Coordinated cell-to-cell communication is thought to play a crucial role in deploying effective immune responses both locally and throughout the whole plant system. This work provides insight into how the defense hormone salicylic acid and its signaling pathway regulate intercellular bridges called plasmodesmata.
Abstract
In plants, mounting an effective innate immune strategy against microbial pathogens involves triggering local cell death within infected cells as well as boosting the immunity of the uninfected neighboring and systemically located cells. Although not much is known about this, it is evident that well-coordinated cell–cell signaling is critical in this process to confine infection to local tissue while allowing for the spread of systemic immune signals throughout the whole plant. In support of this notion, direct cell-to-cell communication was recently found to play a crucial role in plant defense. Here, we provide experimental evidence that salicylic acid (SA) is a critical hormonal signal that regulates cell-to-cell permeability during innate immune responses elicited by virulent bacterial infection in Arabidopsis thaliana. We show that direct exogenous application of SA or bacterial infection suppresses cell–cell coupling and that SA pathway mutants are impaired in this response. The SA- or infection-induced suppression of cell–cell coupling requires an ENHANCED DESEASE RESISTANCE1– and NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1–dependent SA pathway in conjunction with the regulator of plasmodesmal gating PLASMODESMATA-LOCATED PROTEIN5. We discuss a model wherein the SA signaling pathway and plasmodesmata-mediated cell-to-cell communication converge under an intricate regulatory loop.
INTRODUCTION
The basal immune response to microbial pathogens requires accumulation of the defense hormone salicylic acid (SA) (Vlot et al., 2009; Rivas-San Vicente and Plasencia, 2011; Fu and Dong, 2013). Plant protein receptors that recognize pathogen-associated molecular patterns or effectors trigger a mitogen-activated protein kinase cascade and a burst of reactive oxygen species that together activate multiple downstream responses (Wiermer et al., 2005; Spoel and Dong, 2012). The core genetic components known to regulate upstream events of SA biosynthesis include ENHANCED DESEASE RESISTANCE1 (EDS1), PHYTOALEXIN DEFICIENT4, and SENESCENCE-ASSOCIATED GENE101 (Wiermer et al., 2005; Dempsey et al., 2011; Rietz et al., 2011). Lipase-like proteins that are encoded by these regulatory genes function together in specific combinations to enhance defense gene expression. Although the underlying molecular mechanisms are not fully known, EDS1 facilitates the expression of ISOCHORISMATE SYNTHASE1 (ICS1), which encodes an SA biosynthetic enzyme that plays a key role in immunity against bacterial infection and other stresses (Wildermuth et al., 2001; Vlot et al., 2009; Dempsey et al., 2011). Following ICS1-based hyperaccumulation of SA, one of the master regulators of SA signal transduction, NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1), is activated by changes in redox conditions within the cell (Tada et al., 2008). As a transcriptional coactivator, NPR1 migrates into the nucleus and brings about major shifts in gene expression patterns, such as induction of pathogenesis-related genes and secretion pathways (Wang et al., 2005; Fu and Dong, 2013).
The critical role of SA as an immune signal has been well documented (Vlot et al., 2009; Rivas-San Vicente and Plasencia, 2011; Fu and Dong, 2013). Direct application of SA activates various pathogenesis-related gene expressions, induces resistance to virulent microbial pathogens, elicits hypersensitive response cell death, and establishes systemic acquired resistance (Malamy et al., 1990; Ryals et al., 1996; Mur et al., 2008; Shah, 2009; Coll et al., 2011). An elegant recent study predicts that a gradient of SA may form such that cells local to the infection site begin hyperaccumulating SA, priming them for cell death, while their neighboring uninfected cells acquire a boost of immunity owing to a lower concentration of SA building up within them (Fu et al., 2012). The molecular mechanisms determining the cellular boundaries between the dying and healthy neighboring cells have yet to be discovered. However, it is conceivable that well-orchestrated local cell communication would be essential in order to confine the hypersensitive response within infected cells so that the detrimental spread of cell death–triggering signals into neighboring healthy cells is prevented (Rustérucci et al., 2001; Rinne and van der Schoot, 2003; Lee and Lu, 2011). In this view, plants would also require a mechanism that coordinates SA-based defense reactions with intercellular connectivity for the full and safe execution of basal immune responses.
Plasmodesmata (PD) allow for direct cytoplasmic connections in the plant and facilitate local molecular exchange among neighboring cells (Robards and Lucas, 1990; Blackman and Overall, 2001; Oparka and Roberts, 2001; Cilia and Jackson, 2004; Maule, 2008; Lucas et al., 2009; Burch-Smith et al., 2011; Sevilem et al., 2013). As PDs are initially formed during cell division, virtually all cells are born with plasmodesmal connections with their sister cells by default. However, these primary PD connections are not permanently set for the rest of a cell’s life. Rather, they undergo various types of structural modifications and degeneration/regeneration processes to meet the specific needs of cells that may set out rapid expansion, different developmental phases or differentiation, or adaption processes in response to the changes in various physiological and environmental conditions (Ehlers and Kollmann, 2001; Roberts and Oparka, 2003; Lucas and Lee, 2004; Burch-Smith et al., 2011; Burch-Smith and Zambryski, 2012). For instance, PD frequency and density change as cells grow and develop (Gunning, 1978; Seagull, 1983; Ehlers and Kollmann, 1996; Burch-Smith and Zambryski, 2010; Ehlers and van Bel, 2010) or during shifts from vegetative to reproductive phases (Ormenese et al., 2000; Ormenese et al., 2002; Ormenese et al., 2006); PDs differentiate from simple to complex forms (Faulkner et al., 2008; Fitzgibbon et al., 2013); PDs are completely disintegrated during guard cell maturation (Wille and Lucas, 1984); PD permeability undergoes temporal regulation by environmental conditions, such as daylength and temperature (Ormenese et al., 2006; Bilska and Sowinski, 2010; Rinne et al., 2011), etc.
Permeability, dilation, or structure of PDs can be also altered during infection by microbial pathogens (Heinlein, 2002; Benitez-Alfonso et al., 2010; Schoelz et al., 2011; Ueki and Citovsky, 2011). For example, plant viruses spread their infectious materials cell to cell (Benitez-Alfonso et al., 2010) through either regulating PD dilation (Waigmann et al., 1994) or modifying PD structure (van Lent et al., 1991; Pouwels et al., 2003,; 2004). Unlike viruses, bacterial pathogens are mostly epiphytic in their lifestyle, and their mode of infection does not require them to move cell to cell (Hou et al., 2009). However, bacterial infection induces the closure of PD, and a loss of PD regulation or a constitutive closure of PD in Arabidopsis thaliana confers either susceptibility or resistance, respectively, to virulent strains of Pseudomonas syringae (Lee et al., 2011). We have previously proposed that PLASMODESMATA-LOCATED PROTEIN5 (PDLP5) of Arabidopsis acts as a molecular link between the regulation of PD-mediated cell-to-cell coupling and innate immunity (Lee et al., 2011). PDLP5 is a type I transmembrane protein sharing structural and a minimal sequence homology to seven other PD-located proteins (PDLPs) (Thomas et al., 2008). Using immunolocalization in combination with correlative electron microscopy, we mapped the central cavity region as the subdomain of PD that PDLP5 associates with Lee et al. (2011). Interestingly, a recent review reported an immunogold labeling of another PDLP family member, PDLP1, along the length of the PD channel (Maule et al., 2011), which suggests the subdomain of PD that PDLP members are targeted to may not be the same.
More studies are necessary before assigning either specific or redundant functions to each PDLP isoform. However, PDLP5 seems to perform a unique function integral to immune responses among the PDLP isoforms. For example, lack of PDLP5 results in a loss of the regulation of basal PD permeability and an increased susceptibility to bacterial infection, whereas single knockouts of PDLP1, PDLP2, or PDLP3 had no effect on PD permeability (Thomas et al., 2008). PDLP5 alone is transcriptionally and translationally induced by infection with the virulent bacterial pathogen P. syringae pv maculicola (Pma) or by direct application of exogenous SA. The PDLP5 gene is also called HOPW1-1-INDUCED GENE1 , which was named based on its transcriptional induction upon infection by P. syringae secreting HOPW1-1 effector (Lee et al., 2008).
Consistent with the finding that PDLP5 gene induction is regulated by SA, both endogenous and SA-induced expression of PDLP5 transcript were significantly compromised in eds1, npr1, and ics1 mutants (Lee et al., 2011). Moreover, an ectopic overexpression of PDLP5 induces PD closure, SA accumulation, and basal immunity against virulent bacterial pathogens, whereas the loss-of-function mutant pdlp5-1 allows for an abnormally extensive PD permeability and enhanced disease susceptibility (Lee et al., 2011). Finally, an introduction of the bacterial gene encoding SA hydroxylase, NahG, suppressed the outward plant morphological phenotypes associated with PDLP5 overexpression, namely, growth retardation and spontaneous lesion development. Taken together, these data indicate that SA accumulation through a positive feedback regulation plays a crucial role in PDLP5 function.
Here, we report that SA signaling components are required to regulate cell-to-cell connectivity as well as the epistatic relationship between the SA pathway and PDLP5. We show that direct application of SA to Arabidopsis Columbia-0 (Col-0) induces PD callose deposition and closure, while in the absence of PDLP5, application of exogenous SA was not sufficient to induce these responses. Furthermore, SA mutants defective in SA accumulation or signal transduction are compromised in PD closure upon infection by virulent bacterial pathogens, and the capacity of PDLP5 to activate PD callose deposition and closure is largely dependent on the intact SA biosynthesis and signaling pathway. These results together firmly establish that crosstalk between PDLP5 and the SA pathway is essential for regulating PD permeability during a pathogen defense response.
