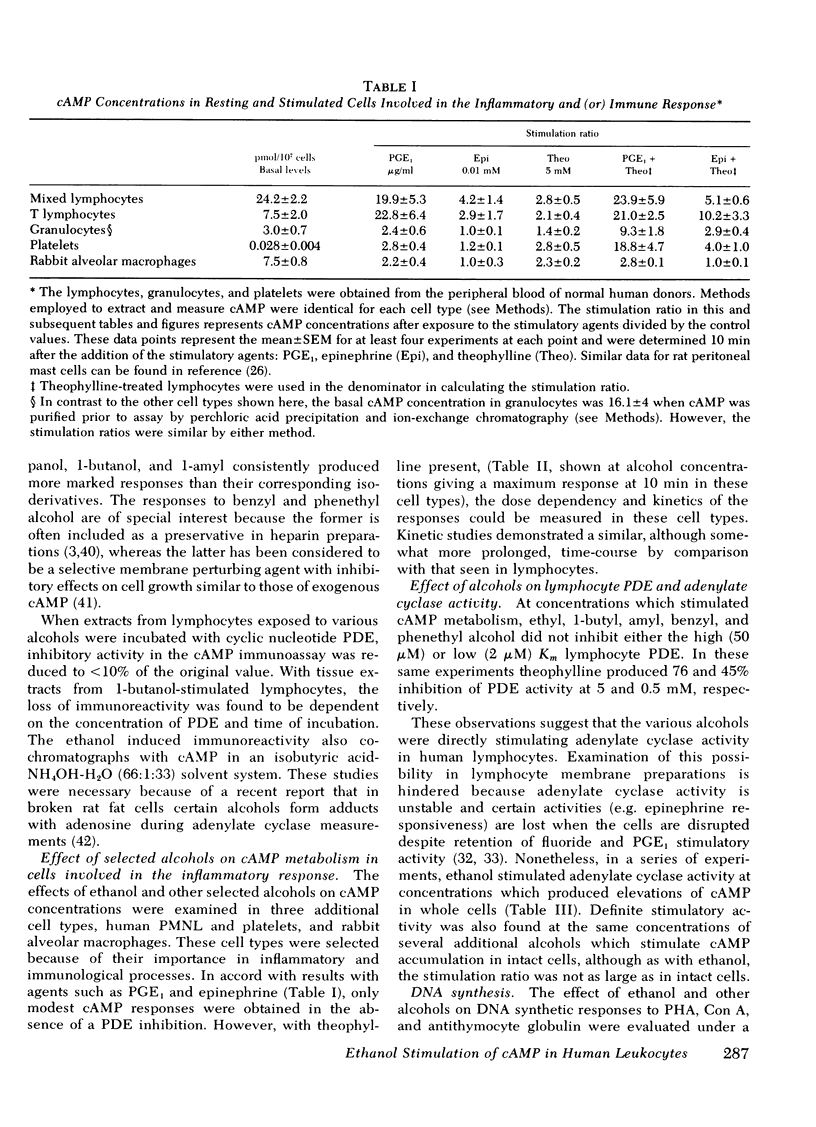
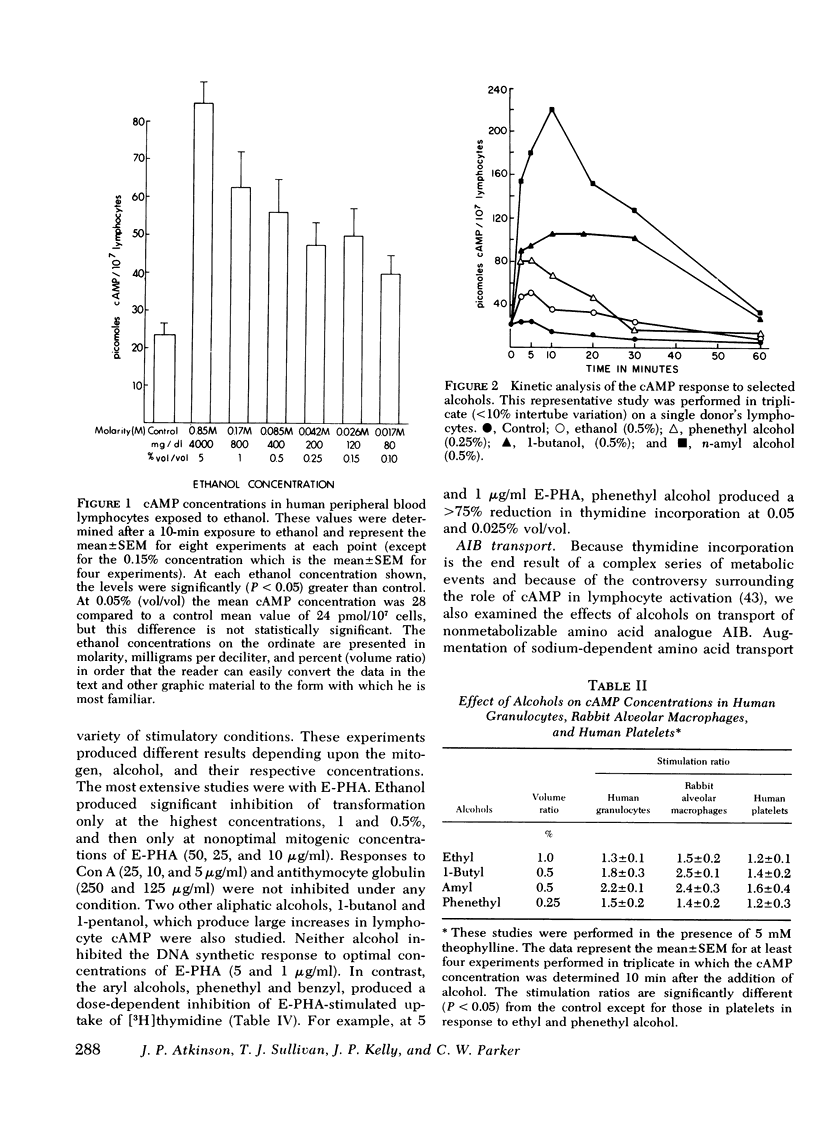
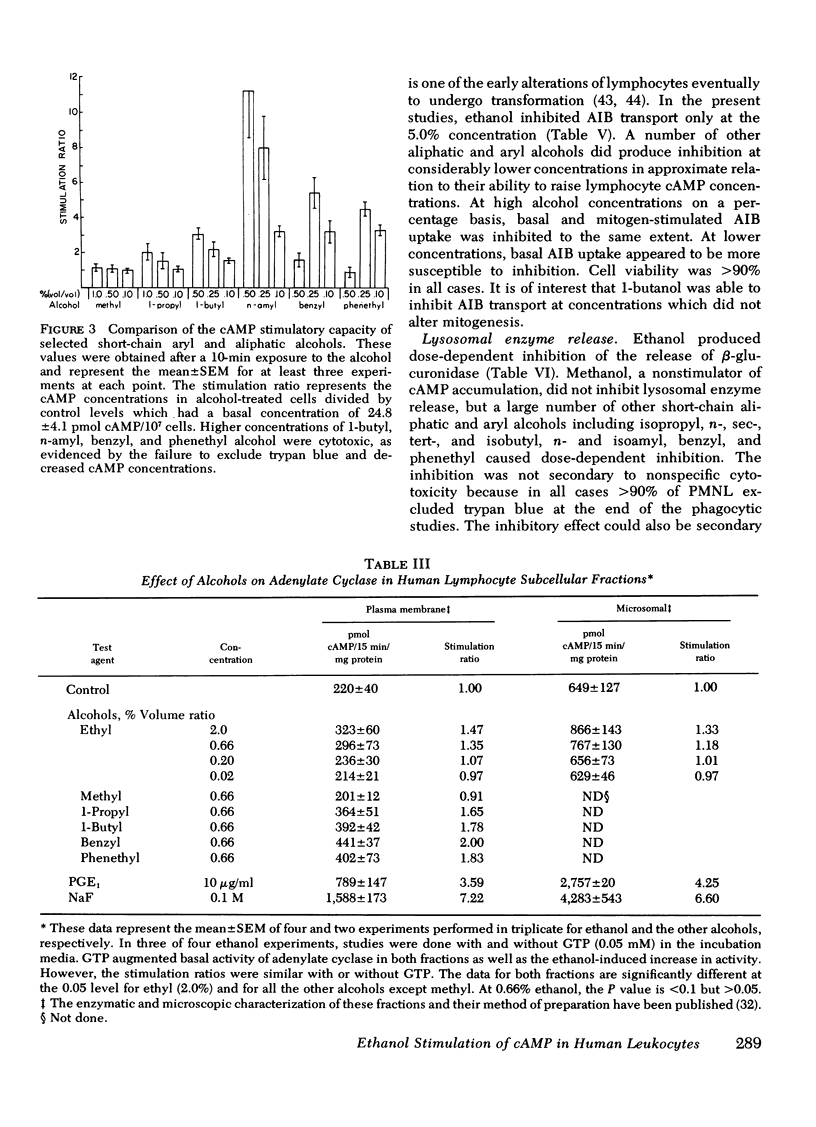
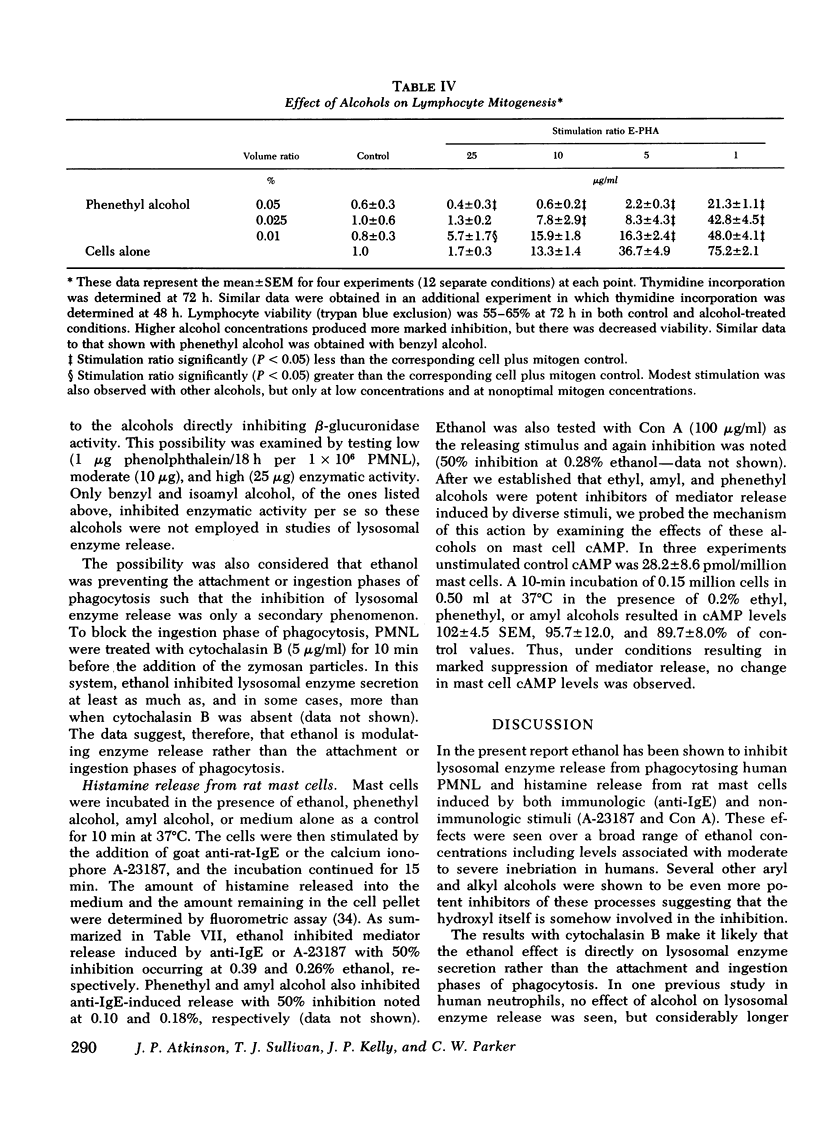
Abstract
In this study ethanol and certain other short-chain aryl (benzyl and phenethyl) and aliphatic (methyl, propyl, butyl, and amyl) alcohols produced up to 10-fold increases in cyclic AMP (cAMP) concentrations in purified human peripheral blood lymphocytes. Ethanol concentrations as low as 80 mg/dl produced significant elevations in lymphocyte cAMP. Significant but less marked augmentation of cAMP in response to alcohols was observed in human platelets, human granulocytes, and rabbit alveolar macrophages. The mechanism of the alcohol-induced cAMP accumulation is probably secondary to membrane perturbation and consequent activation of adenylate cyclase, because ethanol directly stimulated this enzyme in lymphocyte membrane preparations but had no effect on lymphocyte phosphodiesterase activity.
