbia-0 (Col-0; 0% live seed) and moderately compatible C24 (17% live seed) was mapped to seven additive and several more epistatic quantitative trait loci (QTL) (Burkart-Waco et al., 2012). The identity of these QTL and the mode of action of the genes causing incompatibility are unknown. Studies of interploidy (tetraploid × diploid) interspecific Arabidopsis hybrids has revealed that ectopic expression of transcription factors involved in endosperm development contributes to incompatibility (Josefsson et al., 2006; Walia et al., 2009). These results have led to the hypotheses that (1) there are multiple factors governing the incompatibility response, and (2) aberrant gene regulation may underlie incompatibilities in Arabidopsis hybrids.
Here, we further characterize the molecular mechanism of hybrid incompatibility in Arabidopsis and assess TE activation in response to interspecific hybridization. We investigated seed development and the transcriptomes of developing hybrid seeds from incompatible Col-0 × A. arenosa and moderately compatible C24 × A. arenosa crosses. Early embryo development (2 to 6 d) was delayed in both incompatible and compatible hybrids, and differences in endosperm development were limited. Transcriptomes of seeds at 3 d after pollination (DAP) exhibit regulatory disruption affecting genes at the maternal-filial interface, suggesting that the endosperm is the primary cause of incompatibility. We identified 1228 genes differentially expressed between the compatible and incompatible hybrid crosses, including 10 AGAMOUS-LIKE (AGL) transcripts and an overrepresentation of ribosomal, photosynthetic, and defense response genes. Some AGLs and the defense response genes also vary between Col-0 and C24 accessions in self-crosses, suggesting that maternal natural variation is conserved in hybrids at 3 DAP. Maternal transmission of nullimorphic alleles of the misregulated endosperm growth factor HAIKU1 (IKU1) and seed coat transcription factor TRANSPARENT TESTA GLABRA2 (TTG2) was sufficient to improve hybrid seed survival. Our data do not support a role for transposon activation in embryo lethality, but a loss-of-function mutation in an important regulator of pathogen responses, NONEXPRESSOR OF PR genes 1 (NPR1), improved hybrid seed survival, suggesting that a factor central to basal immune responses impacts postzygotic incompatibility in Arabidopsis. Because of the regulatory malfunction at many defense response genes in incompatible hybrids, we propose that hybridization can induce stress responses that result in hybrid necrosis (Bomblies et al., 2007).
RESULTS
Developmental Delays in seed of A. thaliana × A. arenosa Hybrids
We compared seed development of A. thaliana ecotypes Col-0 and C24, which respectively produce 0 to 1% and ∼17% live seed after interspecific hybridization to A. arenosa (Burkart-Waco et al., 2012). Photomicrographs of developing seed from A. thaliana controls and interspecific F1 hybrid seed are shown in Figure 1. A. arenosa embryos grow much slower than A. thaliana (see Supplemental Figure 1 online, scale bar = 60 μM). For example, in A. arenosa, embryos are only one to four celled at 5 DAP, but endosperm proliferates rapidly in comparison to that of A. thaliana and hybrids (Bushell et al., 2003). Staining with vital dyes failed to detect dead cells in any cross at 2 to 6 DAP, although dead seed were clearly visible as early as 9 to 11 DAP, bars = 60 μm (see Supplemental Figure 2 online). Compared with A. thaliana seeds, interspecies hybrid embryos from both ecotypes were similar at 2 DAP, but a few C24 hybrids advanced to higher stages (Figure 1). At all stages beyond this, the hybrid embryos were developmentally delayed and similar between Col-0 and C24, with the exception of a few C24 × A. arenosa embryos displaying developmental progression to heart stage by 6 DAP, whereas no Col-0 embryos made this transition. Endosperm of Col-0 × A. arenosa hybrid seed was delayed at 3 DAP, as shown in Supplemental Figures 3A and 3B online (74.0 nuclei ± se 3.8; t test, P = 0.001 versus 95.6 nuclei ± se 4.1 in selfed Col-0), but C24 interspecific hybrid embryos appeared nearly normal at 3 DAP (82.0 ± se 9.2; t test, P = 0.12 versus 94.5 ± se 4.2 in selfed C24). Interestingly, the maternally derived inner integument was thinner in Col-0 hybrids than in selfed seed (see Supplemental Figure 3E online). In summary, incompatible Col-0 hybrids at 3 DAP displayed subtle reduction of endosperm proliferation and maternal tissue symptoms when compared with C24 hybrids (see Supplemental Figure 3 online). The stronger delay in the hybrid embryos could result from incompatibilities that either affected the embryo and not the endosperm, or the endosperm and, indirectly, the embryo. In addition, embryonic delay may result from the genetic influence of the A. arenosa seed father (see Supplemental Figure 1 online).
Figure 1.
Early Seed Development of A. thaliana and A. thaliana × A. arenosa Hybrids.
(A) Images of seeds 3 to 6 DAP for control crosses (diploid Col-0 and diploid C24) and hybrid crosses (compatible hybrid, diploid A. thaliana C24 × diploid A. arenosa and incompatible hybrid, and diploid A. thaliana Col-0 × diploid A. arenosa). Seeds were cleared with Hoyer’s and imaged on a Leica microscope using differential interference contrast/Nomarski optics. Embryos (EM) are labeled. Both compatible and incompatible hybrids are delayed in development relative to A. thaliana parent. Bars = 60 μm.
(B) Embryo stages (right panel) were divided into eight numbered categories (right y axis), and the number of embryos in each category was recorded for each cross and each developmental stage. The mean proportion (black bars) of total embryos (N) of selfed Col-0 and C24 and crosses of Col-0 and C24 with A. arenosa (left y axis) at 2 to 6 DAP (x axis). Error bars indicate the sd. A. thaliana Col-0 and C24 produced self-cross progeny that are very similar early (3 DAP) in development. Later, C24 selfed progeny developed faster with the majority of embryos one stage ahead of Col-0 by 4 DAP. Thus, despite the relative compatibility of C24 compared with Col-0 (∼17% versus 0.5% live seed), C24 shares the developmental embryo delays with the incompatible 2× Col-0 × 2× A. arenosa hybrid crosses.
RNA Expression Profiling by Sequencing
To identify any molecular abnormalities during early seed development, we performed transcriptome analysis of 3 DAP seed by RNA sequencing. This stage was chosen because hybrid endosperms are relatively normal in C24 and only slightly delayed in Col-0. We chose to compare seeds with similar numbers of endosperm nuclei, rather than selecting seeds by embryo stage. Key epigenetic regulators of seed development are transcribed primarily in the endosperm (Baroux et al., 2002; Gehring et al., 2004; Berger and Chaudhury, 2009), and the abnormal regulation of gene expression in the endosperm has been suggested to underlie interspecific incompatibility (Haig and Westoby, 1991; Gutierrez-Marcos et al., 2003; Josefsson et al., 2006). The high degree of phenotypic variability observed later in seed development, even within a seed class (e.g., seeds at 11 DAP in Supplemental Figure 2B online), suggest that this focus on early development when organ growth was more consistent (see Supplemental Figure 3B online) is less sensitive to spurious secondary effects on growth.
To examine the differences in gene expression between hybrid and control seed, we used high-throughput RNA sequencing, as illustrated in Supplemental Figure 4 online, and our cDNA sequencing statistics are presented in Supplemental Table 1 online. We used 3 DAP seed from Col-0 × A. arenosa and C24 × A. arenosa hybrids and used Col-0, C24, and A. arenosa seed from selfing or intersib matings for controls (see Supplemental Figure 4A online). We compared mapping of sequence reads to a reference cDNA database from A. thaliana alone or from both parents and opted for the first approach (see Methods for technical details). A gene was considered expressed if a mean of four sequence reads (averaged across both replicates) was mapped unequivocally to it. We found 20,768 genes expressed in both Col-0 and C24 (see Supplemental Figure 5 online). In the hybrid endosperm data, 18,483 genes were expressed in both Col-0 × A. arenosa and C24 × A. arenosa (see Supplemental Figure 5 online). Control and hybrid expression profiles were compared with sets of genes whose expression was characterized in different seed compartments (http://seedgenenetwork.net; Le et al., 2010). We refer to these annotated sets of transcripts as “Harada-Goldberg” transcripts, after the senior authors of the Le et al. (2010) study. Greater than 92% of the genes identified as expressed at the globular stage in Le et al. were detected in controls. Similarly, >91% of the genes expressed at the preglobular stage and genes expressed were detected in our hybrid seed transcriptomes. The majority of molecular signatures observed in the hybrid transcriptome analysis matched the seed phenotypes observed in the embryogenesis time course (Figure 1; see Supplemental Figure 5 online). Thus, the observed developmental delay, rather than a generalized loss of transcriptional regulation, is the most likely cause of differences in gene expression between A. thaliana selfed seed and interspecies hybrids.
Comparison of the Transcriptomes of Control and Hybrid Crosses
We compared transcriptional profiles of Col-0 to Col-0 × A. arenosa hybrids and C24 to C24 × A. arenosa hybrids to identify differentially expressed genes. We found expression of 509 genes differed between Col-0 and Col-0 hybrids, and 4206 were differentially expressed between C24 and C24 hybrids (see Supplemental Figure 6 online). Of the 509 differences between Col-0 and Col-0 hybrids, 73% were also found among the set of differences between C24 and C24 hybrids. The numbers of differentially expressed genes in Col-0 and C24 hybrids was unexpected. It is unlikely that disparities between differentially expressed genes is attributed to biological variation within a genotype, as there is a high degree of correlation (≥91%) in expression values between replicates (see Supplemental Figure 7 online). Furthermore, if transcriptional dysregulation is the cause of incompatibility, one would expect the greater degree of transcriptional differences to be found between Col-0 and Col-0 hybrids, which exhibit stronger incompatibilities.
To characterize differentially expressed genes, Gene Ontology (GO) enrichment was assessed relative to the proportions of genes found in the A. thaliana genome (see Supplemental Table 2 online) and a set of 20,768 transcripts with coverage more than four reads in both 3 DAP Col-0 and C24, which we called the “seed genes” (see Supplemental Figure 5 and Supplemental Data Set 1 online). Genes that were differentially expressed in both hybrids (371 genes; see Supplemental Figure 6 online) were enriched for genes classified in cell killing, photosynthesis, cell wall modifications, and defense responses (see Supplemental Tables 2 and 3 and Supplemental Data Set 2 online). Very similar GO categories and fold enrichments were observed for both The Arabidopsis Information Resource and seed gene background sets (see Supplemental Tables 2 and 3 online). For example, we observed a fourfold enrichment for cell killing in differentially expressed transcripts relative to all Arabidopsis genes and a 6.5-fold enrichment relative to seed transcripts (see Supplemental Tables 2 and 3 online). Genes that were specifically differentially expressed between Col-0 and Col-0 hybrid (138 genes; see Supplemental Figure 6 online) also displayed enrichment for cell killing genes and ribosomal protein genes, with both classes induced in hybrids (see Supplemental Tables 2 and 3 online). The genes annotated as “cell killing,” all low molecular weight Cys-rich peptides (CRPs) and defensin-like proteins, are expressed in a manner inconsistent with inducible hypersensitive defenses response since they were also moderately expressed in control seeds (see Supplemental Table 4 online). These results suggest that defense responses, or related pathways, could play a role in the abnormal development of hybrids.