RESULTS
Direct Application of SA Induces PD Closure
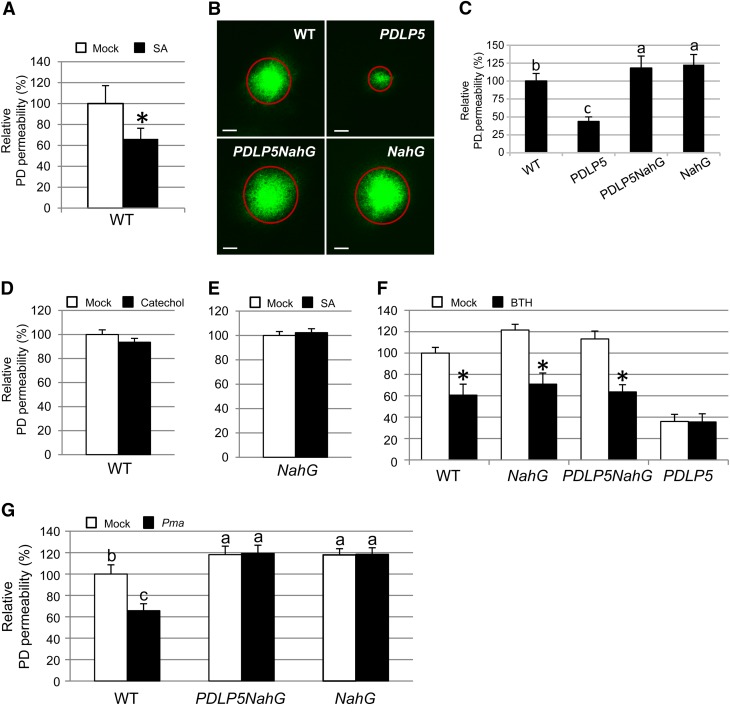
To gain insight into the potential role of SA in modulating cell–cell coupling, we first investigated whether exogenous application of SA has an effect on PD permeability by employing the Drop-and-See (DANS) assay that we developed in a previous study (Lee et al., 2011). The DANS assay uses membrane-permeable, nonfluorescent carboxyfluorescein diacetate as a probe, which acquires fluorescence once released into the cytoplasm through cleavage by cellular esterases and becomes membrane impermeable. The DANS assay has proven to be an effective, noninvasive approach for a real-time, in situ assessment for the extent of molecular diffusion through PD. To test the effect of SA on PD permeability, 3-week-old wild-type Col-0 plants were sprayed with buffer in the absence or presence of SA at 1, 10, or 100 µM, followed by DANS assays on the fourth and fifth rosette leaves (see Supplemental Figure 1 online). Compared with the buffer control, treating wild-type Col-0 plants with 100 µM SA for 24 h resulted in a significant reduction in PD permeability (Figure 1A). Treating the plants with 100 µM SA for 24 or even for 48 h did not cause any obvious stress or yellowing of the plants. Also, there was no cell death detectable by microscopy (see Supplemental Figure 2 online). Based on these results, we chose to use 100 µM SA and a 24-h time point as an optimal treatment condition for our study to assess the effect of SA on PD permeability.
Figure 1.
PD Closure Is Regulated by SA.
(A) Relative PD permeabilities of Col-0 plants 24 h after mock treatment or treatment with 100 μM SA. WT, the wild type.
(B) and (C) Comparison of PD permeabilities of wild-type Col-0, PDLP5, PDLP5NahG, and NahG plants.
(B) Confocal images showing representative CFDA movement in abaxial leaf surfaces. Circles represent the extent of dye diffusion. Bars = 200 μm.
(C) Quantification of CFDA movement. At least 10 plants were used per assay, and more than three repeats were performed.
(D) to (G) DANS assays showing changes in PD permeability in response to various chemicals (100 μM catechol [D], 100 μM SA [E], and 100 μM BTH [F]) and pathogen treatments (Pma ES4326 [OD600 = 0.001] [G]). Three-week-old plants were sprayed with chemicals or infected with Pma. DANS assays were performed at 24 h after treatments by loading CFDA for 5 min on the adaxial surfaces of fourth and fifth leaves and examining the abaxial surface for dye diffusion by a confocal microscopy. At least five individual plants were used per treatment, and two leaves (fourth and fifth) from each plant were subjected to DANS assays. At least two biological repeats were performed for quantification. Asterisks indicate a significant difference (P < 0.001) between two samples. Levels not connected by the same letters are significantly different at the α = 0.05 level according to the LSD test following one-way ANOVA. Bars indicate se.
[See online article for color version of this figure.]
SA Accumulation Is Required for PDLP5-Mediated PD Closure
Having found that SA treatment induces PD closure, we then tested whether SA accumulation is required for the reduced PD permeability phenotype, which is manifested by transgenic plants overexpressing PDLP5 under the control of the 35S promoter (hereafter called PDLP5 plants). To this end, comparative PD permeability assays were performed on 3-week-old wild-type Col-0, PDLP5, NahG, and homozygous F3 progenies of the cross between PDLP5 and NahG plants (hereafter called PDLP5NahG) grown under the same environmental conditions. NahG encodes a bacterial SA hydroxylase (Delaney et al., 1994) and was employed as a genetic means to negate SA hyperaccumulation in the PDLP5 plants. As previously shown in many SA hyperaccumulating mutants (Lorrain et al., 2003; Brodersen et al., 2005), introduction of NahG blocked the spontaneous lesion formation phenotype of PDLP5 plants. Compared with the wild-type Col-0 control, the PD permeability based on the DANS assay is substantially reduced in PDLP5 (Figures 1B and 1C). However, this PD inhibition phenotype also was completely suppressed by the introduction of NahG, indicating that PDLP5 requires SA accumulation to induce the closure of PD.
We predicted that NahG would restore the PD permeability in PDLP5 to a similar level as wild-type Col-0 if NahG simply eliminates the PDLP5-induced SA hyperaccumulation. Surprisingly, PDLP5NahG exhibited greatly enhanced PD permeability, allowing for ∼20% higher diffusion of the fluorescent reporter than the wild type (Figures 1B and 1C). Notably, this level of extensive PD permeability was previously observed in pdlp5-1 (Lee et al., 2011). The level of PDLP5 transcript in PDLP5NahG was comparable to that in the parental line PDLP5 (Lee et al., 2011), eliminating the possibility that the restored PD permeability in PDLP5NahG may reflect a potential fluctuation or instability in PDLP5 expression. Thus, the enhanced PD permeability in PDLP5NahG led us to ask whether the overexpression of NahG alone could enhance PD permeability. Indeed, subsequent DANS assays on NahG plants revealed that an overexpression of NahG alone can significantly enhance the dye diffusion through PD (Figures 1B and 1C). Fluorescent dye absorption on the adaxial surface in transgenic or mutant lines was comparable to that in wild-type plants, as we had shown previously for the PDLP5 and pdlp5-1 (Lee et al., 2011; see Supplemental Figure 3A online). In addition, overall epidermal cell sizes on both adaxial and abaxial sides of PDLP5, NahG, and PDLP5NahG were comparable to those of wild-type Col-0 plants (see Supplemental Figures 3B and 3C online). Thus, the aberrant PD permeability shown in NahG or PDLP5NahG was apparently not due to an alteration in epidermal surface property or cell size.
The ability of NahG to suppress the phenotypes associated with SA hyperaccumulation has been largely attributed to its SA hydroxylase activity, which disables SA accumulation (Lawton et al., 1995). However, there was a report that a certain phenotype manifested by the introduction of NahG is linked to an inappropriate production of an SA degradation by-product, catechol (van Wees and Glazebrook, 2003). We therefore tested the possibility that the enhancing effect NahG has on PD permeability is linked not necessarily to the lack of SA accumulation, but rather to an increased catechol production. The DANS assays performed 24 h after spraying wild-type Col-0 with 100 µM catechol (van Wees and Glazebrook, 2003) demonstrated that this catechol treatment has no effect on the PD permeability (Figure 1D). These results indicate that the inability to accumulate SA, rather than catechol accumulation, attributes to extensive opening of PD in NahG plants.
In contrast with the repressive effect of SA on symplastic dye diffusion in wild-type Col-0 (Figure 1A), the same SA treatment failed to induce PD closure in NahG (Figure 1E), as expected. However, application of an SA agonist, benzo(1,2,3) thiadiazole-7-carbothioic acid (BTH), which is not degradable by NahG, could induce PD closure in NahG to a similar extent shown in BTH-treated wild-type plants (Figure 1F). Moreover, BTH treatment could restore the reduced PD permeability phenotype in PDLP5NahG, whereas such treatment did not have any additional effect in PDLP5 plants. Collectively, our data provide strong experimental evidence that SA both acts as a signal to restrict cell-to-cell coupling and is required for PDLP5-mediated PD closure.
Inability to Accumulate SA Leads to the Loss of the Regulation of PD during Defense
In response to virulent Pma infection, cell-to-cell diffusion through PD becomes highly restricted in Arabidopsis Col-0 (Lee et al., 2011). Having established that exogenous application of SA promotes PD closure, we speculated that the Pma-induced PD closure might also be attributed to cellular activation of SA biosynthesis and accumulation resulting from innate immune responses. To test this hypothesis, the PD permeability of NahG upon Pma infection was examined in comparison to Col-0 wild-type plants; we reasoned that the inability to accumulate SA would make NahG insensitive to Pma infection in terms of the PD response. Indeed, we found that bacterial infection failed to induce PD closure in NahG as well as in PDLP5NahG, while infected wild-type Col-0 exhibited a substantial reduction in PD permeability compared with the mock-treated plants (Figure 1G). The SA deficiency in PDLP5NahG also suppressed the PDLP5 pathogen-resistant phenotype, indicating that SA buildup is in fact required for both the regulation of PD and basal immunity in PDLP5 plants. These results underscore the dual role of SA as a hormonal signal that not only activates defense but also regulate symplastic cell-to-cell connectivity during the plant response to microbial pathogens.