Lysosomal enzyme release, by phagocytosing human leukocytes, and aminoisobutyric acid transport in mitogen-stimulated human lymphocytes were shown to be inhibited by ethanol and other alcohols at concentrations which also elevate cAMP. In general, the magnitude of the inhibition of these inflammatory processes correlated with the ability of the alcohol to elevate cAMP concentrations. Lectin-and anti-thymocyte globulin-induced lymphocyte mitogenesis was inhibited or unaffected depending upon both the concentration and type of mitogenic stimulus and the concentration and type of alcohol utilized. Inflammatory mediator release from rat mast cells also was inhibited by ethanol and certain other alcohols, but whole cell cAMP was not increased. Ethanol may alter these inflammatory responses and other biologic processes at least in part by modulating cellular levels of cAMP.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., GOLD J. PNEUMOCOCCAL BACTEREMIA WITH ESPECIAL REFERENCE TO BACTEREMIC PNEUMOCOCCAL PNEUMONIA. Ann Intern Med. 1964 May;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P., Greene W. C., McNearney T. A., Parker C. W. Studies on the stimulation of cAMP metabolism in human lymphocytes by latex polymers. Exp Cell Res. 1976 May;99(2):395–407. doi: 10.1016/0014-4827(76)90597-8. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P., Udey M. C., Wedner H. J., Parker C. W. Studies on the stimulation of cAMP metabolism by heparin solutions containing benzyl alcohol. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):297–305. [PubMed] [Google Scholar]
- Atkinson J. P., Wedner H. J., Parker C. W. Two novel stimuli of cyclic adenosine 3',5'-monophosphate (cAMP) in human lymphocytes. J Immunol. 1975 Oct;115(4):1023–1027. [PubMed] [Google Scholar]
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Bleich H. L., Boro E. S. Control of lymphocyte function. N Engl J Med. 1976 Nov 18;295(21):1180–1186. doi: 10.1056/NEJM197611182952110. [DOI] [PubMed] [Google Scholar]
- Brayton R. G., Stokes P. E., Schwartz M. S., Louria D. B. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N Engl J Med. 1970 Jan 15;282(3):123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- Eisen S. A., Wedner H. J., Parker C. W. Isolation of pure human peripheral blood T-lymphocytes using nylon wool columns. Immunol Commun. 1972;1(6):571–577. doi: 10.3109/08820137209022965. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. E., Bitensky M. W. Selective activation by short chain alcohols of glucagon responsive adenyl cyclase in liver. Endocrinology. 1970 Nov;87(5):1075–1081. doi: 10.1210/endo-87-5-1075. [DOI] [PubMed] [Google Scholar]
- Greene H. L., Herman R. H., Kraemer S. Stimulation of jejunal adenyl cyclase by ethanol. J Lab Clin Med. 1971 Sep;78(3):336–342. [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Opposing effects of mitogenic and nonmitogenic lectins on lymphocyte activation. Evidence that wheat germ agglutinin produces a negative signal. J Biol Chem. 1976 Jul 10;251(13):4017–4025. [PubMed] [Google Scholar]
- Ignarro L. J., Lint T. F., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils. Effects of autonomic agents on enzyme release, phagocytosis, and cylic nucleotide levels. J Exp Med. 1974 Jun 1;139(6):1395–1414. doi: 10.1084/jem.139.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Kimura H., Kuriyama K. Changes in activity and hormonal sensitivity of brain adenyl cyclase following chronic ethanol administration. Experientia. 1972 Nov 15;28(11):1322–1323. doi: 10.1007/BF01965322. [DOI] [PubMed] [Google Scholar]
- Kemp A., Berke G. Effects of heparin and benzyl alcohol on lymphocyte-mediated cytotoxicity in vitro. Cell Immunol. 1973 Jun;7(3):512–515. doi: 10.1016/0008-8749(73)90215-3. [DOI] [PubMed] [Google Scholar]
- Kuo W. N., Hodgins D. S., Kuo J. F. Adenylate cyclase in islets of Langerhans. Isolation of islets and regulation of adenylate cyclase activity by various hormones and agents. J Biol Chem. 1973 Apr 25;248(8):2705–2711. [PubMed] [Google Scholar]