Gene Misregulation Is Limited to Particular Seed Compartments
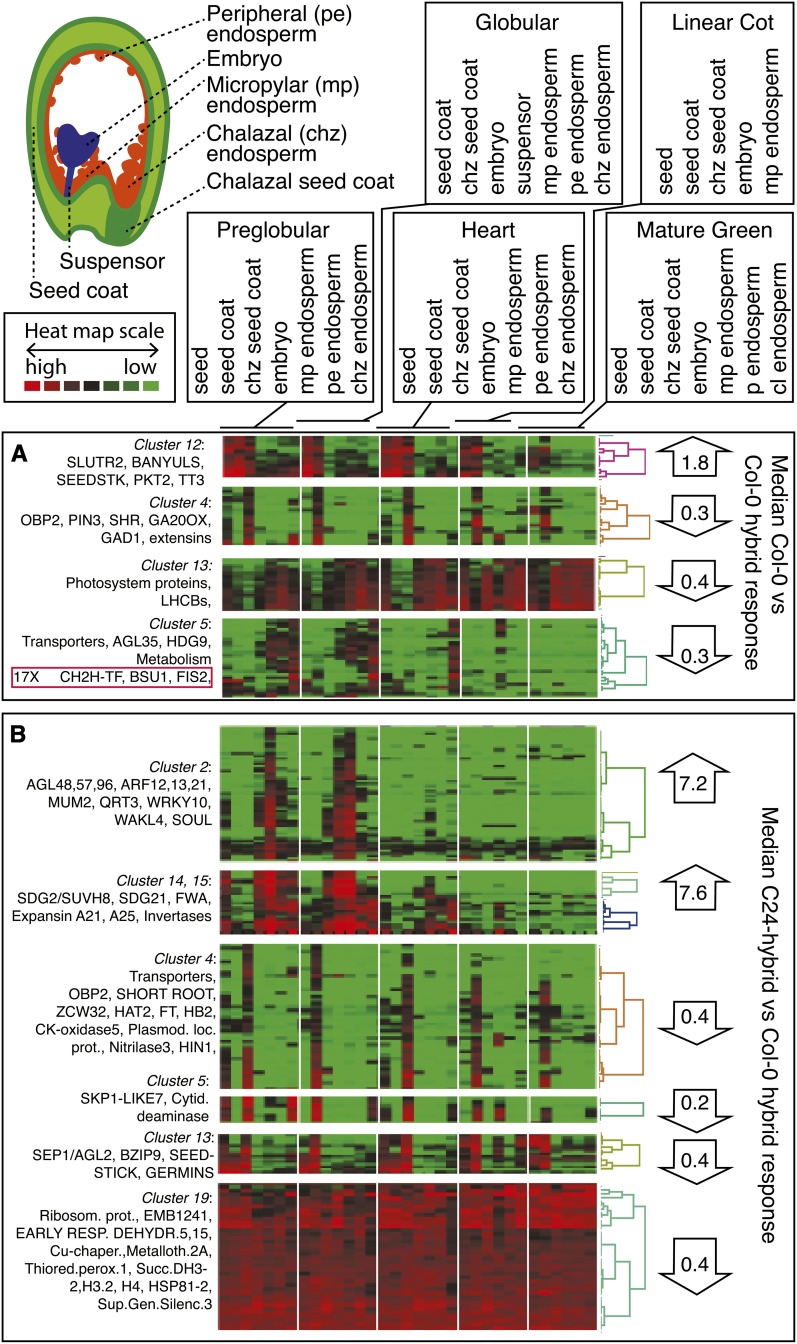
To assess the role of the hybridization-sensitive genes during seed development, we took the set of genes that were differentially regulated between Col-0 and Col-0 × A. arenosa and analyzed their patterns of tissue-specific expression at different stages of normal seed development using the Harada-Goldberg laser capture microdissection (LCM) seed expression data set (Figure 2). Of the 509 genes that were significantly up- or downregulated between Col-0 and Col-0 hybrids, 396 were represented in the LCM set. Some of these genes clustered together as developmentally coexpressed in their normal regulation, and these clusters responded to hybridity in a consistent manner. For example, a set of genes expressed preferentially in the general seed coat with functions in secondary metabolism (primarily flavonoid biosynthesis) was downregulated in hybrids. By contrast, a set of genes expressed specifically in the chalazal end of the seed coat, which included extensins and the cell identity and layer specification factor SHORT ROOT (Levesque et al., 2006), were coordinately overexpressed in hybrids. The regulatory changes in seed coat gene expression in the hybrids confirm the intimate connection between fertilization products and maternal tissue (Garcia et al., 2005; Dilkes et al., 2008). A set of genes preferentially expressed in the endosperm adjacent to the chalazal end were upregulated in hybrids and included transporters and endosperm transcription factors. Notably, three of these clusters that were upregulated in response to hybridization (such as photosynthetic genes) remain relatively constant in normal A. thaliana from preglobular to heart stage (Figure 2, heat map), inconsistent with a heterochronic malfunction resulting in hybrid lethality.
Figure 2.
Hybridization Disrupts Specific Spatio-Temporal Patterns of Seed Development.
The seed compartments in which hybridization sensitive genes were normally expressed were identified using the Harada-Goldberg LCM seed expression data set, and the expression patterns were used to cluster the 396 represented genes out of the 509 that were significantly up- or downregulated between Col-0 and Col-0 × A. arenosa (A) and between the incompatible Col-0 hybrid and the compatible C24 hybrid (B). The heat maps represent patterns of expression for genes (rows) across seed compartments (columns) at five developmental stages from 2 DAP (preglobular) to 10 DAP (mature green; see definitions at top). The median fold change for each cluster ([A], control/hybridization; [B], compatible/incompatible) is displayed on the right.
(A) Interspecific hybridization causes decreased expression of seed coat genes that specify the accumulation of secondary metabolites, mostly flavonoids. Other gene clusters are impacted in the opposite way; for example, those expressed preferentially in endosperm and eventually in the embryo and encoding photosynthetic genes. Two additional clusters, one preferentially expressed in chalazal endosperm and the other in chalazal seed coat, illustrate acceleration of the chalazal pole program. Interestingly, three well-known regulators in the chalazal endosperm diverged from the cluster trend and were poorly expressed in the hybrids (box in bottom left).
(B) Compatible ecotype C24 differs distinctly from incompatible ecotype Col-0, resembling the normal developmental pattern. For example, embryonic and micropylar gene clusters display enhanced response, while the chalazal pole overexpression induced by hybridity (A) is diminished.
Hybrids Display Unique Seed Gene Expression Signatures Relative to Both A. thaliana and A. arenosa Parents
Nonadditive gene regulation was tested by comparing the transcriptomes of the hybrids and seed mother to the seed transcriptome of A. arenosa seed at 3 DAP. The quality of the latter RNA sequencing libraries was lower than those derived from selfed and hybridized A. thaliana (see Supplemental Table 1 and Supplemental Figure 7 online; see Methods), resulting in fewer high-quality reads. Nonetheless, the comparison revealed nonadditivity for a majority (293) of the 509 genes significantly regulated between A. thaliana versus Col-0 hybrid (see Supplemental Data Set 3 online). Of these 293, over 200 were regulated in the same direction (i.e., up in hybrid relative to both parents) and included endosperm transcription factors IKU1, HDG9, and HDG10. The overall response, which was enriched relative to our seed genes for cell killing (P < 0.0001), defense response (P = 0.004), and translation (P = 0.02), suggested that hybrids resembled the seed parent more than the pollen parent. Col-0 hybrid and C24 hybrid shared 84% of the differentially expressed genes between Col-0 hybrid and A. arenosa (3592 and 3975, respectively) with over half (2305/3592) upregulated in hybrid relative to A. arenosa, about twice more than those observed between Col-0 hybrid and Col-0.
To better understand the influence of each parent on the hybrid, we identified genes whose regulation was conserved between one parent and the hybrid, but differed from the other parent. Relative to seed genes, 3298 A. thaliana–like genes were predominately upregulated in the hybrid relative to the A. arenosa parent (2373 of 3298 genes, mean fold change 24×). These were enriched in secondary metabolic processes (100 genes, twofold enrichment, P < 0.0001), phenylpropanoid biosynthesis (47 genes, twofold enrichment, P < 0.0001), and aromatic compound biosynthesis (55 genes, twofold enrichment, P < 0.001). The 214 A. arenosa–like genes were not distinctly regulated (either up or down) relative to A. thaliana (see Supplemental Data Set 3 online). They contained many photosystem proteins (upregulation relative to Col-0), corresponding to those observed in cluster analysis (Figure 2). In common with the A. arenosa pollen parent, the hybrid appeared deficient for polycomb protein FERTILIZATION INDEPENDENT SEED2 (FIS2), imprinted transcription factor AGAMOUS-LIKE (AGL96) (Wolff et al., 2011), and SEEDSTICK.
The overall pattern of differential gene expression between hybrids and parents is consistent with a release of gene suppression in Col-0 hybrids. Complications introduced by mapping specificity and reduced read numbers in the A. arenosa libraries relative to the A. thaliana and hybrid data sets are unlikely to account for frequent upregulation in Col-0 hybrid relative to A. arenosa.
Aberrant Expression of Endosperm-Localized Transcription Factors
We investigated misregulated transcription factors that are involved in endosperm development. FIS2, HAIKU1, SEEDSTICK/AGL11, SHORT ROOT, HOMEODOMAIN GLABROUS9 (HDG9), HDG10, and ZHOUPI/RETARDED GROWTH OF EMBRYO1 (Chaudhury et al., 1997; Luo et al., 2000; Kondou et al., 2008; Yang et al., 2008; Mizzotti et al., 2012) were all misregulated in comparison to control crosses. A subset of these genes, FIS2, HDG9, and HDG10, is coexpressed during development (Figure 2; see Supplemental Figure 8A online). The expression of both ZHOUPI and FIS2 was sixfold to 20-fold higher in A. thaliana control crosses and the expression of HDG9/10 and IKU1 was lower in control crosses (Figure 2; see Supplemental Figure 8B online). Comparison of hybrid FIS2 expression to our previous studies (Walia et al., 2009) and the Harada-Goldberg Ath1 data (Le et al., 2010) indicates that FIS2 expression remains relatively constant through early seed development (Figure 2; see Supplemental Figure 8C online). ZHOUPI, a factor required for embryonic cuticle formation and embryo-endosperm separation, is reduced 15-fold in Col-0 hybrids (P = 0.048) versus sixfold in C24 hybrids (P < 0.05). Correspondingly, its target ABNORMAL LEAF SHAPE1 (ALE1), which encodes a subtilisin-like protein required for cuticle formation, is reduced in Col-0 hybrids (10×, P = 0.06) and not in C24-hybrids (0.64×, P = 0.9). The observed reduction in expression of two important seed development factors, ZHOUPI and FIS2, may compromise seed function.
To explore the role of the transcription factors that underwent abnormal activation, such as IKU1, we tested whether maternal transmission of loss-of-function mutations affected seed survival. Since the pollen-parent in our hybrid crosses was wild-type A. arenosa, only haploinsufficiency, additive, or maternal effects could be observed. Because of the effect of hybridity on maternal seed coat and genes involved in the flavonoid pathway, we included in the analysis the maternally expressed factor TRANSPARENT TESTA GLABRA2 (TTG2) (Johnson et al., 2002; Garcia et al., 2005), which also affects interploidy hybrid lethality (Dilkes et al., 2008), and IKU2 because of its relationship to IKU1 and endosperm development (Garcia et al., 2003, 2005). The genetic backgrounds of these mutants, Col-0 and Landsberg erecta-0 (Ler-0), influence percentage of live seed. Col-0 × A. arenosa hybrids displayed strong incompatibility. Similarly to hybrids of ecotype C24, hybrids of ecotype Ler-0 had partial fertility. Comparisons were always made to the respective control. Our results are presented in Table 1 and Figure 3. We found no effects from the inactivation of several candidates (Table 1). However, loss of IKU1 in Ler-0 background significantly improved seed survival (iku1 × A. arenosa, 7.1% live seed versus 4% live seed in Ler-0 × A. arenosa control) and maternal TTG2 loss tripled seed survival (Figure 3A). However, T-DNA mutation in the gene IKU2 did not significantly improve overall seed survival. The double mutant of iku2 ttg2 was not significantly different from the single ttg2 mutant for %normal + green + viviparous (%NGV) seed, consistent with additive maternal effects at least for %NGV (Figure 3A), although the ratio of %G to %N is altered. The TTG2 and IKU1 phenotype confirms the role of maternal-filial communication in regulating incompatibility (Dilkes et al., 2008).