SA Induces PD Callose Deposition
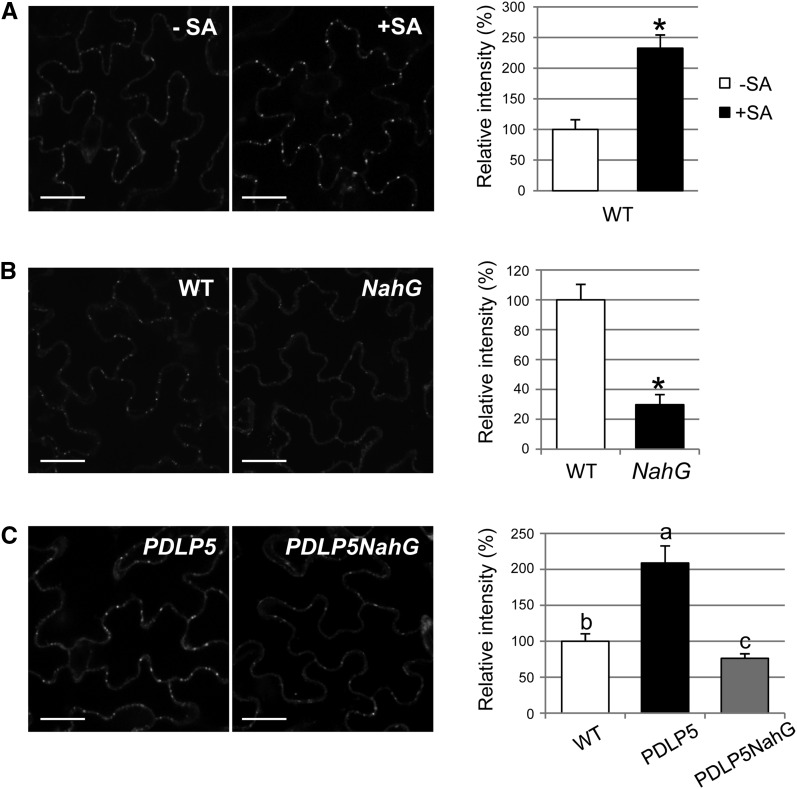
Pma infection was previously found to stimulate callose deposition at PD (Lee et al., 2011), but at that time, it was unknown which signaling molecules or pathway regulated that response. However, in light of the results described above, it seemed prudent to test the possibility that callose deposition restricting PD during the defense response to Pma was also regulated by SA. To this end, we examined the effect of SA on PD callose deposition by treating 3-week-old Col-0 plants with 100 µM SA spray for 24 h, followed by aniline blue staining for callose detection in the rosette leaves and quantification of the fluorescence foci intensity per square micron. Compared with the mock control, the leaves treated with SA accumulated over twofold higher PD callose (Figure 2A). This result together with the finding that PD permeability is highly enhanced in NahG suggested that a lack of SA accumulation may avert deposition of PD callose in NahG. Indeed, the basal PD callose deposition in NahG was substantially lower, comprising only 30% of the wild-type level (Figure 2B). Moreover, consistent with the suppressive effect of NahG on the reduced PD permeability phenotype of PDLP5, NahG also eliminated the phenotype of PDLP5 associated with hyperaccumulation of PD callose (Figure 2C). These data support the idea that SA is a crucial hormonal factor regulating cell-to-cell permeability via recruiting callose to PD.
Figure 2.
PD Callose Deposition Is Dependent on SA Accumulation.
(A) Confocal images showing callose staining at PD (left panels) and quantification of PD callose level (right) in wild-type (WT) Col-0 mock treated (-SA) or treated with 100 μM SA (+SA). Abaxial surfaces of the fourth and fifth leaves of 3.5-week-old plants were imaged by a confocal microscopy following aniline blue staining. Bars = 20 μm.
(B) Callose level in NahG compared with wild-type plants.
(C) Suppression of PD callose accumulation in PDLP5 by NahG. At least three individual plants were used per treatment and fourth and fifth leaves from each plant were subjected to aniline blue staining. Two biological repeats were performed for quantification. Asterisks indicate a significant difference (P < 0.0001) between two samples by t test. Levels not connected by same letters are significantly different at the α = 0.05 level based on LSD test following one-way ANOVA. Bars indicate se.
SA Does Not Rescue the Enhanced PD Permeability Phenotype of pdlp5-1
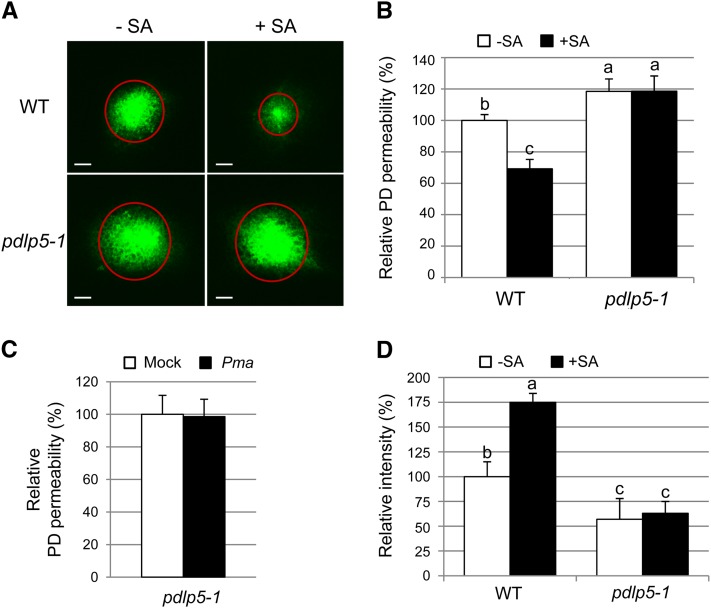
Since a high PDLP5 expression level within cells stimulates SA accumulation, one could argue that the restricted PD phenotype of PDLP5 is due simply to SA hyperaccumulation triggering closure through another, PDLP5-independent mechanism. If this were indeed true, then supplying pdlp5-1 with exogenous SA would induce a near PDLP5-level of PD closure because the loss of PDLP5 would not stop SA from exerting its PD-closing function. To test this possibility, we performed DANS assays on Col-0 and pdlp5-1 that were either mock treated or treated with 100 µM SA. In stark contrast with the wild-type control, the SA-induced PD closure response was fully impaired in pdlp5-1, which retained a higher PD permeability even in the presence of exogenously supplied SA (Figures 3A and 3B). We next addressed whether PDLP5 was essential for closing PD during a response to bacterial pathogen by performing DANS assays on pdlp5-1 following Pma infection. This experiment showed that Pma-induced PD closure is also fully impaired in pdlp5-1 (Figure 3C). This result, together with the loss of SA sensitivity demonstrated in pdlp5-1 (Figures 3A and 3B), indicates that PDLP5 is indeed a key molecular player for translating SA signaling to PD closure during defense responses. Collectively, our data provide strong experimental evidence that PDLP5 and SA exert their effect on PD in an interdependent manner such that restriction of PD requires both components.
Figure 3.
PDLP5 Is Essential for SA-Induced PD Closure and Callose Deposition.
(A) to (C) DANS dye loading assays showing impairment of induced PD closure response in pdlp5-1 upon either 100 μM SA treatment ([A] and [B]) or Pma infection (C). Representative confocal images of abaxial leaf surfaces show the effects of SA treatment on the extents of dye diffusion (A). WT, the wild type. Bars = 200 μm.
(D) Lack of SA-induced PD callose accumulation in pdlp5-1. More than five and three individual plants were used for DANS assays and aniline blue staining, respectively. At least two biological repeats were performed for quantification. All images were taken from leaf number 4 and leaf number 5. Levels not connected by same letters are significantly different at the α = 0.05 level based on LSD test following one-way ANOVA. Bars indicate se.
[See online article for color version of this figure.]
PDLP5 Is an Essential Molecular Link between the SA-Based Defense Response and PD Callose Deposition and Closure
Numerous reports have presented a correlation between PD closure and callose deposition (Radford et al., 1998; Simpson et al., 2009; Lee et al., 2011; Vatén et al., 2011; Zavaliev et al., 2011; Koh et al., 2012), supporting the general consensus that enhanced PD callose deposition is indicative of PD closure and, thus, inhibition of cell-to-cell coupling. Our study has shown that the relative amount of PD callose deposition is inversely correlated with the extent of PD permeability but directly correlated with the level of PDLP5 expression (Lee et al., 2011). However, it was unclear to us whether the increase in PD callose level during infection was also dependent on PDLP5 or any other factors (such as SA hyperaccumulation). We addressed this question by examining PD callose levels in pdlp5-1 following application with exogenous SA. If pdlp5-1 responds normally to the SA treatment in terms of elevated PD callose level, it would mean that SA, not PDLP5, is responsible for augmenting PD callose. However, while wild-type Col-0 responded to exogenous SA by inducing a significant amount of PD callose deposition, this response was also fully impaired in pdlp5-1 (Figure 3D). This result confirms that the induced PD callose response upon SA accumulation is indeed dependent on PDLP5, further supporting that PDLP5-mediated PD closure is most likely mediated through callose deposition at PD.