- Lagarde A., Colobert L. Cyclic 3',5'-AMP phosphodiesterase of human blood lymphocytes. Biochim Biophys Acta. 1972 Aug 28;276(2):444–453. doi: 10.1016/0005-2744(72)91006-6. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Mashiter K., Mashiter G. D., Field J. B. Effects of prostaglandin E1, ethanol and TSH on the adenylate cyclase activity of beef thyroid plasma membranes and cyclic AMP content of dog thyroid slices. Endocrinology. 1974 Feb;94(2):370–376. doi: 10.1210/endo-94-2-370. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. W., Sullivan T. J., Wedner H. J. Cyclic AMP and the immune response;. Adv Cyclic Nucleotide Res. 1974;4(0):1–79. [PubMed] [Google Scholar]
- Petrack B., Ma D., Sheppy F. Formation of a novel nucleotide by fat cell preparations containing adenylate cyclase. J Biol Chem. 1974 Jun 10;249(11):3661–3663. [PubMed] [Google Scholar]
- Pick E. Cyclic AMP affects macrophage migration. Nat New Biol. 1972 Aug 9;238(84):176–177. doi: 10.1038/newbio238176a0. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Parker C. W. Adenylate cyclase activity in lymphocyte subcellular fractions. Characterization of non-nuclear adenylate cyclase. Biochem J. 1977 Mar 15;162(3):473–482. doi: 10.1042/bj1620473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell M. F., Leevy C. M. Lymphocyte transformation and alcoholic liver injury. Gastroenterology. 1972 Dec;63(6):1020–1025. [PubMed] [Google Scholar]
- Spagnuolo P. J., MacGregor R. R. Acute thanol effect on chemotaxis and other components of host defense. J Lab Clin Med. 1975 Jul;86(1):24–31. [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stolc V. Restrained adenyl cyclase in human neutrophils: stimulation of cyclic adenosine 3':5'-monophosphate formation and adenyl cyclase activity by phagocytosis and prostaglandins. Blood. 1974 May;43(5):743–748. [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Cyclic AMP phosphodiesterase activation by the histamine releasing agent, compound 48-80. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1334–1339. doi: 10.1016/s0006-291x(73)80040-3. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Eisen S. A., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. II. Studies on the relationship between intracellular cyclic AMP concentrations and histamine release. J Immunol. 1975 May;114(5):1480–1485. [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Kulczycki A., Jr, Parker C. W. Modulation of cyclic AMP in purified rat mast cells. III. Studies on the effects of concanavalin A and anti-IgE on cyclic AMP concentrations during histamine release. J Immunol. 1976 Sep;117(3):713–716. [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Stenson W., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. I. Responses to pharmacologic, metabolic, and physical stimuli. J Immunol. 1975 May;114(5):1473–1479. [PubMed] [Google Scholar]
- Tague L. L., Shanbour L. L. Effects of ethanol on gastric mucosal adenosine 3', 5' monophosphate (cAMP). Life Sci. 1974 Mar 16;14(6):1065–1073. doi: 10.1016/0024-3205(74)90231-8. [DOI] [PubMed] [Google Scholar]
- Tisman G., Herbert V. In vitro myelosuppression and immunosuppression by ethanol. J Clin Invest. 1973 Jun;52(6):1410–1414. doi: 10.1172/JCI107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;Series 47:72–98. [PubMed] [Google Scholar]
- Wedner H. J., Dankner R., Parker C. W. Cyclic GMP and lectin-induced lymphocyte activation. J Immunol. 1975 Dec;115(6):1682–1687. [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Adenylate cyclase activity in lymphocyte subcellular fractions. Characterization of a nuclear adenylate cyclase. Biochem J. 1977 Mar 15;162(3):483–491. doi: 10.1042/bj1620483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedner H. J., Parker C. W. Lymphocyte activation. Prog Allergy. 1976;20:195–300. [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Wright J. A., Ceri H., Lewis W. H. Similar growth of Chinese hamster cells in the presence of phenethyl alcohol or dibutyryl cyclic AMP. Nat New Biol. 1973 Jul 18;244(133):84–86. doi: 10.1038/newbio244084a0. [DOI] [PubMed] [Google Scholar]