Table 1. Characterization of Candidate Genes.
| Category | Gene | Locus | Meana Col-0 | Mean C24 | Mean Aa | Mean Col-0 X Aa | Mean C24 X Aa | Seed Phenotype At x Aac |
|---|---|---|---|---|---|---|---|---|
| Seed transcription factors | FIS2 | AT2G35670 | 115.4 | 222.5 | 2.2 | 13.3 | 11.1 | ns |
| PHERES1 | AT1G65330 | 303.6 | 90.9 | 0.0 | 551.1 | 1368.1 | 9.9e | |
| TTG2 | AT2G37260 | 771.1 | 705.0 | 84.5 | 758.5 | 614.3 | 15.28d | |
| IKU1 | AT2G35230 | 200.0 | 171.0 | 44.5 | 488.0 | 519.6 | 7.1e | |
| IKU2 | AT3G19700 | 16.1 | 8.0 | 0.0 | 5.2 | 6.7 | ns | |
| AGL2 | AT1G15800 | 10.3 | 9.8 | 8.9 | 13.8 | 19.1 | ns | |
| AGL25 | AT5G10140 | 12.2 | 56.9 | 211.3 | 51.9 | 35.4 | ns | |
| AGL12 | AT1G71692 | 102.3 | 62.4 | 2.2 | 26.7 | 54.8 | ns | |
| AGL27 | AT1G77080 | 330.6 | 3.4 | 522.5 | 115.3 | 118.9 | ns | |
| AGL29 | AT2G34440 | 24.6 | 8.9 | 44.5 | 43.5 | 53.4 | ns | |
| AGL31b | AT5G65050 | 546.1 | 107.5 | 84.5 | 223.3 | 257.8 | ns | |
| AGL42 | AT5G62165 | 22.0 | 4.0 | 88.9 | 1.8 | 7.0 | ns | |
| AGL48b | AT2G40210 | 48.1 | 43.2 | 0.0 | 104.1 | 167.8 | ns | |
| AGL62 | AT5G60440 | 147.6 | 83.0 | 2.2 | 332.2 | 617.0 | 10.9d | |
| AGL87 | AT1G22590 | 50.8 | 107.7 | 2.2 | 85.4 | 131.3 | ns | |
| AGL96b | AT5G06500 | 109.5 | 96.5 | 0.0 | 147.0 | 224.9 | – | |
| VRN2b | AT4G16845 | 366.7 | 139.9 | 244.6 | 182.1 | 169.7 | ns | |
| VIM5 | AT1G57800 | 95.8 | 90.6 | 11.1 | 707.2 | 947.4 | ns | |
| HDG9 | AT5G17320 | 189.2 | 229.5 | 6.7 | 816.1 | 1179.9 | – | |
| HDG10 | AT1G34650 | 67.2 | 10.2 | 17.8 | 222.1 | 241.6 | – | |
| Defense response | PCC1 | AT3G22231 | 1.4 | 0.3 | 0.0 | 0.0 | 0.4 | ns |
| NPR1 | AT1G64280 | 43.4 | 18.2 | 37.8 | 18.1 | 30.5 | 1.8d | |
| NBS-LRR | AT3G44630 | 152.6 | 28.0 | 6.7 | 59.0 | 31.8 | ns | |
| RPS1 | AT3G07040 | 30.6 | 0.0 | 13.3 | 4.7 | 2.7 | ns | |
| EDS1 | AT3G48090 | 29.2 | 12.3 | 100.0 | 19.1 | 14.6 | ns | |
| CDR2b | AT5G33340 | 32.7 | 6.4 | 4.4 | 84.4 | 109.0 | ns | |
| NDR1 | AT3G20600 | 10.9 | 8.0 | 26.7 | 4.7 | 3.5 | ns | |
| SID2 | AT1G74710 | 78.3 | 67.3 | 229.0 | 125.6 | 156.8 | ns | |
ns, not significant; –, did not test mutant.
The mean number of read counts (normalized to library size) from two biological replicates.
Benjamini-Hochberg adjusted P value from differential expression analysis of Col-0 hybrid versus C24
hybrid in DESeq.
The percentage of live seed at maturity when crossed to A. arenosa.
Significant difference in live seed in comparison to Ler-0 × Aa (4.9% live seed, n = 832) in a Student’s t test at P < 0.05.
Significant difference in live seed in comparison to Col-0 × Aa (0.8% live seed, n = 3893) in a Student’s t
test at P < 0.05.
Figure 3.
Endosperm Transcription Factors and Defense Response Regulators Affect Hybrid Seed Survival.
(A) Ler-0 and mutants in Ler-0 background (x axis) were crossed as seed parents to A. arenosa, and the %NGV (i.e., live) was scored for each genotype (y axis). Ler-0 (n = 832 seeds), iku1 (n = 1617 seeds), iku2 (n = 2836 seeds), phe1 (n = 1920 seeds), ttg2 (n = 2404), and ttg2iku2 (n = 922 seeds). Decreasing expression of apparently activated transcription factors improves seed survival.
(B) The mean frequency of normal, viviparous, green, and NGV (normal + green + viviparous) seed (y axis) in control Col-0 and 1/8E/5 crosses as well as np1-1, sid2, and npr1-5 mutants (x axis). npr1-1, npr1-5, and sid2 were different from Col-0 for mean %NGV, as determined by Student’s t test at P < 0.05. Additionally, npr1-1 and npr1-5 had significantly higher %viviparous seed (P < 0.05). The ecotype background of wild-type control and npr1-5 are the same. Line 1/8E, described as Nossen-0 (Shah et al., 1997), was determined in this study to be an unknown ecotype after original seed stocks were obtained and genotyped. The mean and se are given for each cross. Letters indicate lines that were significantly different from control in a Student’s t test at P < 0.05.
Natural Variation for Defense Response Genes
We previously identified quantitative genetic variation in seed survival between Col-0 hybrids and C24 hybrids (Burkart-Waco et al., 2012). To connect this variation and the observed differences in expression, we identified those genes differentially expressed between the two ecotypes in the 3 DAP seed and silique libraries (two replicates each). A set of 1228 genes was differentially expressed between the two hybrids (Padj < 0.05) seed libraries and 753 for siliques (see Supplemental Figure 9 online). In addition, 1291 genes were differentially expressed between the Col-0 and C24 control seed libraries (Padj < 0.05). Supplemental Tables 5 and 6 online report the top six most significant GO enrichment terms (Padj < 0.05) as well as predictive protein motif (for full GO characterization, see Supplemental Data Set 2 online). Genes that were differentially expressed between Col-0 and C24 were enriched for many different environmental responses as well as secondary metabolism and defense response genes. Natural variation between Col-0 and C24 has been previously observed for freezing tolerance (Korn et al., 2008) and defense response gene activation in response to cucumber mosaic virus infection (Postnikova et al., 2011). Like Col-0 and C24, Col-0 × A. arenosa and C24 × A. arenosa displayed differential defense response, including Leucine-rich repeat (LRR) proteins (see Supplemental Tables 5 and 6 online). Of the 1291 differentially expressed genes between Col-0 and C24, 738 genes (57%) had higher expression in C24 and 553 genes in Col-0 (see Supplemental Figure 9A online). The ratio of high Col-0:high C24 was significantly different from that observed in hybrid crosses (see Supplemental Figure 9A online) where, out of 1228 differentially expressed genes, 537 genes (44%) exhibited higher expression in C24 × A. arenosa and 691 genes higher expression in Col-0 × A. arenosa (χ2 test; P = 1.78E-22). This was also observed for two biological replicates of both Col-0 and C24 hybrid 3 DAP siliques (see Supplemental Data Set 4 and Supplemental Figure 9A online). Therefore, relative to controls, in both seeds and siliques there was a net derepression of Col-0 genes in the hybrid state for both seeds and siliques, in particular for GO-enriched transcript (see Supplemental Tables 5 and 6 online).
Immune response genes were prominent among those that displayed natural variation posthybridization. The 303 transcripts (see Supplemental Figure 9C online) that were upregulated in Col-0 (relative to C24) and Col-0 × A. arenosa (relative to C24 × A. arenosa) were highly enriched for RNAs encoding proteins associated with apoptosis and defense response GO categories (see Supplemental Data Set 2 online). However, these classifications may require refinement as many of the putative proteins associated with defense are uncharacterized nucleotide binding site (NBS)-LRR genes or Cys-rich proteins for which developmental roles are possible (Marshall et al., 2011). The 250 seed transcripts that were uniquely upregulated in Col-0 hybrid also displayed defense response and cell killing signatures (P < 0.0001). Although a defense response signature was observed for genes that were high in C24 hybrid (relative to Col-0 hybrid), C24 (relative to Col-0) displayed no enrichment (see Supplemental Data Set 2 online). The upregulation of defense and immune response genes was even more predominant in Col-0 hybrid silique transcriptomes as there was a 13.5-fold enrichment in apoptotic factors (see Supplemental Data Set 2 online). Together, this indicates that Col-0 and C24 have differential defense responses and perhaps immune response genes are activated in Col-0 and to a lesser degree in C24 hybrids posthybridization (see Supplemental Data Set 2 online). Therefore, natural variation in defense response factors present in maternal parents is preserved after hybridization and may play a role in incompatibility.
Suppressing Basal Defense Response Partially Rescues Hybrid Crosses
Natural variation for defense response between Col-0 and C24 led to the hypothesis that differential activation of defense response genes affects hybrid seed survival. We used mutants to explore the role of major defense pathways. Mutant loci included both master regulators and downstream pathogen effectors. NONEXPRESSOR OF PR GENES (NPR1), which controls systemic acquired resistance through the expression of pathogenesis-related proteins, was a likely candidate gene as it is activated in response to stress and is known to promote cell death via salicylic acid signaling (Mukhtar et al., 2009; Spoel et al., 2009). Seed survival after hybridization of npr1 mutants is presented in Figure 3, Table 1, and Supplemental Table 7 online. Both npr1-1 and npr1-5 mutants produced more viviparous seed than their corresponding control crosses (Figure 3B; see Supplemental Table 7 online). Inactivation of SALICYLIC ACID INDUCTION-DEFICIENT2 (SID2), which encodes the enzyme isochorismate synthase (Wildermuth et al., 2001) required for salicylic acid synthesis, also had a beneficial effect on seed survival, a twofold increase, consistent with the npr1 mutant phenotype (Table 1; see Supplemental Table 7 online). Taken together, these results are consistent with a negative effect of defense and stress response activation in response to hybridization across ecotypes and a potential role for salicylic acid–dependent defense signaling in Arabidopsis hybrid seed death.