SA Defense Pathway Mutations Are Epistatic to PDLP5
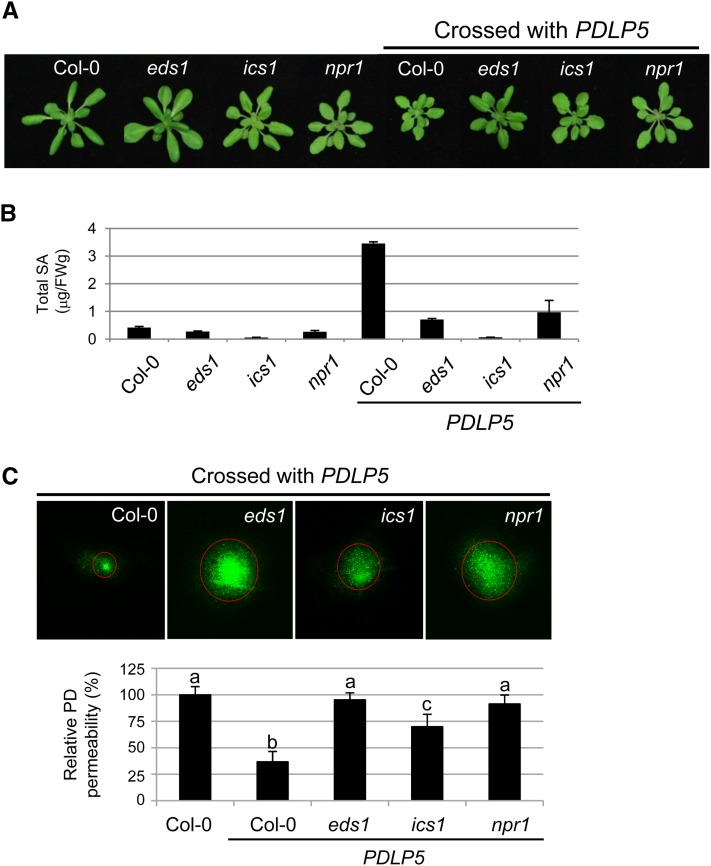
Based upon the data described above, it is clear that SA regulates PD permeability in conjunction with PDLP5. To gain further insight into the functional relationship between PDLP5 and SA, we determined epistatic relationships between the genetic components of SA pathway and PDLP5 using PDLP5 and SA mutants. We chose the SA mutants eds1-2, ics1-1, and npr1-1, which lack the key upstream regulator of SA signaling pathway EDS1, SA biosynthetic enzyme ICS1, and SA downstream regulator NPR1, respectively (Cao et al., 1994; Aarts et al., 1998; Wildermuth et al., 2001). We isolated homozygous F3 progenies of PDLP5eds1, PDLP5ics1, and PDLP5npr1 and then examined which genetic mutations can suppress the PD permeability phenotype in PDLP5. The homozygosity of the SA pathway mutations and the homogeneous PDLP5 expression level in those double mutants were confirmed by transcript analysis using RT-PCR (see Supplemental Figure 4A online). Morphological comparisons of PDLP5eds1, PDLP5 ics1, and PDLP5 npr1 to the PDLP5 parental line showed that all three mutations suppressed the stunted growth phenotype of PDLP5 to a certain extent (Figure 4A). Furthermore, the highly elevated SA level found in the PDLP5 parental line was also diminished (Figure 4B), with a subsequent reduction or abolition of the SA hyperaccumulation marker PR1 (see Supplemental Figure 4B online), confirming that abnormal induction of PR1 in PDLP5 depends on these genetic components. These results indicate that PDLP5-regulated positive SA feedback must work through the basal SA defense pathway components EDS1, ICS1, and NPR1.
Figure 4.
SA Mutants Are Epistatic to PDLP5.
(A) Morphologic phenotypes of eds1, ics1, npr1, and their crosses with PDLP5: PDLP5eds1, PDLP5ics1, and PDLP5npr1, respectively.
(B) Total SA levels in each genetic background.
(C) DANS assays showing PD permeability in SA mutants in PDLP5 background along with wild-type control. Representative confocal images of abaxial leaf surfaces are shown. For each genetic background, at least five individual plants were used for DANS assays with at least two biological repeats. Levels not connected by same letters are significantly different at the α = 0.05 level based on the LSD test following one-way ANOVA. Bars indicate se.
[See online article for color version of this figure.]
We next examined PD permeability phenotype in PDLP5eds1, PDLP5ics1, and PDLP5npr1. Fluorescent dye absorption on the adaxial surface and overall epidermal cell sizes on both adaxial and abaxial sides in SA mutants and crosses were comparable to those in wild-type plants (see Supplemental Figure 3 online). Subsequent DANS assays on these plants demonstrated that all three mutations were able to suppress the restricted PD permeability in PDLP5 (Figure 4C). However, whereas both eds1 and npr1 were able to fully suppress the reduction in PD permeability, ics1 was only partially effective. This result suggests that PDLP5-mediated PD closure requires an EDS1- and NPR1-dependent SA pathway.
Basal PD Permeability Is Normal in SA Mutants
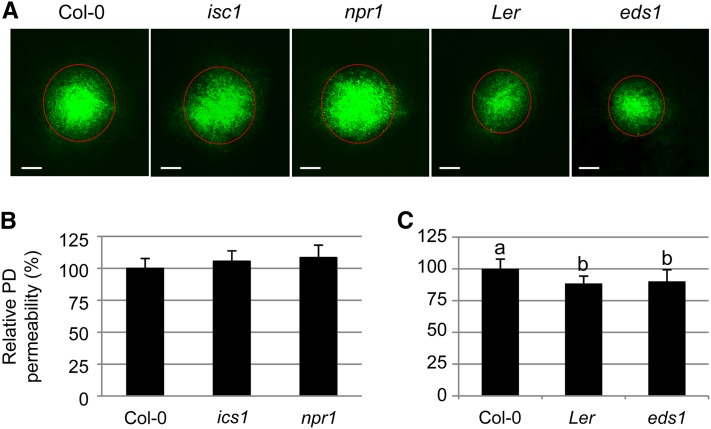
Considering a potential mechanism by which SA acts on PD, we propose two simple possibilities. One is that SA is a chemical agonist that directly affects PDLP5 or other PD components. The other is that SA acts indirectly as a hormonal signal that activates a downstream signaling pathway(s) on which PDLP5 activity/function relies. To gain insight into the mechanistic relationship between SA and PD permeability, we decided to investigate the latter possibility by examining PD permeability phenotypes of SA mutants. To this end, basal PD permeability was first measured by performing DANS assays on the SA pathway mutants eds1, ics1, and npr1. This test showed that the basal level of PD opening in these mutants under normal growth conditions was comparable to that of wild-type controls (Figures 5A and 5B). Notably, eds1 showed a slightly lower PD permeability than wild-type Col-0, a result that was perplexing considering the endogenous SA level in this mutant was lower than Col-0 (Figure 4B). Since eds1 is derived from the Landsberg erecta (Ler) ecotype, we subsequently tested whether the low PD permeability detected in eds1 reflects a difference between Ler and Col-0 ecotypes. Indeed, the DANS assay showed that wild-type Ler plants exhibited slightly lower PD permeability compared with wild-type Col-0, and the PD permeability of eds1 was comparable to wild-type Ler (Figure 5C).
Figure 5.
Basal PD Permeability in SA Mutants Is Normal.
(A) Representative confocal images of abaxial leaf surfaces showing basal PD permeability in Col-0, ics1, npr1, Ler, and eds1. Bars = 200 μm.
(B) and (C) Quantitative comparison of PD permeability in ics1 and npr1 compared with wild-type Col-0 (B) and eds1 compared with wild-type Ler (C). For each genetic background, at least five individual plants were used for DANS assays with at least two biological repeats. Levels not connected by same letters are significantly different at the α = 0.05 level based on LSD test following one-way ANOVA. Bars indicate se.
[See online article for color version of this figure.]
Induced PD Closure Response Is Compromised in SA Mutants
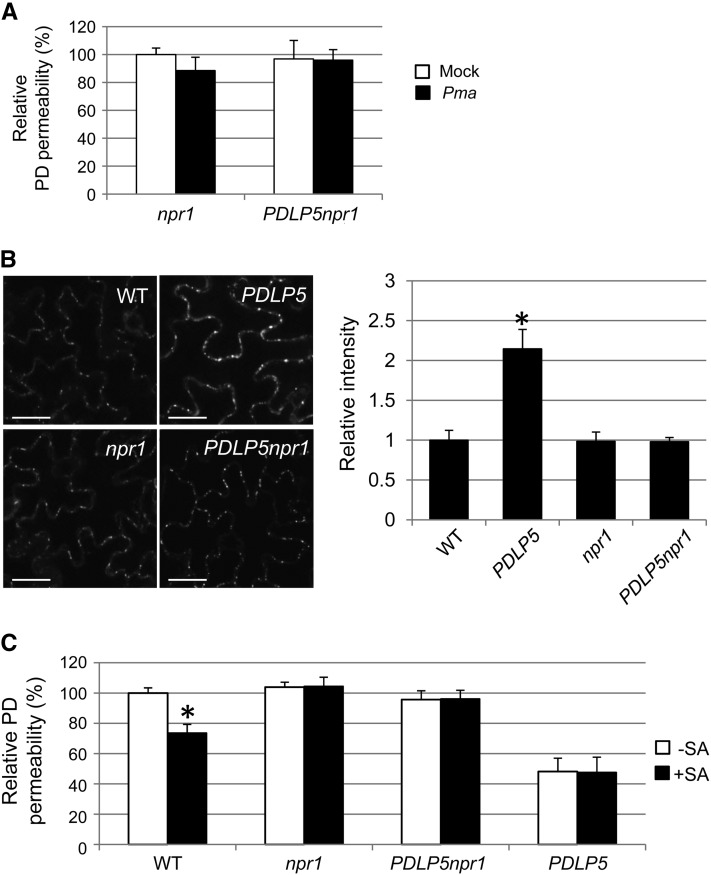
Since NPR1 is the most downstream regulator, responsible for regulating many of the critical genetic responses to SA accumulation during defense (Wang et al., 2005), we decided to determine if NPR1 was also essential for regulating the pathogen-induced reduction in PD permeability. Indeed, the PD closure response normally induced by Pma infection was fully impaired in the absence of functional NPR1 either in wild-type or PDLP5 backgrounds (Figure 6A). Furthermore, quantitative analyses of aniline blue–stained PD callose revealed that basal PD callose levels in the wild type and npr1-1 were comparable to each other. However, the loss of NPR1 could fully suppress the hyper PD callose accumulation phenotype in PDLP5npr1 (Figure 6B). These data establish the dependence of PDLP5 on NPR1 in modulating PD callose accumulation and, hence, PD closure during immune responses.