The Activity of Genes That Are Normally Associated with Polar Compartments Differentiates Compatible from Incompatible Responses
To connect seed tissue differentiation to the incompatibility response, we clustered the 1228 genes that differed in expression between the hybrids according to the tissue and temporal series provided by the Harada-Goldberg LCM study (Figure 2). Interestingly, clusters of genes defined by common regulation in normal seed development responded consistently. C24 hybrids displayed ∼8 times higher expression of genes associated with micropylar endosperm, suspensor and embryo, averaging above the mean expression of self-cross controls, while Col-0 hybrids averaged below the mean expression of controls. These genes encoded potential regulators AGL48, AGL57, AGL96, and AUXIN RESPONSE FACTOR12 (ARF12), ARF13, ARF21, FLOWERING WAGENINGEN FWA, SUVH8, and WRKY DNA-BINDING PROTEIN10 WRKY10. Genes whose normal expression was associated with the chalazal region had an opposite response, displaying higher expression in Col-0 hybrids. Therefore, relative overexpression of genes normally localized to the chalazal pole was associated with the worst outcome.
Investigation of AGL Proteins
AGL proteins, such as AGL62, play an important role in endosperm development (Kang et al., 2008; Shirzadi et al., 2011), and differential regulation of AGL genes is associated with deleterious effects in interspecific-interploidy Arabidopsis hybrid fitness (Josefsson et al., 2006; Walia et al., 2009). We found that 10 AGLs, including the previously documented endosperm cluster, were differentially expressed between the Col-0 hybrid and C24 hybrid seed (see Supplemental Table 8 online). AGL expression was also confirmed in hybrid silique analysis for five of 10 genes. Only two of these genes were differentially expressed between control crosses (at Padj < 0.05). However, the magnitude of expression in hybrid and control crosses was similar and in the same direction for some of these genes (see Supplemental Table 8 online), suggesting that natural variation between Col-0 and C24 may underlie the differences in AGL2, AGL25 (also known as FLOWERING LOCUS C), AGL27, and AGL31 expression. T-DNA knockouts did not improve seed survival significantly (Table 1; see Supplemental Table 9 online), suggesting lack of maternal effect or redundancy in interspecific seed survival for select AGL. In addition, AGL48, AGL49, and AGL96 were differentially regulated in hybrid crosses but did not vary between controls (see Supplemental Table 8 online). For example, AGL96 was undetectable in Col-0 hybrids and A. arenosa, suggesting potential for paternal influence on AGL regulation.
Transposon Activation Is Sporadic in Hybrid 3 DAP Seed
TE genes are reported to be induced in incompatible hybrid crosses (McClintock, 1984; Josefsson et al., 2006). To address the potential role of transposable elements and the connected natural variation in hybrid seed death, we surveyed relative expression level in the early seed transcriptome. We considered both 3900 genes annotated as transposable element genes (TEGs; such as AT1G37170) as well as 31,189 A. thaliana TEs (such as AT1TE21600) in the TAIR10 genome release, to which we aligned reads that did not map to the genes. Our findings are presented in Supplemental Table 10 and Supplemental Figure 10 online. We found that ∼3000 TEs were expressed in both hybrid and A. thaliana seed libraries. TE expression was low for both hybrid and control crosses (mean nine to 26 reads for TE and four to 36 reads for TEG) relative to the mean read count per gene in our libraries, which ranged from 300 to 400 reads and median 22 to 56 reads (see Supplemental Table 10 online). To account for variability between TE features expression, we summed read counts between replicates instead of averaging them and then analyzed fold change in Col-0 hybrid relative to Col-0. We identified relatively few TEs that were differentially expressed between hybrid and A. thaliana controls. Expression of these TEs was equally probable to be high in Col-0 or in Col-0 hybrid (see Supplemental Figure 10 online). This trend was also identified for three differentially expressed TE genes that were part of the 509 differentially expressed genes Col-0 versus Col-0 hybrid; two out of three were high in Col-0 (see Supplemental Table 11 online).
To determine how natural variation affected transposon expression, we compared Col-0 and C24 to identify differentially expressed members of the TE set. We found 56 TEG among the 1228 differentially expressed genes identified during hybridization to A. arenosa. In self crosses, 39 TEG could be identified among the 1291 differentially expressed genes. There was a large overlap between the differentially expressed hybrid and control TEG (33 out of 39 control TEG found in hybrid set), suggesting that some transposons maintain their normal pattern of regulation in the hybrid state.
We were concerned that our transposon analysis might be affected by mapping bias potentially underreporting TEs of A. arenosa. If TEs were too divergent to map on the reference genome (which is maternal), expression analysis could favor maternal elements. Paternal elements, such as Athila, which are known to be progressively more misregulated in hybrid crosses at 4 to 8 DAP (Josefsson et al., 2006), could be missed. To identify TEs that were differentially expressed without mapping reads to a reference, we queried the cDNA sequence for encoded peptide motifs characteristic of candidate TE proteins. Common TE motifs were identified for Copia and Athila elements (see Supplemental Table 12 online). We found that the number of reads with TE motifs were lower or comparable in hybrid versus control crosses for all motifs tested. This analysis included the Athila elements that are progressively activated at 4 to 8 DAP in hybrid seed development. In conclusion, both reference-dependent expression analysis (see Supplemental Figure 10 and Supplemental Tables 10 and 11 online) and ab initio counting of TE sequence motifs (see Supplemental Table 12 online) indicate that the majority of TEs maintained their normal expression pattern early after hybridization.
The overall repression of transposons was maintained after hybridization, but it is possible that TEs may still contribute to differential gene regulation through cis-regulatory action (Okamura and Nakai, 2008; Sakai et al., 2011). To test whether there was an association between differentially expressed genes and proximity to cis-linked transposons, we calculated both 5′ and 3′ distance of each gene to the nearest TE. Then, we used the Student’s t test to evaluate the hypothesis that the mean distance of differentially expressed genes to nearest transposon is not different from the mean distance calculated for a random sampling of genes of the same size. Differentially expressed genes from both hybrid and control crosses were significantly closer to TEs than one might expect by chance (Figure 4), though this association was not enhanced by hybridity.
Figure 4.
Association between Differentially Expressed Genes and TEs.
The distribution of the distance in base pairs (y axis) from genes of interest to the nearest 5′ transposon (dark gray) or 3′ transposon (light gray) was determined for each gene set (x axis). Differentially expressed hybrid and control genes were compared with two random gene sets of equal size (n = 1148 and n = 1236, randomly selected from the set of seed genes we identified in our transcriptome analysis). Comparison of the mean distance from both 5′ and 3′ with a Student’s t test determined that both hybrid and control genes are closer on average to TE than a random sample of genes (P < 0.0001). Furthermore, the dispersion of candidate genes was less than the random sample set. Genes that were classified as TEs were excluded from the analysis.
In conclusion, there was no indication of genomic shock in either compatible or incompatible hybrid crosses as most TEs maintained their wild-type expression pattern after hybridization. Differentially expressed genes are closer to TEs than expected by chance (Figure 4), which is consistent with a cis-effect of TEs on neighboring genes. Thus, Col-0 and C24 differ in the regulatory mechanism(s) affecting both transposon expression and gene expression, and the differential repression exerted by Col-0 or C24 is maintained in the hybrid state. Evaluated from the regulation of silenced elements, there is little evidence at 3 DAP of widespread differential epigenetic restructuring of silent TEs in the endosperm. Absence of early transposon activation indicates that deregulation of developmental pathways precedes TE activation and weakens the hypothesis that the latter play a role in seed death.
DISCUSSION
Hybrid Incompatibility Subverts Regulation of Endosperm and Seed Coat
Hybrid incompatibility has been attributed to multiple mechanisms, including immune responses, genomic shock resulting in TEs activation, and regulatory disruption of normal developmental pathways. To help discriminate between these mechanisms, we investigated the early regulatory changes associated with hybrid incompatibility and manifested in developing seed by comparing selfed and hybrid seed in two ecotypes differing by the degree of incompatibility. Embryonic delay in comparison to maternal self crosses was obvious at 3 DAP, while endosperm development appeared normal or nearly normal in compatible hybrids. This contrasts with the endosperm phenotype of incompatible interploidy crosses, which displayed accelerated proliferation (Scott et al., 1998; Dilkes et al., 2008). Endosperm overproliferated relative to the cognate embryo but not relative to seed age (Figure 1; see Supplemental Figure 3 online). These observations could have three explanations: First, the hybrid embryo displays additive regulation developing at a rate intermediate between the parental ones. Second, hybrid incompatibility is manifested predominantly in the embryo. Third, hybrid incompatibility affects early endosperm function but not its growth. Physiological impairment of the endosperm results in delayed embryo development. To distinguish these three hypotheses, we undertook analysis of the transcriptome.
Comparison of transcriptomes in control and hybrids revealed dramatic changes in genes normally associated with the endosperm and with the seed coat (Figure 2). Furthermore, it indicated that genes that are normally localized in the chalazal compartment undergo a distinct regulatory fate, suggesting that the chalazal compartment responds differently from the rest of the seed. Regulatory changes in the seed coat were indicated by the suppression of a gene cluster specifying proteins of secondary metabolism that are preferentially expressed during normal development in the general seed coat (Figure 2). At the same time, a cluster of coregulated genes expressed preferentially in the chalazal end seed coat was upregulated in incompatible Col-0 relative to controls. This suggests that the transcriptional response observed in hybrids is affected along the polarity axis of the seed.
Disruption of seed coat function is accompanied by increase in gene clusters that are preferentially expressed in endosperm or in chalazal endosperm. Some of these, such as the set of photosynthetic genes preferentially expressed in endosperm during seed development (Figure 2; see Supplemental Tables 2 and 3 online), could depend on pollen-parental influence. Underrepresentation of embryonic cells in the seed probably hindered detection of embryonic changes. Taken together with the rest of the regulatory changes, these data provide a molecular explanation for the time-proven practice of embryo rescue to achieve interspecific hybridization (Sharma et al., 1996). A probable scenario is one in which regulatory dysfunction in the endosperm affects both the maternal seed coat, which downregulates the secondary metabolite gene cluster, and the embryo, whose growth is compromised.
A Pathway Mediating Maternal-Zygotic Communication in the Seed Affects Hybrid Incompatibility
The gene encoding HAIKU1, an endosperm factor affecting seed size, was upregulated in hybrids. HAIKU1 interacts with HAIKU2 and maternal factor TTG2 to regulate seed size through endosperm growth (Luo et al., 2005; Wang et al., 2010). TTG2 variation affects seed death in interploidy crosses within A. thaliana (Dilkes et al., 2008). We show that both HAIKU1 and TTG2 knockouts have beneficial effects on hybrid seed survival (Table 1, Figure 3), indicating that overexpression of HAIKU1 in hybrids contributes to seed death. Inactivation of this pathway either on the maternal side (TTG2) or on the filial side (IKU1) has comparable effects. However, improvement in seed survival is only partial, consistent with multilocus incompatibility where each gene contributes to a fraction of the phenotypic variance observed in the population (Burkart-Waco et al., 2012).
The finding that the upregulated gene IKU1 (and the connected gene TTG2) improved seed survival (Table 1, Figure 3) is consistent with the aberrant expression of this gene affecting hybrid seed lethality. Furthermore, the delay observed in our developmental analysis (Figure 1; see Supplemental Figures 1 to 3 online) most likely reflects a perturbation in development due to hybridization. Yet, the transcriptional differences observed cannot be explained simply by heterochrony (i.e., not the result of extending normal development [preglobular versus globular embryo]), as the wild-type expression level of many of disregulated factors remained constant during early seed development (Figure 2).
Critical Endosperm Factors Are Downregulated
The expression levels of FIS2 and ZHOUPI, which encode seed factors required for normal seed development, were significantly suppressed in both hybrids. For Col-0 and C24, control (self-cross) expression of ZHOUPI was 16 and 6 times that of the hybrid, respectively; expression of FIS2 was 20 and 11 times (see Supplemental Figure 8 online). ZHOUPI encodes a basic helix-loop-helix endosperm transcription factor that regulates development of the embryo-endosperm interface through targets, including ALE1. Loss of either ALE1 or ZHOUPI results in shriveled seed whose embryos lack a proper cuticle (Yang et al., 2008). ALE1 expression was significantly reduced in Col-0 hybrids, but not in C24 hybrids, consistent with ZHOUPI regulation. FIS2 encodes a subunit of the POLYCOMB REPRESSIVE COMPLEX (PRC). Its reduction might explain the previously reported alterations in the expression of Polycomb-regulated PHERES (Josefsson et al., 2006) and perhaps HDG9 and IKU1 misexpression (see Supplemental Figure 8C online). Interestingly, low FIS2 and ZHOUPI expression in A. arenosa could potentially explain its low expression in the hybrid. The A. arenosa expression pattern could result from its different developmental strategy in early seed development, namely, rapid endosperm proliferation coupled with delayed embryo growth (see Supplemental Figure 1 online; Bushell et al., 2003). Alternately, the mechanism governing ZHOUPI and FIS2 misexpression may also be responsible for HDG10 and IKU1 derepression. A candidate pathway is the one responsible for DNA methylation, as both FIS2 and HDG9 are imprinted via DNA methylation and maternally expressed (Jullien et al., 2006; Gehring et al., 2009). The relationship between expression level and function in the seed is unknown. Reduction of the ZHOUPI target ALE1 in Col-0 hybrids and not in C24 hybrids could contribute to their differential response. However, this hypothesis will have to be evaluated by transgenic supplementation to establish whether these changes are causal to the incompatibility syndrome and whether the stronger suppression in Col-0 is significant.
Early Deregulation of Select AGL Genes
Analysis of natural variation between Col-0 and C24 hybrids identified 1228 genes that were differentially expressed. To evaluate the contribution of natural variation to interspecific seed survival, we prioritized candidate genes by seed compartmentalization and potential role in other incompatibility syndromes. We identified 10 AGL genes, preferentially expressed in the endosperm (Figure 2) that displayed accession-dependent expression. Loss-of-function mutations in these factors did not impact seed survival (Table 1; see Supplemental Table 8 online), although is difficult to know whether this is caused by lack of maternal effect or haploinsufficiency or because AGL31 and VRN2, which are known to affect vernalization and flowering, are not required for seed development (Ratcliffe et al., 2003; Yan et al., 2004). Other AGLs (for example AGL96 and AGL40), which display differential regulation relative to A. thaliana crosses and polarity in compartmentalization, remain to be investigated, in particular endosperm-localized and imprinted AGL96 (Köhler et al., 2012), whose chromosomal localization is consistent with one of the incompatibility QTL described by Burkart-Waco et al. (2012) (Table 2) and appears to be deficient like FIS2 protein.
Table 2. QTL Candidates.
| Category | Gene | Locus | Chra | Startb | Endc | QTL Candidated | QTLe |
|---|---|---|---|---|---|---|---|
| Seed transcription factors | FIS2 | AT2G35670 | Chr2 | 14,992,565 | 14,996,757 | + | FOE |
| PHERES1 | AT1G65330 | Chr1 | 24,266,481 | 24,267,321 | + | FEE | |
| TTG2 | AT2G37260 | Chr2 | 15644840 | 15647066 | − | ||
| IKU1 | AT2G35230 | Chr2 | 14,842,293 | 14,844,435 | + | FOE | |
| IKU2 | AT3G19700 | Chr3 | 6,843,662 | 6,847,276 | + | FII | |
| VRN2 | AT4G16845 | Chr4 | 9,476,143 | 9,479,879 | + | OGR | |
| Defense response genes | NPR1 | AT1G64280 | Chr1 | 23,852,748 | 23,855,567 | + | FEE |
| NDR1 | AT3G20600 | Chr3 | 7,194,846 | 7,195,737 | − | FII | |
| SID2 | AT1G74710 | Chr1 | 28,070,295 | 28074149 | + | FEE | |
| AGL proteins | AGL12 | AT1G71692 | Chr1 | 26,952,645 | 26,955,128 | + | FEE |
| AGL29 | AT2G34440 | Chr2 | 14,526,950 | 14,527,690 | + | FOE | |
| AGL96 | AT5G06500 | Chr5 | 1,982,444 | 1,983,173 | + | BSK |
Chr, chromosome.
Gene start position.
Gene end position.
Candidate gene based on position and mutant hybrid seed survival.
QTL associated with natural variation for interspecific seed between Col-0 and C24 (described in Burkart-Waco et al., 2012).
A Defense-Like Response Involving NPR1 Mediates Hybrid Seed Death
Defense genes are activated in response to hybridity (see Supplemental Tables 2 and 3 online). Stress-related responses have been reported previously from the analysis of somatic tissue such as leaves (Chen and Ni, 2006), but cause and effect was not clear from these observations. The genes classified in this response encompass genes encoding NBS-LRR and defensin/CRPs. A few of these proteins, including NPR1, are confirmed defense factors. Our expression analysis uncovered natural variation for these stress-related genes’ expression and this variation is maintained after hybridization being evident at 3 DAP (see Supplemental Tables 5 and 6 online). Deregulation of defense response pathways contributes to seed death as suppressing defense responses, either through inactivation of the sensor NPR1 or of the salicylic acid biosynthetic enzyme encoded by SID2, increases seed survival (Figure 3B; see Supplemental Table 7 online). NPR1 and SID2 activity was deleterious for hybrid survival, but we found no evidence that this response was mediated by the negative interaction of two factors resulting in hybrid necrosis such as observed by Bomblies et al. (2007). The contribution of NPR1 to A. thaliana × A. arenosa seed survival is modest, consistent with the finding that at least seven QTL explain variation between C24 and Col-0 hybrids (Burkart-Waco et al., 2012).
Notwithstanding the GO classification suggesting activation of apoptotic pathways (see Supplemental Tables 2 and 3 online), no induction of pathogenesis-related proteins or of bona fide apoptosis markers was detected. Studies in maize (Zea mays) indicate that CRPs are involved in nutrient transfer between endosperm and embryo (Magnard et al., 2000; Balandín et al., 2005; Marshall et al., 2011), suggesting a similar developmental role in Arabidopsis. It is possible that the induced NBS-LRR, including NPR1, may serve a regulatory role during seed development. NPR1 is known to interact with TTG2 in tobacco (Nicotiana tabacum; Li et al., 2012); perhaps in Arabidopsis it senses disrupting events in the cell and signals the maternal seed coat or endosperm to subvert development.
TEs Do Not Undergo Widespread Activation in the Early Incompatibility Response but Are Involved in Regulatory Changes
If epigenetic remodeling of heterochromatin leading to catastrophic activation of TEs triggers seed death, increased expression of TEs should be manifest at an early stage. We failed to find such a smoking gun. Mean and distribution of expression of TEs in controls and hybrid crosses were essentially the same (see Supplemental Tables 10 and 11 and Supplemental Figure 10 online). Alignment-independent motif analysis indicated that there is not an overrepresentation of Copia or Athila motifs in 3 DAP hybrid seed (see Supplemental Table 12 online), making it unlikely that we failed to detect changes derived from expression of the paternal genome. We identified modest differences in transposon suppression between treatments (23 TEG that were differentially expressed between hybrids but not controls), indicating that some TEs were differentially regulated after hybridization. The outcome of such divergence affected selected TEs, but it also had repercussions on gene expression because regulated genes were physically closer to TEs than expected (Figure 4).
A Summary View of Genetic and Molecular Events Observed during Postzygotic Incompatibility
Collectively, considerable information has been gained on the A. thaliana × A. arenosa postzygotic incompatibility syndrome from developmental, molecular, and genetic analyses (Comai, 2000; Bushell et al., 2003; Josefsson et al., 2006; Walia et al., 2009). Genetically, the largest effect on hybrid seed survival is mediated by maternal dosage, whereby doubling the maternal contribution rescues the cross yielding 70 to 90% live seeds. This indicates that a maternal factor or factors is deficient in the diploid cross studied here. Deficiency in this unknown factor (factors) affects endosperm 3 DAP. Possible candidates are P4-siRNAs, a 24-nucleotide small RNA class dependent on RNA Pol IV, that are contributed maternally and are candidate factors for this (Mosher et al., 2009; Lu et al., 2012) as they are associated with AGL and TE induction (Lu et al., 2012). Here, we find (Figure 5) that at 3 DAP, well before endosperm overproliferation, Athila retroelement activation, and seed shrinking, there is ZHOUPI and FIS2 downregulation. This downregulation provides a potentially attractive explanation for the shrunken embryo phenotype, which may depend on the ZHOUPI-ALE1 pathway, and for endosperm overproliferation, which may depend on AGL regulation by FIS2 (Kang et al., 2008; Walia et al., 2009). A role for FIS2, and its connection to the hypothetical loss of PRC function, is consistent with its location within the FOE incompatibility QTL (Burkart-Waco et al., 2012). An additional FOE QTL candidate is IKU1 (Table 2), a factor affecting endosperm and seed size, whose role is supported by the increased compatibility of the hybrid cross in the iku1 mutant background. The function of IKU1 in incompatibility is further supported by the comparable effect of a mutation in TTG2, a maternal factor required for function of the IKU1 pathway. The activity of the TTG2-IKU1 pathway in interspecific incompatibility is supported by loss in expression of secondary metabolite genes in the seed coat. This alteration of the expression program in the maternal tissue must result from a signal emanating from the hybrid zygotes, most likely the endosperm, and underscores the similarity to intraspecific incompatibility (Dilkes et al., 2008). The complexity of the syndrome is underscored by the function of the NPR1-SID2 pathway, the activation of defense-like responses and the parental genotype–dependent activation of AGL genes. It is possible that all these responses may be caused by a single event, perhaps the loss of FIS2 regulation. According to this hypothesis, the incompatibility phenotype may result from PRC2-dependent disruption of downstream pathways, including TTG-IKU1, NPR1-SID2, and certain AGLs. Modulation of these pathways, either through genetic manipulations or through natural variation could explain the variation in phenotype, such as observed between Col-0 and C24. Alternatively, the dosage dependent factor(s) deficient in the seed mother may have multiple independent effects that contribute to incompatibility. However, our 3 DAP transcriptome analysis found no evidence of early widespread TE activation. This is inconsistent with the hypothesis that parental imbalance has a primary and frequent effect on heterochromatin maintenance (Dilkes and Comai, 2004; Madlung et al., 2005; Martienssen, 2010; Lu et al., 2012).
Figure 5.
Summary of Developmental and Molecular Events during Normal Development and Interspecific Hybridization.
The top part of the figure illustrates normal seed development in the early steps (days 1 to 6) after pollination. The chalazal end of the seed is organized in chalazal seed coat (maternal) and chalazal endosperm (zygotic), which are thought to form a conduit for nutrient transmission from the seed mother to the zygotes. The bottom part of the figure illustrates the main developmental and molecular changes observed in this study (3 DAP) or reported in the literature (4, 5, and 6 DAP). The results, summarized in this figure, demonstrate that regulatory disruption is evident well before endosperm proliferation and activation of Athila retroelements.
[See online article for color version of this figure.]
In summary, our analysis of seed development in this incompatible system has revealed the effect on seed survival of disruption in early communication between the zygote(s) and the maternal tissues enveloping it. Subversion of multiple key regulators in the endosperm is accompanied by the activation of photosynthetic and ribosomal genes, and a cluster of NBS-LRR, one of which, NPR1, acts through the salicylate pathway to suppress seed survival. The decreased expression of a critical PRC2 member, FIS2, suggests future paths of investigation.