Figure 6.
NPR1 Is Required for PDLP5-Mediated PD Closure and Callose Deposition.
(A) Lack of PD closure response in npr1 and PDLP5npr1 to Pma infection.
(B) Basal PD callose level in npr1 and PDLP5npr1 is normal. Abaxial surfaces of the fourth and fifth leaves of 3.5-week-old plants were imaged by a confocal microscopy following aniline blue staining. WT, the wild type. Bars = 20 μm.
(C) PD permeability in npr1, PDLP5npr1, and PDLP5 is insensitive to 100 μM SA treatment. More than five and three individual plants were used per treatment for DANS assays and aniline blue staining, respectively. At least two biological repeats were performed for quantification. Asterisks indicate a significant difference (P < 0.001) between two samples by t test.
Although signaling downstream of SA is largely dependent on NPR1, some SA responses are NPR1 independent (Ferrari et al., 2003; Blanco et al., 2005). To determine whether SA-induced PD closure relies solely on an NPR1-dependent pathway, we performed additional DANS assays using npr1 and PDLP5npr1 following SA application. This experiment showed that exogenous SA application was not able to complement the loss of NPR1 (Figure 6C), thus validating that SA-induced PD closure requires NPR1 or its downstream factor(s). By contrast, SA application restored normal PD closure response in PDLP5eds1 and PDLP5ics1 (see Supplemental Figure 5 online).
In conclusion, we demonstrated that SA is a critical signaling molecule for regulating cell-to-cell connectivity and that SA-directed PD closure requires PDLP5 as the molecular link between the SA pathway and PD modulation. PDLP5 functions to close PD during immune responses by working simultaneously with an NPR1-dependent pathway to trigger a high level of callose deposition at PD during infection. And without either PDLP5 or NPR1, Arabidopsis cannot close PD in response to pathogen infection.
DISCUSSION
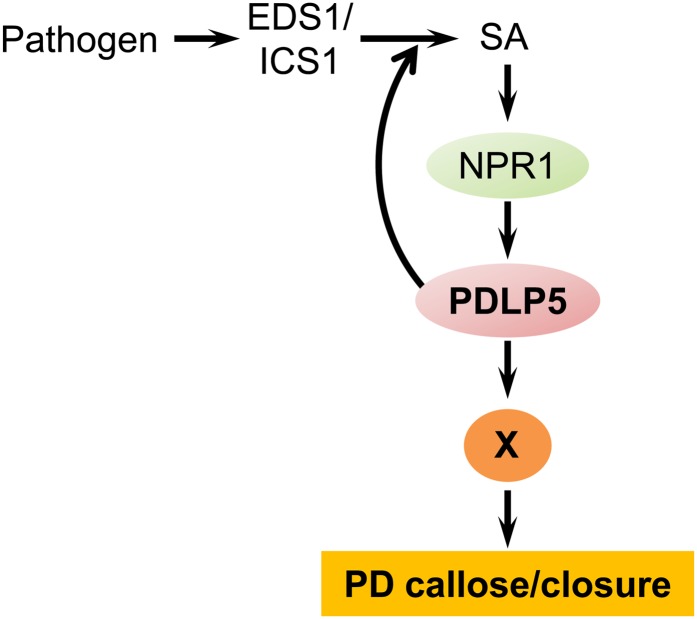
We had shown in a recent study that the SA pathway plays a role in regulating PD by upregulating a PD inhibitor, PDLP5, during immune responses against bacterial pathogens (Lee et al., 2011). In this article, we provided experimental evidence supporting that an accumulation of SA is critical to block PD in Arabidopsis and established an epistatic relationship between SA biosynthetic/signaling components and PDLP5. First, exogenous application of SA or BTH induces PD callose deposition, which results in PD closure. Second, defects in SA accumulation or signaling in NahG and npr1, respectively, compromise the ability to close PD in response to either SA treatment or bacterial infection. Third, in the absence of PDLP5, plants were also not able to stimulate PD callose deposition; thus, there was no PD closure after treatment with exogenous SA or during bacterial infection. Finally, dissection of epistatic relationships revealed that the physiological and cellular phenotypes associated with overexpression of PDLP5, such as SA hyperaccumulation, increased PD callose deposition, and severe restriction of PD permeability, are suppressed in eds1, ics1, npr1, and NahG. Taken together, these data provide insight into how PD closure is regulated during defense. Namely, bacterial pathogen infection triggers the basal SA defense pathway, and SA accumulation activates both NPR1 and PDLP5, which must work in tandem to produce a complete PD closure response, via increasing PD callose deposition. This response is reinforced by a positive feedback loop through the SA defense pathway, which requires the components EDS1, ICS1, and NPR1 (Figure 7).
Figure 7.
An Illustrated Model Demonstrating the Crosstalk between PDLP5-Mediated PD Regulation and SA Defense Signaling.
Pathogen infection induces PDLP5 expression via an EDS1/ICS/NPR1-dependent SA pathway. A positive feedback loop between PDLP5 and SA accumulation requires EDS1, ICS1, and NPR1. Hyperaccumulation of PDLP5 leads to callose-dependent PD closure. This PDLP5 function requires NPR1, perhaps via a yet unknown “factor X.”
[See online article for color version of this figure.]
SA Accumulation via ICS1 Is Required but Not Sufficient for PDLP5-Mediated PD Closure
In Arabidopsis, SA is synthesized primarily via the isochorismate-using pathway in the chloroplast and secondarily via the Phe ammonia-lyase pathway. ICS1, a biosynthetic enzyme in the isochorismate pathway, is not required for the maintenance of the basal level of SA but is mainly responsible for SA accumulation during bacterial infection (Wildermuth et al., 2001). Interestingly, the SA levels in PDLP5eds1 and PDLP5npr1 are elevated but are not as high as in the PDLP5 parental line. By contrast, PDLP5ics1 was found to have a similar SA level to that of ics1 alone (Figure 4B), which indicates that SA accumulation in PDLP5 is fully dependent on ICS1 function. However, while the basal PD permeability of eds1, ics1, and npr1 was no different from that in each of their wild-type ecotypes (either Col-0 or Ler; Figure 5), both the eds1 and npr1 mutations fully suppressed PDLP5-induced PD closure, whereas ics1 could only partially suppress the PDLP5-restricted PD phenotype (Figure 4C). These data suggest the presence of an additional factor(s) independent of ICS1-amplified SA that is responsible for PD closure via PDLP5 through EDS1- or NPR1-triggered changes in defense signaling. We speculate that factors from both ICS1-dependent and -independent pathways may work together to produce the maximum activity/function of PDLP5.
Do SA and PDLP5 Impede Dye Movement through Induction of Cell Death or PD Modification?
We have shown in our previous study that overexpression of PDLP5 results in hyperaccumulation of SA and spontaneous lesions (Lee et al., 2011). It is possible that cell death might have impeded dye movement in PDLP5 plants to some extent apart from or in addition to PD callose deposition. The PDLP5 line used in this study was an intermediate line that has a mild cell death phenotype. Moreover, cell death in PDLP5 plants progresses with aging, becoming visible at 4 to 5 weeks after germination starting from the edges of the oldest leaves. All our DANS assays and callose staining were done on the central regions of the fourth and fifth rosette leaves of 3-week-old plants, in which there are few to no lesions. Also, treatment with 100 µM SA for 24 h or longer did not cause lesions to form but still resulted in a reduction in PD permeability. Based on these pieces of circumstantial evidence, it appears that cell death might be a later response than or eventual outcome of PD closure.
What might be the direct role that SA and SA pathways play in regulating PD? Could SA affect the subcellular localization of PDLP5? We tested this possibility, but PDLP5 localization at PD remained the same 24 or even 48 h after SA treatment (see Supplemental Figure 6 online). It is possible that SA or SA pathways may induce a component that PDLP5 requires for its function; our data indicate that PDLP5 cannot function to close PD in the absence of this SA-dependent component, factor X (Figure 7). SA was shown to facilitate secondary PD formation in Arabidopsis seedlings grown on SA-containing medium for several days (Fitzgibbon et al., 2013). It would be interesting to determine whether there is a close link between structural modification of PD and the SA pathway during immune responses and whether SA together with PDLP5 induces PD closure through such modification.