METHODS
Plant Growth
All plants were grown under long-day conditions (16 h light at 21°C and 8 h dark at 18°C) in a controlled environment facility at the University of California, Davis, CA. For time-course and expression analysis, the female Col-0 (seed stock ID CS6673) and C24 (seed stock ID CS22620) diploid ecotypes of Arabidopsis thaliana were hemizygous for a male sterility construct (Paul et al., 1992) and were gifts from Rod Scott (University of Bath, UK). Diploid Arabidopsis arenosa var Strecno was originally collected by Marcus Koch near Strecno Castle in Slovakia and was provided to us by M. Lysak. The male-sterile A. thaliana lines were used as seed parent, and A. arenosa was used as the pollen donor for all interspecific crosses as the reciprocal cross is prevented by unilateral pollen incompatibility (Lewis and Crowe, 1958). A single pair of A. arenosa plants was used for all crosses in these experiments to reduce genetic variation due to heterozygosity in A. arenosa lines. Crosses were performed at 10 am to reduce circadian biases by pollinating fully developed stigmas by hand; no emasculation was necessary because of the male sterility construct.
Mutant Analysis
Unless otherwise noted, all mutant lines are listed in Supplemental Table 13 online and were ordered from the ABRC. NahG and eds1-1 were gifts from the R. Michelmore laboratory at the University of California, Davis; the npr1-5 line and parent line (1/8E/5) were gifts from the D. Klessig laboratory at Cornell. NPR1 DNA was amplified from each line (primers 5′-GAGGACACATTGGTTATACTC-3′ and 5′-CAAGATCGAGCAGCGTCATCTTC-3′), and codominant cleaved amplified polymorphic sequence analysis was used to determine the genotype at the NPR1 locus (Shah et al., 1997, 1999). The npr1-1 mutant lacks NlaIII restriction site, and the npr1-5 allele lacks NlaIV as described (Cao et al., 1994; Shah et al., 1997, 1999). All other mutant lines were genotyped with standard primers as provided by the SIGNAL database (http://signal.salk.edu/). After genotyping, unopened buds were emasculated and allowed to develop for 24 h. Mutants and controls (same ecotype as mutant line) were then pollinated with A. arenosa pollen, and seed phenotypes were assessed as previously described (Burkart-Waco et al., 2012). Each cross was performed in triplicate unless otherwise noted. Mutant effect on seed phenotype was assessed by comparison of mean between the wild type crossed to A. arenosa and mutant hybrids with the Student’s t test.
Microscopy
To score embryo and endosperm stage, siliques were harvested at 2, 3, 4, 5, and 6 DAP and dissected with a hypodermic needle under a stereomicroscope. Seeds were cleared using Hoyer’s medium (chloral hydrate:distilled water:glycerol [80:30:10 g]), mounted, and imaged using differential interference contrast microscopy. Peripheral endosperm nuclei were imaged from up to seven optical sections through whole mounted seeds. Endosperm nuclei were counted in a section of the seed (about half the peripheral endosperm area as determined by pixel counts in Adobe Photoshop CS3). The number of nuclei was then scaled to reflect the total endosperm area, which was also determined graphically in Photoshop. Seed coat measurements, from Photoshop CS3 (Adobe) pixel counts, were made on one optical section of mounted seed (Col-0, n = 12 seed at 2 DAP and n = 26 seed at 3 DAP; C24, n = 10 seed at 2 DAP and n = 22 seed at 3 DAP; Col-0 hybrid, n = 8 seed at 2 DAP and n = 19 seed at 3 DAP; C24 hybrid, n = 7 seed at 2 DAP and n = 7 seed at 3 DAP). Development images were taken on a Leica DM-6000 (Leica Microsystems) with Nomarski optics using a DFC 350 FX camera (Leica). Images were processed with Leica Application Suite V3 (Leica Microsystems) and Illustrator CS4 (Adobe).
Tissue Preparation for Transcriptome Profiling
Two biological replicates were prepared for each condition for transcriptome analysis (see Supplemental Figure 4A online). Immature seeds or siliques were harvested from ColA9 × A. arenosa, C24A9 × A. arenosa, ColA9 × Col-0, C24A9 × C24, and A. arenosa × A. arenosa and immediately frozen in liquid nitrogen. Total RNA was obtained using Plant RNA reagent (Invitrogen). Frozen tissue was placed in a 1-mL Dounce tissue grinder (Omni International) with 200 μL plant RNA reagent. The tissue was ground for ∼30 s and then the extract was transferred to a clean tube. The grinder was washed with 300 μL of plant RNA reagent, which was combined with the tissue extract. RNA extraction proceeded as described in the manufacturer’s protocol and was followed by DNase I (Invitrogen) digestion at 37°C for 60 min according to the manufacturer’s recommendation to remove any DNA contamination. RNA was eluted in 20 μL DNase-free water, and concentration was determined using a NanoDrop 1000 spectrophotometer. RNA was stored at −80°C for downstream applications.
Illumina Sequencing
Approximately 10 μg of total RNA was processed in preparation for Illumina Sequencing using a homemade kit. All enzymatic reactions followed manufacturer’s protocol unless otherwise noted, and a list of reagents is provided in Supplemental Table 14 online. mRNA was purified from total RNA using Dynabeads (Invitrogen), which purify poly(A)-containing transcripts. Then, cDNA was synthesized with random priming using Superscript III (Invitrogen) followed by heat inactivation for 5 min at 85°C. Second-strand cDNA was then synthesized as follows: We combined 5× second-strand buffer (200 mM Tris-HCl, pH 7, 22 mM MgCl2, and 425 mM KCl), water, and 10 mM deoxynucleotide triphosphates with the first-strand mix. The reaction was incubated on ice for 5 min and then DNA polymerase I (NEB) and RNaseH (NEB) were added and the reaction was incubated at 16°C for 2.5 h. The double-stranded cDNA was cleaned using Agencourt AMPure XP (Beckman Coulter Genomics) with a 1.8:1 (v/v) AMPure to initial reaction volume ratio. After cDNA synthesis, each library was chemically fragmented using 1 μL of NEB’s dsDNA Fragmentase for 30 min at 37°C and cleaned with AMPure. Fragmentation was followed by end repair with NEB’s End Repair Module Enzyme Mix, and A-base overhangs, which are needed for adaptor ligation, were added with Klenow (NEB). End repair and A-base addition were both followed by AMPure cleanup. Barcoded Illumina adaptors for controls and regular Illumina adaptors for controls (see Supplemental Table 15 online) were ligated at room temperature using NEB Quick Ligase. To remove adapter contamination in library sequencing, we size-selected our libraries using AMPure. Changing the amount of AMPure buffer changes fragment size selection. We used 0.1:1 (v/v), AMPure:initial reaction volume to obtain fragments (cDNA insert + adaptor) greater than 300 bp and eluted in 20 μL Qiagen elution buffer. Ten microliters of each library was enriched using 15 μL Phusion 2x HF master mix (NEB), 3 μL water, and 2 μL 5 μM premixed PE primers (see Supplemental Table 15 online) with the following reaction conditions: 30 s at 98°C; 14 cycles of 10 s at 98°C, 30 s at 65°C, and 30 s at 72°C; final extension with 5 min at 72°C. Enriched libraries were purified with AMPure (0.8:1 v/v; AMPure library), and quality and quantity were assessed using the Agilent BioAnalyzer. Libraries were sequenced according to the manufacturer’s instructions. The hybrids were sequenced using Illumina’s HiSequation 2000 (100-bp paired-end reads), and the controls were sequenced using Illumina’s HiSequation 2000 (100-bp paired-end reads) or Illumina’s Genome Analyzer II (40-bp single-end reads).
Computational Analysis
For an overview of the bioinformatics pipeline, see Supplemental Figure 4B online.
Sequence Preprocessing
All sequence preprocessing was done with custom Python scripts available at the Comai lab wiki (http://comailab.genomecenter.ucdavis.edu/index.php/Barcoded_data_preparation_tools). We chose to process forward reads only, so as not to inflate count scores. Sequences were trimmed using four separate scripts to remove adaptors, remove barcodes, convert Illumina 1.5+ fastq to sanger, and remove ambiguous bases. Reads that were shorter than 35 bp were discarded along with sequence with base quality score lower than Phred 20.
Alignment
To assess gene expression, each biological replicate was aligned separately to both TAIR10 cDNA (available for download at ftp://ftp.Arabidopsis.org//home/tair/Sequences//blast_datasets/TAIR10_blastsets/TAIR10_cdna_20101214) using Burrows-Wheeler Aligner version 0.5.8c (Li and Durbin, 2009). We also aligned unmapped reads to a set of ∼31K transposons annotated in the TAIR10 genomic gff file (available at http://www.Arabidopsis.org). After alignment, read counts were generated using a custom Python script, Sam Gene Counter (http://comailab.genomecenter.ucdavis.edu/index.php/Hybrid_Transcriptome). Sequences that mapped to more than one gene model of the same gene were saved and counted as one read count, while sequences that mapped to more than one gene were excluded. See Supplemental Table 1 online for mapping efficiency and Supplemental Figure 7 online for the high degree of correlation in read counts between biological replicates. Read count/locus tables for both genes and TE are available at the Gene Expression Omnibus (GSE42957).
A. arenosa Processing
A. arenosa libraries were processed as described in the alignment section. After alignment, we found a high degree of repetitive reads (reads with same start position) even if all mid-procedure quality control steps were consistent with good progress. Because of the small library size, we assumed this was because of reduced RNA complexity. To avoid count inflation, we ran an overamplification detection script (http://comailab.genomecenter.ucdavis.edu/index.php/Hybrid_Transcriptome) to remove all but one copy of repeated reads. Then, counts from the filtered FASTAQ files were generated as described above. The low quality of these libraries likely depends on some intrinsic feature of the A. arenosa seed: Multiple attempts employing different methods of RNA purification and performed by different individuals resulted in severely to moderately flawed results (as evaluated by sequencing) while virtually all other RNA sequencing libraries yielded satisfactory results.
Expression Analysis
Differential expression was assessed in R (version 2.14.1) using the R package DESeq (version 1.6.1; http://bioconductor.org/packages/release/bioc/html/DESeq.html), which is designed to test differential expression of count data from two biological replicates taking into account both technical and biological variability (Anders and Huber, 2010). A negative binomial distribution with parametric fit was used to assess differential expression based on variance estimates. All read counts were normalized to a library size factor calculated by DESeq, which takes into account the read count for each library and then scales read counts up or down to normalize between all libraries. We set a threshold of Benjamini-Hochberg (Benjamini, 1995; Benjamini and Yekutieli, 2005) adjusted P < 0.05 to call differentially expressed genes.
Paternal Gene Analysis
To assess paternal gene expression, we aligned each replicate to an A. arenosa cDNA reference using a set of 40,205 novel ESTs. The tags were assembled from Illumina reads obtained by sequencing cDNA. The cDNA was prepared from RNA isolated from flowering plants of A. arenosa using Velvet, CLC, and CAP3 (Huang and Madan, 1999). Most contigs (∼28K) had predicted A. thaliana orthologs with 90% identity across >400 bp. The remaining contigs did not have a good BLAST match. All reads that did not map to A. thaliana cDNA were mapped to Aa-cDNA to maximize mapping efficiency. We found that <5% of reads mapped to A. arenosa contigs from each condition (Col-0 = 397,885 reads; C24 = 389,359 reads; Col-0 hybrid = 736,394 reads; C24 hybrid = 1,000,408 reads) and 66% of the mapping matches were to genes that lacked an orthologous assignment. Because of the small proportion of total reads that mapped to A. arenosa contigs and the observation that A. thaliana and A. thaliana hybrids mapped to A. arenosa cDNA, we decided that A. thaliana mapping was sufficient for expression analysis.
GO and Reference Gene Set Comparisons
To identify the set of genes that are normally expressed in preglobular and globular stage seed, we looked at the set overlap between our Burrows-Wheeler Aligner alignments in comparison to genes identified by the Goldberg and Harada laboratories using the Affymetrix array (Ath1, GEO Platform GPL198). All data are publically available at http://seedgenenetwork.net under the Arabidopsis project. MAS5 presence/absence calls were used to assess gene expression in any seed compartment of preglobular, globular stage, heart, linear cotyledon, and mature green (statistical algorithms were as specified by the microarray manufacturer, http://www.affymetrix.com). All the Ath1 annotated genes are present in Arabidopsis TAIR10 cDNA, so comparisons between Harada-Goldberg and our gene set were possible without annotation conversions. To assess biological significance, we looked at GO enrichment using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources version 6.7 (http://david.abcc.ncifcrf.gov/) (Huang et al., 2009a, 2009b), which is a bioinformatics tool to functionally characterize gene enrichment based on annotations and functional domains. We used (1) the full Arabidopsis gene set background gene set and (2) a set of genes expressed in both Col-0 and C24 3 DAP seed with coverage more than four reads (see Supplemental Data Set 1 online) with maximum enrichment threshold of Padj (Benjamini) < 0.05. Significant predictive protein signatures from InterPro (http://www.ebi.ac.uk/interpro/) identified by DAVID were also taken into account in the functional analysis. To assess gene coexpression during development, we use the data set available at AtGenExpress (http://www.weigelworld.org/resources/microarray/AtGenExpress) (Schmid et al., 2005) to obtain expression values during development and JMP 9.0 (SAS) statistical suite to perform multivariate analysis.
Transposon Analysis
Transposons in the A. thaliana genome are annotated in two different ways: 3900 TEGs are included in the gene set and thus listed in the TAIR10_GFF3_gene.gff file. In addition, a 31K set of TEs is classified by the notation AT0TE00000 found in the TAIR10_GFF3_genes_transposons.gff. Both GFF files are available for download from The Arabidopsis Information Resource (http://www.Arabidopsis.org).
To assess proximity of differentially expressed genes to TEs, coordinates for each gene in the Arabidopsis genome (28,496 genes) as well as TE map position (for ∼31K TAIR10 TE) and origin of replication were obtained from The Arabidopsis Information Resource (http://www.Arabidopsis.org) using the TAIR10 GFF file. For all annotated genes that were not TEGs, we calculated the minimum distances in base pairs between their 5′ and 3′ ends (with respect to transcription) and the closest flanking (in either direction) TE. This file is available at (http://comailab.genomecenter.ucdavis.edu/index.php/Hybrid_Transcriptome). We determined distance information for seed genes, 19,820 genes out of the 20,768 having more than four reads in both Col-0 and C24 (see Supplemental Figure 5 online), and differential expressed genes between hybrid (Col-0 hybrid versus C24 hybrid) and control sets (Col-0 versus C24). The null hypothesis that mean 5′ distance and mean 3′ distance of differentially expressed gene was not closer to TE was tested with a Student’s t test using a random sample of seed genes with n equal to the number of differentially expressed genes as a control gene set. This random sampling was replicated 10 times to reduce sampling effects; only one of the 10 samples is displayed in Figure 4.
Motif analysis was employed to determine if common or specific TE motifs (see Supplemental Table 12 online) were overrepresented in any library. Peptide motifs common to many elements were selected from alignments of Arabidopsis copia or gypsy sequences. The motifs were tested for specificity by BLASTP analysis against Arabidopsis protein. To identify motifs in the RNA sequencing data, the first 30 bases of each read, regardless of its alignment behavior, were translated into six possible frames, and the occurrence of the target motifs was scored with a custom Python script. Control motifs were derived by inverting the real ones, such as the ELVISLIVES motif producing a SEVILSIVLE control. The counts were normalized to account for the total number of reads in the scored library, which ranged from 9M to 23M.
Accession Numbers
Raw and processed sequence data can be found at the Gene Expression Omnibus repository (GSE42957). A list of Arabidopsis accession numbers is provided in Supplemental Table 13 online.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Comparison of A. arenosa to Hybrid and A. thaliana Embryos at 5 DAP.
Supplemental Figure 2. Phenotypic Variability of Shriveled Hybrid Seed.
Supplemental Figure 3. Peripheral Endosperm Nuclei of 3 DAP Arabidopsis Seed.
Supplemental Figure 4. Experimental Design for Differential Expression Analysis.
Supplemental Figure 5. Expression Profiles from Transcriptome Analysis of High and Low Compatibility Hybrids.
Supplemental Figure 6. Comparison of Differential Expression between Hybrids and Controls.
Supplemental Figure 7. Correlation between Read Counts in Biological Replicates.
Supplemental Figure 8. Misregulation of Endosperm Transcription Factors Posthybridization.
Supplemental Figure 9. Characterization of Natural Variation for Gene Expression.
Supplemental Figure 10. Expression Analysis of Transposable Elements during Hybridization.
Supplemental Table 1. Library Statistics.
Supplemental Table 2. Gene Ontology Enrichment of Differentially Expressed Genes between Control and Hybrids Relative to TAIR10.
Supplemental Table 3. Gene Ontology Enrichment of Differentially Expressed Genes between Control and Hybrids Relative to Seed Genes.
Supplemental Table 4. Gene Expression Level of Genes with Predictive “Cell Killing” Function.
Supplemental Table 5. Gene Ontology Enrichment of Differentially Expressed Genes in 3 DAP Seed between Col-0 and C24 Relative to TAIR10.
Supplemental Table 6. Gene Ontology Enrichment of Differentially Expressed Genes in 3 DAP Seed between Col-0 and C24 Relative to Seed Genes.
Supplemental Table 7. Interspecific Seed Phenotypes of Defense Response Mutant Lines.
Supplemental Table 8. Expression Analysis of AGAMOUS-LIKE Genes.
Supplemental Table 9. Interspecific Seed Phenotypes of Mutant AGAMOUS-LIKE Genes in T-DNA Lines.
Supplemental Table 10. Transposable Element Read Count Statistics.
Supplemental Table 11. Differential Expression Analysis of Transposable Element Genes.
Supplemental Table 12. Kmer Analysis of Transposable Element Expression (Hits/10M Reads).
Supplemental Table 13. Mutant Line Accession Number.
Supplemental Table 14. Reagents for Library Preparation.
Supplemental Table 15. Adaptor and Barcode Sequences.
Supplemental Data Set 1. Seed Genes.
Supplemental Data Set 2. Gene Ontology Data Sets.
Supplemental Data Set 3. A. arenosa Comparisons.
Supplemental Data Set 4. Shared DESeq Genes between Seeds and Siliques.
Acknowledgments
Illumina sequencing was primarily performed by UC Davis Genome Center DNA Technologies Core Facility. We thank Henriette O'Geen for technical support and assistance with sequencing. We also thank Jennifer Monson-Miller for optimizing our Illumina library protocol and Meric Lieberman and Victor Missirian for insight into computational analysis. We thank Isabelle Henry, Siobhan Brady, and Daniel Kliebenstein for their intellectual contributions to the project and for comments on the article. We thank the Harada Lab and Ryan Kirkbride for sharing their seed microarray data prior to publication. We thank Daniel Klessig, Rod Scott, and M. Lysak for seed and thank Dennis Lewis and the staff at the UC Davis Controlled Environment Facility for excellent plant growth facilities. We also thank anonymous reviewers for helpful suggestions. This work was funded by National Institutes of Health R01 GM076103-01A1 (dosage-dependent regulation in hybridization) grant to L.C.
AUTHOR CONTRIBUTIONS
D.B.-W. planned and performed research, analyzed data, and wrote the article. K.N. contributed new computational tools, analyzed data, and edited the methods section. B.D. performed research and edited the article. C.J. performed research and edited the article. L.C. designed research, contributed new computational tools, analyzed data, and wrote the article.
Glossary
- Col-0
Columbia-0
- QTL
quantitative trait loci
- DAP
days after pollination
- GO
Gene Ontology
- CRP
Cys-rich peptide
- LCM
laser capture microdissection
- Ler-0
Landsberg erecta-0
- %NGV
%normal + green + viviparous
- NBS
nucleotide binding site
- LRR
Leucine-rich repeat
- TEG
transposable element genes
- TE
transposable element
References
- Anders S., Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandín M., Royo J., Gómez E., Muniz L.M., Molina A., Hueros G. (2005). A protective role for the embryo surrounding region of the maize endosperm, as evidenced by the characterisation of ZmESR-6, a defensin gene specifically expressed in this region. Plant Mol. Biol. 58: 269–282 [DOI] [PubMed] [Google Scholar]
- Baroux C., Spillane C., Grossniklaus U. (2002). Genomic imprinting during seed development. Adv. Genet. 46: 165–214 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. (2005). Quantitative trait loci analysis using the false discovery rate. Genetics 171: 783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg H. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J.R. Stat. Soc., B 57: 289–300 [Google Scholar]
- Berger F., Chaudhury A. (2009). Parental memories shape seeds. Trends Plant Sci. 14: 550–556 [DOI] [PubMed] [Google Scholar]
- Bomblies K., Lempe J., Epple P., Warthmann N., Lanz C., Dangl J.L., Weigel D. (2007). Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 5: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart-Waco D., Josefsson C., Dilkes B.R., Kozloff N., Torjek O., Meyer R., Altmann T., Comai L. (2012). Hybrid incompatibility in Arabidopsis is determined by a multiple-locus genetic network. Plant Physiol. 158: 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell C., Spielman M., Scott R.J. (2003). The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. Plant Cell 15: 1430–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Bowling S.A., Gordon A.S., Dong X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A.M., Ming L., Miller C., Craig S., Dennis E.S., Peacock W.J. (1997). Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94: 4223–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.J. (2010). Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 15: 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.J., Ni Z. (2006). Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. Bioessays 28: 240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J.Y., Hung Y.S., Lin K.H., Lee H.Y., Leu J.Y. (2010). Multiple molecular mechanisms cause reproductive isolation between three yeast species. PLoS Biol. 8: e1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J.Y., Leu J.Y. (2010). Speciation through cytonuclear incompatibility: Insights from yeast and implications for higher eukaryotes. Bioessays 32: 401–411 [DOI] [PubMed] [Google Scholar]
- Comai L. (2000). Genetic and epigenetic interactions in allopolyploid plants. Plant Mol. Biol. 43: 387–399 [DOI] [PubMed] [Google Scholar]