Basal PD Callose/Permeability Requires SA in Conjunction with PDLP5 but Not npr1
In contrast with ics1, NahG fully suppressed the restricted-PD phenotype in PDLP5. This result is not too surprising, as several previous reports have documented that NahG and ics1 do not always suppress phenotypes associated with SA hyperaccumulation in a similar manner (Nawrath and Métraux, 1999; Heck et al., 2003; Brodersen et al., 2005; Jagadeeswaran et al., 2007; Vogelmann et al., 2012). One obvious possibility is that NahG is more efficient at eliminating SA in the PDLP5 background than ics1, considering their efficacies in suppressing the PDLP5 phenotype. Along this line, we could argue that SA is the major factor PDLP5 requires to close PD and that SA synthesized independently of ICS1 might play a role in both maintaining basal PD permeability and responding to pathogen infection. Thus, NahG is more potent than ics1 in suppressing PDLP5 because it degrades SA from all sources within the plant, while ics1 only prevents ICS1-dependent SA accumulation. Consistent with this idea, treating NahG plants with nondegradable SA analog BTH induced PD closure, as was also the case for BTH-treated wild-type plants.
While the highly enhanced basal PD permeability in the NahG background was not seen in ics1, eds1, or npr1, the permeability observed in the NahG background was quite similar to that in pdlp5-1 (Figure 3). An intriguing question here is whether there is a common factor that NahG and pdlp5-1 share to open PD beyond the basal level. One may logically suggest that the enhanced PD permeability in pdlp5-1 has to do with greatly reduced or eliminated SA, similar to NahG. However, our previous study has already shown that the SA content in pdlp5-1 was not different from that in the wild type (Lee et al., 2011), underscoring that a loss of PDLP5 results in an enhancement in basal PD permeability regardless of having a normal SA level. Another possibility is that there is not sufficient SA in the NahG plants to maintain normal levels of PDLP5. Indeed, RT-PCR data show that the PDLP5 expression level in NahG is lower than that in ics1 and the wild type (see Supplemental Figure 7 online), supporting the pdlp5-1 phenotype in NahG (i.e., enhanced PD permeability). However, given that PDLP5 alone does not close PD in the absence of SA accumulation, we conclude that PDLP5 or SA cannot work alone but rather both are required to close PD. In the case of npr1, the level of SA and/or PDLP5 must still be above the threshold required for maintaining basal PD callose accumulation/permeability. The fact that basal PD callose/permeability is abolished in both NahG and pdlp5-1 (Figures 2C and 3D) but not in npr1 is consistent with our conclusion that basal PD regulation requires PDLP5 only in conjunction with SA but not NPR1.
SA-Induced PD Callose Accumulation via Specific Callose Synthases?
Callose accumulation in planta is induced in response to fungal infection at the penetration site, upon elicitor treatment and bacterial infection, and by other developmental or mechanical factors (Jacobs et al., 2003; Nishimura et al., 2003; DebRoy et al., 2004; Xie et al., 2011). How might SA pathways and PDLP5 specifically affect PD callose accumulation? SA has been implicated in upregulating a subset of Arabidopsis callose synthase (CALS) transcripts in an NPR1-dependent manner (Dong et al., 2008). It is tempting to speculate that the loss of PD callose in PDLP5npr1 is due to the lack of expression of the same NPR1-dependent CALS genes, CALS1/GSL6 and CALS12/GSL5/PMR4, described by Dong et al. (2008). However, knocking down those CALS genes by RNA interference was reported earlier not to affect callose accumulation either at the cell plate or PD (Jacobs et al., 2003; Nishimura et al., 2003), eliminating the possibility that these isoforms are involved in PD callose deposition. Increases in CALS transcript levels may not necessarily correlate with upregulation of callose synthase activity because callose synthase requires additional protein components to form an active complex and other factors for catalytic activation (Brownfield et al., 2009). Also, callose deposition is regulated by the balance between callose synthase and hydrolase activities (Beffa et al., 1996; Levy et al., 2007; Zavaliev et al., 2011); thus, the role of β-1,3-glucanses would need to be taken into consideration.
Recently, two Arabidopsis CALS genes, CALS10/GSL8 and CALS3/GSL12, were shown to affect PD callose accumulation and play important roles during plant development (Guseman et al., 2010; Vatén et al., 2011). The loss-of-function mutant of CALS10/GSL8, chor, is compromised for callose deposition at the cell plate, cell wall, and PD and is seedling lethal. Knocking out CALS3/GSL12 did not alter the level of PD callose deposition, but gain-of-function mutations caused hyperaccumulation of PD callose in root cells. Another isoform, CALS7/GSL7, was shown to be specifically expressed in phloem and required for both basal and wound-induced callose deposition at the sieve plates (Xie et al., 2011). Collectively, these data suggest that specific CALS genes may be responsible for PD callose deposition in certain tissues in specific developmental stages and/or in response to physiological cues or environmental challenges. In this study, we showed that direct application of SA to Arabidopsis leaves induces a substantial amount of PD callose, which suggests that the PD callose induction by bacterial infection that we previously reported (Lee et al., 2011) is likely mediated through elevated SA concentration. In addition, the hyperaccumulation of PD callose induced by PDLP5 overexpression was suppressed in npr1, underscoring the critical role of this component (Figure 6). Currently, we are investigating which CALS/hydrolases might be responsible for SA-induced PD callose accumulation during immune responses and the mechanisms by which they may regulate both basal and induced PD permeability in plants.
Crosstalk between Defense Signaling and PD Regulation via PDLP5
Based upon the results presented in this study, we propose a model illustrating how PDLP5 is integrated into the defense signaling cascade to regulate cell-to-cell connectivity in Arabidopsis (Figure 7). Bacterial pathogen infection activates the basal immune pathway through EDS1 and ICS1, stimulating SA biosynthesis, which leads to the upregulation of PDLP5 gene expression, which is partially dependent upon NPR1. However, the high expression of PDLP5 also triggers a feedback loop through the same pathway, soon causing a hyperaccumulation of SA in the tissue, which reinforces the defense response. What happens next will require further exploration to gather essential details, but from the experimental evidence that we have at hand so far, PD closure during an SA-based defense response absolutely requires both NPR1 and PDLP5. Since it is highly unlikely that NPR1 and PDLP5 interact directly, there is probably an NPR1-dependent “factor X” that must work together with PDLP5 for proper PD callose accumulation and closure during SA-dependent defense. Further biochemical characterization of PDLP5 and identification of the genes that are responsible for the PD callose deposition during immune responses should provide insight into the mechanism by which the restriction of PD is achieved during basal immunity.
METHODS
Plant Materials and Growth Conditions
Seeds of the Arabidopsis thaliana mutants eds1-2, ics1, and npr1-1 were provided by the H. Bais lab (University of Delaware) and transgenic NahG seeds from the X. Dong lab (Duke University). Plants were grown in soil in a 22°C, 60% humidity, 16/8-h-light/dark growth chamber. Crosses were made by removing the sepals, petals, and immature stamens from an unopened bud of an SA pathway mutant and then coating the stigma with pollen from either PDLP5 or pdlp5-1 plants. Segregating F2 seeds from the offspring of the crosses were screened with specific primers for each mutant (see Supplemental Table 1 online) using RT-PCR (see section below), and F3 seeds used in this study were collected from double homozygous mutants.
Genomic and RT-PCR
Total genomic DNA was isolated using DNA extraction buffer containing 200 mM Tris-HCl, pH 8.0, 250 mM NaCl, 25 mM EDTA, pH 8.0, and 0.5% SDS, followed by isopropanol precipitation. Genomic DNA resuspended with nano-water was treated with 10 µg/mL RNase A solution (Sigma-Aldrich) at 65°C for 15 min, and 100 to 300 ng DNA was used as template per PCR. Total RNAs were isolated using the Trizol (Invitrogen) method for RT: RNAs were first treated with 2 units of DNaseI (New England Biolabs) for 20 min at 37°C, and 1 µg of each RNA sample was used in a 20 μL total RT reaction containing 0.5 mM deoxynucleotide triphosphate, 0.5 µg oligo(dT), 20 units of Murine RNase inhibitor (NEB), and 20 units of M-MuLV reverse transcriptase (NEB) at 42°C for 1 h. One-twentieth of the cDNAs from RT was used per PCR reaction as follows. Both genomic and RT-PCRs were performed in a 25-μL reaction volume using gene-specific primers (see Supplemental Table 1 online) and Taq DNA polymerase (GenScript). All PCRs were performed using a Bio-Rad DNA Engine Peltier thermal cycler. The genomic PCR amplification profile was three cycles of 94°C for 1 min, 52°C for 2 min, and 72°C for 6 min followed by 25 cycles of 94°C for 1 min, 60°C for 2 min, and 72°C for 6 min. The RT-PCR profile was 28 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 35 s. Samples were resolved in ethidium bromide–stained 1% agarose gels and visualized and imaged with an Alpha-Imager HP, followed by densitometric quantification using the Image J-NIH program. At least three biological and three technical repeats were performed per sample for quantification.
Confocal Microscopy Imaging, DANS Assay, and Callose Quantification
Plant samples were imaged on a Zeiss AxioObserver inverted light microscope using an LSM 510 META scanhead on a Zeiss LSM 5 LIVE DUO confocal microscope as described before (Lee et al., 2011). Dye loading assays and callose staining were performed on fourth and fifth rosette leaves of 3-week-old plants as described previously (Lee et al., 2011) in the presence or absence of chemical treatments. Briefly, a droplet of 1 mM 5(6)-carboxyfluorescein diacetate (CFDA) was loaded on the adaxial leaf surface of intact Arabidopsis plants for 5 min, followed by removal of dye by pipetting and imaging the abaxial leaf surface under a Fluar ×5/0.25 objective lens, using 488-nm laser excitation with a 505- to 550-nm band-pass emission filter. Aniline blue stains were detected using a C-Apochromat ×40/1.20-W Korr UV-VIS-IR objective and 405-nm laser excitation with a 420- to 480-nm band-pass emission filter. For the effect of bacterial infection, dye loading assays and callose staining were performed on systemic rosette leaves (fourth and fifth) 24 h after infection by bacterial infiltration on lower leaves.