- Comai L., Tyagi A.P., Winter K., Holmes-Davis R., Reynolds S.H., Stevens Y., Byers B. (2000). Phenotypic instability and rapid gene silencing in newly formed Arabidopsis allotetraploids. Plant Cell 12: 1551–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilkes B.P., Comai L. (2004). A differential dosage hypothesis for parental effects in seed development. Plant Cell 16: 3174–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilkes B.P., Spielman M., Weizbauer R., Watson B., Burkart-Waco D., Scott R.J., Comai L. (2008). The maternally expressed WRKY transcription factor TTG2 controls lethality in interploidy crosses of Arabidopsis. PLoS Biol. 6: 2707–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. (1936). Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., Fitz Gerald J.N., Berger F. (2005). Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell 17: 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., Saingery V., Chambrier P., Mayer U., Jürgens G., Berger F. (2003). Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 131: 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M., Bubb K.L., Henikoff S. (2009). Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324: 1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M., Choi Y., Fischer R.L. (2004). Imprinting and seed development. Plant Cell 16 (Suppl): S203–S213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Marcos J.F., Pennington P.D., Costa L.M., Dickinson H.G. (2003). Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M., Lu J., Tian L., Ramachandran V., Kasschau K.D., Chapman E.J., Carrington J.C., Chen X., Wang X.J., Chen Z.J. (2009). Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids. Proc. Natl. Acad. Sci. USA 106: 17835–17840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D., Westoby M. (1991). Genomic imprinting in endosperm: Its effects on seed development in crosses between species and between different ploidies of the same species, and its implications for the evolution of apomixis. Phil. Trans. R. Soc. Lond. B. 333: Issue 1266, 1–13 [Google Scholar]
- Hochholdinger F., Hoecker N. (2007). Towards the molecular basis of heterosis. Trends Plant Sci. 12: 427–432 [DOI] [PubMed] [Google Scholar]
- Huang W., Sherman B.T., Lempicki R.A. (2009a). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Huang W., Sherman B.T., Lempicki R.A. (2009b). Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Madan A. (1999). CAP3: A DNA sequence assembly program. Genome Res. 9: 868–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken M.J., Zhang N.W., McHale L.K., Pelgrom K., den Boer E., Lindhout P., Michelmore R.W., Visser R.G., Niks R.E. (2009). Rin4 causes hybrid necrosis and race-specific resistance in an interspecific lettuce hybrid. Plant Cell 21: 3368–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.S., Kolevski B., Smyth D.R. (2002). TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14: 1359–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson C., Dilkes B., Comai L. (2006). Parent-dependent loss of gene silencing during interspecies hybridization. Curr. Biol. 16: 1322–1328 [DOI] [PubMed] [Google Scholar]
- Jullien P.E., Kinoshita T., Ohad N., Berger F. (2006). Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18: 1360–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I.H., Steffen J.G., Portereiko M.F., Lloyd A., Drews G.N. (2008). The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. Plant Cell 20: 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkush K., Feldman M., Levy A.A. (2003). Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat. Genet. 33: 102–106 [DOI] [PubMed] [Google Scholar]
- Köhler C., Wolff P., Spillane C. (2012). Epigenetic mechanisms underlying genomic imprinting in plants. Annu. Rev. Plant Biol. 63: 331–352 [DOI] [PubMed] [Google Scholar]
- Kondou Y., Nakazawa M., Kawashima M., Ichikawa T., Yoshizumi T., Suzuki K., Ishikawa A., Koshi T., Matsui R., Muto S., Matsui M. (2008). RETARDED GROWTH OF EMBRYO1, a new basic helix-loop-helix protein, expresses in endosperm to control embryo growth. Plant Physiol. 147: 1924–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn M., Peterek S., Mock H.P., Heyer A.G., Hincha D.K. (2008). Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ. 31: 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger U., Lippman Z.B., Zamir D. (2010). The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 42: 459–463 [DOI] [PubMed] [Google Scholar]
- Krüger J., Thomas C.M., Golstein C., Dixon M.S., Smoker M., Tang S., Mulder L., Jones J.D. (2002). A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296: 744–747 [DOI] [PubMed] [Google Scholar]
- Le B.H., et al. (2010). Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 107: 8063–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M.P., Vernoux T., Busch W., Cui H., Wang J.Y., Blilou I., Hassan H., Nakajima K., Matsumoto N., Lohmann J.U., Scheres B., Benfey P.N. (2006). Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 4: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D., Crowe L.K. (1958). Unilateral interspecific incompatibility in flowering plants. Heredity 12: 233–256 [Google Scholar]
- Li B., Gao R., Cui R., Lu B., Li X., Zhao Y., You Z., Tian S., Dong H. (2012). Tobacco TTG2 suppresses resistance to pathogens by sequestering NPR1 from the nucleus. J. Cell Sci. 125: 4913–4922 [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Z.B., Zamir D. (2007). Heterosis: Revisiting the magic. Trends Genet. 23: 60–66 [DOI] [PubMed] [Google Scholar]
- Lu J.Z., Zhang C., Baulcombe D.C., Chen Z.J. (2012). Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 109: 5529–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Bilodeau P., Dennis E.S., Peacock W.J., Chaudhury A. (2000). Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 97: 10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Dennis E.S., Berger F., Peacock W.J., Chaudhury A. (2005). MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl. Acad. Sci. USA 102: 17531–17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A., Tyagi A.P., Watson B., Jiang H., Kagochi T., Doerge R.W., Martienssen R., Comai L. (2005). Genomic changes in synthetic Arabidopsis polyploids. Plant J. 41: 221–230 [DOI] [PubMed] [Google Scholar]
- Magnard J.L., Le Deunff E., Domenech J., Rogowsky P.M., Testillano P.S., Rougier M., Risueño M.C., Vergne P., Dumas C. (2000). Genes normally expressed in the endosperm are expressed at early stages of microspore embryogenesis in maize. Plant Mol. Biol. 44: 559–574 [DOI] [PubMed] [Google Scholar]
- Maheshwari S., Barbash D.A. (2011). The genetics of hybrid incompatibilities. Annu. Rev. Genet. 45: 331–355 [DOI] [PubMed] [Google Scholar]
- Marshall E., Costa L.M., Gutierrez-Marcos J. (2011). Cysteine-rich peptides (CRPs) mediate diverse aspects of cell-cell communication in plant reproduction and development. J. Exp. Bot. 62: 1677–1686 [DOI] [PubMed] [Google Scholar]
- Martienssen R.A. (2010). Heterochromatin, small RNA and post-fertilization dysgenesis in allopolyploid and interploid hybrids of Arabidopsis. New Phytol. 186: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. (1984). The significance of responses of the genome to challenge. Science 226: 792–801 [DOI] [PubMed] [Google Scholar]
- Mizuno N., Shitsukawa N., Hosogi N., Park P., Takumi S. (2011). Autoimmune response and repression of mitotic cell division occur in inter-specific crosses between tetraploid wheat and Aegilops tauschii Coss. that show low temperature-induced hybrid necrosis. Plant J. 68: 114–128 [DOI] [PubMed] [Google Scholar]
- Mizzotti C., Mendes M.A., Caporali E., Schnittger A., Kater M.M., Battaglia R., Colombo L. (2012). The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J. 70: 409–420 [DOI] [PubMed] [Google Scholar]
- Mosher R.A., Melnyk C.W., Kelly K.A., Dunn R.M., Studholme D.J., Baulcombe D.C. (2009). Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature 460: 283–286 [DOI] [PubMed] [Google Scholar]
- Mukhtar M.S., Nishimura M.T., Dangl J. (2009). NPR1 in plant defense: It’s not over ‘til it’s turned over. Cell 137: 804–806 [DOI] [PubMed] [Google Scholar]
- Muller, H. (1942). Isolating Mechanisms, Evolution and Temperature. In Biological Symposia. (Tempe, Arizona: Jaques Cattell Press) pp. 71–125. [Google Scholar]
- Ng D.W., Lu J., Chen Z.J. (2012). Big roles for small RNAs in polyploidy, hybrid vigor, and hybrid incompatibility. Curr. Opin. Plant Biol. 15: 154–161 [DOI] [PubMed] [Google Scholar]
- Okamura K., Nakai K. (2008). Retrotransposition as a source of new promoters. Mol. Biol. Evol. 25: 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H.A. (1995). The population genetics of speciation: The evolution of hybrid incompatibilities. Genetics 139: 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W., Hodge R., Smartt S., Draper J., Scott R. (1992). The isolation and characterisation of the tapetum-specific Arabidopsis thaliana A9 gene. Plant Mol. Biol. 19: 611–622 [DOI] [PubMed] [Google Scholar]
- Postnikova O.A., Minakova N.Y., Boutanaev A.M., Nemchinov L.G. (2011). Clustering of pathogen-response genes in the genome of Arabidopsis thaliana. J. Integr. Plant Biol. 53: 824–834 [DOI] [PubMed] [Google Scholar]
- Ratcliffe O.J., Kumimoto R.W., Wong B.J., Riechmann J.L. (2003). Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15: 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg L.H., Blackman B.K. (2010). Speciation genes in plants. Ann. Bot. (Lond.) 106: 439–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Mizuno H., Kawahara Y., Wakimoto H., Ikawa H., Kawahigashi H., Kanamori H., Matsumoto T., Itoh T., Gaut B.S. (2011). Retrogenes in rice (Oryza sativa L. ssp. japonica) exhibit correlated expression with their source genes. Genome Biol. Evol. 3: 1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M., Davison T.S., Henz S.R., Pape U.J., Demar M., Vingron M., Schölkopf B., Weigel D., Lohmann J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Scott R.J., Spielman M., Bailey J., Dickinson H.G. (1998). Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125: 3329–3341 [DOI] [PubMed] [Google Scholar]
- Shah J., Kachroo P., Klessig D.F. (1999). The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 11: 191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J., Tsui F., Klessig D.F. (1997). Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol. Plant Microbe Interact. 10: 69–78 [DOI] [PubMed] [Google Scholar]
- Shaked H., Kashkush K., Ozkan H., Feldman M., Levy A.A. (2001). Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13: 1749–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Kaur R., Kumar K. (1996). Embryo rescue in plants—A review. Euphytica 89: 325–337 [Google Scholar]
- Shirzadi R., Andersen E.D., Bjerkan K.N., Gloeckle B.M., Heese M., Ungru A., Winge P., Koncz C., Aalen R.B., Schnittger A., Grini P.E. (2011). Genome-wide transcript profiling of endosperm without paternal contribution identifies parent-of-origin-dependent regulation of AGAMOUS-LIKE36. PLoS Genet. 7: e1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad P.V., Dunn R.M., Santos B.A., Bassett A., Baulcombe D.C. (2012). Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. EMBO J. 31: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel S.H., Mou Z., Tada Y., Spivey N.W., Genschik P., Dong X. (2009). Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137: 860–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer N.M., Stupar R.M. (2007). Allelic variation and heterosis in maize: How do two halves make more than a whole? Genome Res. 17: 264–275 [DOI] [PubMed] [Google Scholar]
- Ungerer M.C., Strakosh S.C., Zhen Y. (2006). Genome expansion in three hybrid sunflower species is associated with retrotransposon proliferation. Curr. Biol. 16: R872–R873 [DOI] [PubMed] [Google Scholar]
- Walia H., Josefsson C., Dilkes B., Kirkbride R., Harada J., Comai L. (2009). Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Curr. Biol. 19: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Garcia D., Zhang H., Feng K., Chaudhury A., Berger F., Peacock W.J., Dennis E.S., Luo M. (2010). The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. 63: 670–679 [DOI] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Wolff P., Weinhofer I., Seguin J., Roszak P., Beisel C., Donoghue M.T., Spillane C., Nordborg M., Rehmsmeier M., Köhler C. (2011). High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis endosperm. PLoS Genet. 7: e1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E., Takashi T., Morinaka Y., Lin S., Wu J., Matsumoto T., Kitano H., Matsuoka M., Ashikari M. (2010). Gain of deleterious function causes an autoimmune response and Bateson-Dobzhansky-Muller incompatibility in rice. Mol. Genet. Genomics 283: 305–315 [DOI] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Blechl A., Tranquilli G., Ramakrishna W., SanMiguel P., Bennetzen J.L., Echenique V., Dubcovsky J. (2004). The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Johnston N., Talideh E., Mitchell S., Jeffree C., Goodrich J., Ingram G. (2008). The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development 135: 3501–3509 [DOI] [PubMed] [Google Scholar]
- Zhang C., Ng D.W., Lu J., Chen Z.J. (2012). Roles of target site location and sequence complementarity in trans-acting siRNA formation in Arabidopsis. Plant J. 69: 217–226 [DOI] [PubMed] [Google Scholar]