Chemical Treatments, Bacterial Infection, and SA Measurement
For SA and catechol treatments, 3-week-old plants grown in soil were sprayed with 100 μM SA, BTH, or catechol dissolved in double-distilled water containing 0.01% Silwet-77 or 10 mM MES, pH 5.8, and samples were collected 24 h after treatment. Pma ES4326 infection was performed as described before (Lee et al., 2011). Briefly, 3- to 4-week-old Arabidopsis plants were infected by infiltration (OD600 = 0.001) and incubated for 2 d before DANS and callose quantification assays. Statistical difference between samples was analyzed using a t test at P < 0.001 or analysis of variance (ANOVA) in conjunction with Fisher’s LSD test.
Total SA content was measured by HPLC analysis using 25-d-old plant extracts according to Wang et al. (2011). Briefly, 100 to 200 mg of whole plant tissues was extracted with methanol, dried, and resuspended in 500 μL of 100 mM sodium acetate, pH 5.5. For total SA measurement, 40 units of β-glucosidase (Sigma-Aldrich G-0395) were added to a set of duplicated tubes to digest glucosyl-conjugated SA for 1.5 h at 37°C. All samples were precipitated with an equal volume of 10% TCA and centrifuged at 10,000g for 10 min. The supernatant was further extracted twice with 1 mL of extraction solvent (ethylacetate:cyclopentane:2-propanol 100:99:1, v/v). The top phase was collected and dried in a fume hood overnight. The residual fraction was resuspended in 0.5 mL of 55% methanol by vortex, filtrated through a 0.2-mm nylon spin-prep membrane (Fisher 07-200-389), and subjected to reverse-phase HPLC analysis using an RF2000 fluorescence detector.
Accession Numbers
DNA sequence data from this article can be found in the GenBank/EMBL database under accession numbers AT1G70690, AT1G74710, AT3G48090, AT1G64280, and X83926.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. DANS Assays on Mock- or SA-Treated Wild-Type Col-0 Plants.
Supplemental Figure 2. SA Treatment for 24 or 48 h Does Not Induce Microlesion Formation.
Supplemental Figure 3. Confocal Images Showing Adaxial Leaf Surfaces 5 min after Loading with Fluorescent Dye in DANS Assays.
Supplemental Figure 4. Genotyping and Marker Gene Phenotyping to Confirm Mutations in Desired Genes and Homozygosity of Crosses.
Supplemental Figure 5. PD Closure Response in PDLP5ics1 and PDLP5eds1 Plants upon 100 μM SA Treatment.
Supplemental Figure 6. SA Treatment Has No Effect on PDLP5 Subcellular Localization.
Supplemental Figure 7. RT-PCR Showing the Relative Expression Level of PDLP5.
Supplemental Table 1. PCR Primers.
Acknowledgments
This research was supported by grants provided by the National Science Foundation (IOB 0954931) and partially by the National Center for Research Resources (5P30RR031160-03) and the National Institute of General Medical Sciences (8 P30 GM103519-03) from the National Institutes of Health to J.-Y.L. Publically available Arabidopsis mutant lines were obtained from the ABRC.
AUTHOR CONTRIBUTIONS
X.W., R.S., W.C., and C.Z. performed research. X.W., R.S., H.L., and J.-Y.L. analyzed the data. J.-Y.L. directed the research and wrote the article.
Glossary
- SA
salicylic acid
- PD
plasmodesmata
- PDLP
PD-located protein
- Pma
P. syringae pv maculicola
- Col-0
Columbia-0
- BTH
benzo(1,2,3) thiadiazole-7-carbothioic acid
- Ler
Landsberg erecta
- CFDA
5(6)-carboxyfluorescein diacetate
- ANOVA
analysis of variance
References
- Aarts N., Metz M., Holub E., Staskawicz B.J., Daniels M.J., Parker J.E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 10306–10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffa R.S., Hofer R.M., Thomas M., Meins F., Jr (1996). Decreased susceptibility to viral disease of [beta]-1,3-glucanase-deficient plants generated by antisense transformation. Plant Cell 8: 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y., Faulkner C., Ritzenthaler C., Maule A.J. (2010). Plasmodesmata: Gateways to local and systemic virus infection. Mol. Plant Microbe Interact. 23: 1403–1412 [DOI] [PubMed] [Google Scholar]
- Bilska A., Sowinski P. (2010). Closure of plasmodesmata in maize (Zea mays) at low temperature: A new mechanism for inhibition of photosynthesis. Ann. Bot. (Lond.) 106: 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman L.M., Overall R.L. (2001). Structure and function of plasmodesmata. Aust. J. Plant Physiol. 28: 709–727 [Google Scholar]
- Blanco F., Garretón V., Frey N., Dominguez C., Pérez-Acle T., Van der Straeten D., Jordana X., Holuigue L. (2005). Identification of NPR1-dependent and independent genes early induced by salicylic acid treatment in Arabidopsis. Plant Mol. Biol. 59: 927–944 [DOI] [PubMed] [Google Scholar]
- Brodersen P., Malinovsky F.G., Hématy K., Newman M.A., Mundy J. (2005). The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol. 138: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield, L., Doblin, M., Fincher, G., and Basic, A. (2009). Biochemical and molecular properties of biosynthetic enzymes for (1,3)-β-glucans in embryophytes, chlorophytes and rhodophytes. In Chemistry, Biochemistry, and Biology of (1,3)-β-Glucans and Related Polysaccahrides, A. Basic, ed (New York: Academic Press), pp. 283–327. [Google Scholar]
- Burch-Smith T.M., Stonebloom S., Xu M., Zambryski P.C. (2011). Plasmodesmata during development: Re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 248: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith T.M., Zambryski P.C. (2010). Loss of INCREASED SIZE EXCLUSION LIMIT (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Curr. Biol. 20: 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith T.M., Zambryski P.C. (2012). Plasmodesmata paradigm shift: Regulation from without versus within. Annu. Rev. Plant Biol. 63: 239–260 [DOI] [PubMed] [Google Scholar]
- Cao H., Bowling S.A., Gordon A.S., Dong X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia M.L., Jackson D. (2004). Plasmodesmata form and function. Curr. Opin. Cell Biol. 16: 500–506 [DOI] [PubMed] [Google Scholar]
- Coll N.S., Epple P., Dangl J.L. (2011). Programmed cell death in the plant immune system. Cell Death Differ. 18: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebRoy S., Thilmony R., Kwack Y.B., Nomura K., He S.Y. (2004). A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA 101: 9927–9932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney T.P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., Gaffney T., Gut-Rella M., Kessmann H., Ward E., Ryals J. (1994). A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Dempsey, D.A., Vlot, A.C., Wildermuth, M.C., and Klessig, D.F. (2011). Salicylic acid biosynthesis and metabolism. The Arabidopsis Book 9:e0156, /10.1199/tab.0156. [DOI] [PMC free article] [PubMed]
- Dong X., Hong Z., Chatterjee J., Kim S., Verma D.P. (2008). Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229: 87–98 [DOI] [PubMed] [Google Scholar]
- Ehlers K., Kollmann R. (1996). Formation of branched plasmodesmata in regenerating Solanum nigrum protoplasts. Planta 199: 126–138 [Google Scholar]
- Ehlers K., Kollmann R. (2001). Primary and secondary plasmodesmata: Structure, origin, and functioning. Protoplasma 216: 1–30 [DOI] [PubMed] [Google Scholar]
- Ehlers K., van Bel A.J.E. (2010). Dynamics of plasmodesmal connectivity in successive interfaces of the cambial zone. Planta 231: 371–385 [DOI] [PubMed] [Google Scholar]
- Faulkner C., Akman O.E., Bell K., Jeffree C., Oparka K. (2008). Peeking into pit fields: A multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell 20: 1504–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Plotnikova J.M., De Lorenzo G., Ausubel F.M. (2003). Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J., Beck M., Zhou J., Faulkner C., Robatzek S., Oparka K. (2013). A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis. Plant Cell 25: 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.Q., Dong X. (2013). Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 64: 839–863 [DOI] [PubMed] [Google Scholar]
- Fu Z.Q., Yan S., Saleh A., Wang W., Ruble J., Oka N., Mohan R., Spoel S.H., Tada Y., Zheng N., Dong X. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning B.E.S. (1978). Age-related and origin-related control of numbers of plasmodesmata in cell-walls of developing Azolla roots. Planta 143: 181–190 [DOI] [PubMed] [Google Scholar]
- Guseman J.M., Lee J.S., Bogenschutz N.L., Peterson K.M., Virata R.E., Xie B., Kanaoka M.M., Hong Z.L., Torii K.U. (2010). Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 137: 1731–1741 [DOI] [PubMed] [Google Scholar]
- Heck S., Grau T., Buchala A., Métraux J.P., Nawrath C. (2003). Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J. 36: 342–352 [DOI] [PubMed] [Google Scholar]
- Heinlein M. (2002). The spread of tobacco mosaic virus infection: Insights into the cellular mechanism of RNA transport. Cell. Mol. Life Sci. 59: 58–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Yang Y., Zhou J.M. (2009). The multilevel and dynamic interplay between plant and pathogen. Plant Signal. Behav. 4: 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jacobs A.K., Lipka V., Burton R.A., Panstruga R., Strizhov N., Schulze-Lefert P., Fincher G.B. (2003). An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15: 2503–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran G., Raina S., Acharya B.R., Maqbool S.B., Mosher S.L., Appel H.M., Schultz J.C., Klessig D.F., Raina R. (2007). Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J. 51: 234–246 [DOI] [PubMed] [Google Scholar]
- Koh E.J., Zhou L., Williams D.S., Park J., Ding N., Duan Y.P., Kang B.H. (2012). Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with “Candidatus Liberibacter asiaticus”. Protoplasma 249: 687–697 [DOI] [PubMed] [Google Scholar]
- Lawton K., Weymann K., Friedrich L., Vernooij B., Uknes S., Ryals J. (1995). Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol. Plant Microbe Interact. 8: 863–870 [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Lu H. (2011). Plasmodesmata: The battleground against intruders. Trends Plant Sci. 16: 201–210 [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Wang X., Cui W., Sager R., Modla S., Czymmek K., Zybaliov B., van Wijk K., Zhang C., Lu H., Lakshmanan V. (2011). A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23: 3353–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.W., Jelenska J., Greenberg J.T. (2008). Arabidopsis proteins important for modulating defense responses to Pseudomonas syringae that secrete HopW1-1. Plant J. 54: 452–465 [DOI] [PubMed] [Google Scholar]
- Levy A., Erlanger M., Rosenthal M., Epel B.L. (2007). A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 49: 669–682 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Vailleau F., Balagué C., Roby D. (2003). Lesion mimic mutants: Keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 8: 263–271 [DOI] [PubMed] [Google Scholar]
- Lucas W.J., Ham B.K., Kim J.Y. (2009). Plasmodesmata - Bridging the gap between neighboring plant cells. Trends Cell Biol. 19: 495–503 [DOI] [PubMed] [Google Scholar]
- Lucas W.J., Lee J.Y. (2004). Plasmodesmata as a supracellular control network in plants. Nat. Rev. Mol. Cell Biol. 5: 712–726 [DOI] [PubMed] [Google Scholar]
- Malamy J., Carr J.P., Klessig D.F., Raskin I. (1990). Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004 [DOI] [PubMed] [Google Scholar]
- Maule A.J. (2008). Plasmodesmata: Structure, function and biogenesis. Curr. Opin. Plant Biol. 11: 680–686 [DOI] [PubMed] [Google Scholar]
- Maule A.J., Benitez-Alfonso Y., Faulkner C. (2011). Plasmodesmata - Membrane tunnels with attitude. Curr. Opin. Plant Biol. 14: 683–690 [DOI] [PubMed] [Google Scholar]
- Mur L.A., Kenton P., Lloyd A.J., Ougham H., Prats E. (2008). The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 59: 501–520 [DOI] [PubMed] [Google Scholar]
- Nawrath C., Métraux J.P. (1999). Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M.T., Stein M., Hou B.H., Vogel J.P., Edwards H., Somerville S.C. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301: 969–972 [DOI] [PubMed] [Google Scholar]
- Oparka K.J., Roberts A.G. (2001). Plasmodesmata. A not so open-and-shut case. Plant Physiol. 125: 123–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormenese S., Bernier G., Périlleux C. (2006). Cytokinin application to the shoot apical meristem of Sinapis alba enhances secondary plasmodesmata formation. Planta 224: 1481–1484 [DOI] [PubMed] [Google Scholar]
- Ormenese S., Havelange A., Bernier G., van der Schoot C. (2002). The shoot apical meristem of Sinapis alba L. expands its central symplasmic field during the floral transition. Planta 215: 67–78 [DOI] [PubMed] [Google Scholar]
- Ormenese S., Havelange A., Deltour R., Bernier G. (2000). The frequency of plasmodesmata increases early in the whole shoot apical meristem of Sinapis alba L. during floral transition. Planta 211: 370–375 [DOI] [PubMed] [Google Scholar]
- Pouwels J., Kornet N., van Bers N., Guighelaar T., van Lent J., Bisseling T., Wellink J. (2003). Identification of distinct steps during tubule formation by the movement protein of Cowpea mosaic virus. J. Gen. Virol. 84: 3485–3494 [DOI] [PubMed] [Google Scholar]
- Pouwels J., van der Velden T., Willemse J., Borst J.W., van Lent J., Bisseling T., Wellink J. (2004). Studies on the origin and structure of tubules made by the movement protein of Cowpea mosaic virus. J. Gen. Virol. 85: 3787–3796 [DOI] [PubMed] [Google Scholar]
- Radford J.E., Vesk M., Overall R.L. (1998). Callose deposition at plasmodesmata. Protoplasma 201: 30–37 [Google Scholar]
- Rietz S., Stamm A., Malonek S., Wagner S., Becker D., Medina-Escobar N., Vlot A.C., Feys B.J., Niefind K., Parker J.E. (2011). Different roles of Enhanced Disease Susceptibility1 (EDS1) bound to and dissociated from Phytoalexin Deficient4 (PAD4) in Arabidopsis immunity. New Phytol. 191: 107–119 [DOI] [PubMed] [Google Scholar]
- Rinne P.L.H., van der Schoot C. (2003). Plasmodesmata at the crossroads between development, dormancy, and defense. Can. J. Bot. 81: 1182–1197 [Google Scholar]
- Rinne P.L.H., Welling A., Vahala J., Ripel L., Ruonala R., Kangasjärvi J., van der Schoot C. (2011). Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23: 130–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M., Plasencia J. (2011). Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 62: 3321–3338 [DOI] [PubMed] [Google Scholar]
- Robards A.W., Lucas W.J. (1990). Plasmodesmata. Annu. Rev. Plant Physiol. 41: 369–419 [Google Scholar]
- Roberts A.G., Oparka K.J. (2003). Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26: 103–124 [Google Scholar]
- Rustérucci C., Aviv D.H., Holt III B.F., Dangl J.L., Parker J.E. (2001). The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13: 2211–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J.A., Neuenschwander U.H., Willits M.G., Molina A., Steiner H.Y., Hunt M.D. (1996). Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelz J.E., Harries P.A., Nelson R.S. (2011). Intracellular transport of plant viruses: Finding the door out of the cell. Mol. Plant 4: 813–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagull R.W. (1983). Differences in the frequency and disposition of plasmodesmata resulting from root cell elongation. Planta 159: 497–504 [DOI] [PubMed] [Google Scholar]
- Sevilem I., Miyashima S., Helariutta Y. (2013). Cell-to-cell communication via plasmodesmata in vascular plants. Cell Adhes. Migr. 7: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. (2009). Plants under attack: Systemic signals in defence. Curr. Opin. Plant Biol. 12: 459–464 [DOI] [PubMed] [Google Scholar]
- Simpson C., Thomas C., Findlay K., Bayer E., Maule A.J. (2009). An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 21: 581–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel S.H., Dong X. (2012). How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12: 89–100 [DOI] [PubMed] [Google Scholar]
- Tada Y., Spoel S.H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., Zuo J., Dong X. (2008). Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.L., Bayer E.M., Ritzenthaler C., Fernandez-Calvino L., Maule A.J. (2008). Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 6: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki S., Citovsky V. (2011). To gate, or not to gate: Regulatory mechanisms for intercellular protein transport and virus movement in plants. Mol. Plant 4: 782–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent J., Storms M., van der Meer F., Wellink J., Goldbach R. (1991). Tubular structures involved in movement of cowpea mosaic virus are also formed in infected cowpea protoplasts. J. Gen. Virol. 72: 2615–2623 [DOI] [PubMed] [Google Scholar]
- van Wees S.C., Glazebrook J. (2003). Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J. 33: 733–742 [DOI] [PubMed] [Google Scholar]
- Vatén A., et al. (2011). Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21: 1144–1155 [DOI] [PubMed] [Google Scholar]
- Vlot A.C., Dempsey D.A., Klessig D.F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vogelmann K., Drechsel G., Bergler J., Subert C., Philippar K., Soll J., Engelmann J.C., Engelsdorf T., Voll L.M., Hoth S. (2012). Early senescence and cell death in Arabidopsis saul1 mutants involves the PAD4-dependent salicylic acid pathway. Plant Physiol. 159: 1477–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann E., Lucas W.J., Citovsky V., Zambryski P. (1994). Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc. Natl. Acad. Sci. USA 91: 1433–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Weaver N.D., Kesarwani M., Dong X. (2005). Induction of protein secretory pathway is required for systemic acquired resistance. Science 308: 1036–1040 [DOI] [PubMed] [Google Scholar]
- Wang G.F., Seabolt S., Hamdoun S., Ng G., Park J., Lu H. (2011). Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering time in Arabidopsis. Plant Physiol. 156: 1508–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiermer M., Feys B.J., Parker J.E. (2005). Plant immunity: The EDS1 regulatory node. Curr. Opin. Plant Biol. 8: 383–389 [DOI] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Wille A.C., Lucas W.J. (1984). Ultrastructural and histochemical studies on guard cells. Planta 160: 129–142 [DOI] [PubMed] [Google Scholar]
- Xie B., Wang X., Zhu M., Zhang Z., Hong Z. (2011). CalS7 encodes a callose synthase responsible for callose deposition in the phloem. Plant J. 65: 1–14 [DOI] [PubMed] [Google Scholar]
- Zavaliev R., Ueki S., Epel B.L., Citovsky V. (2011). Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248: 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]