Abstract
Cryptococcus neoformans is a pathogenic yeast that commonly infects immunocompromised individuals, yet has developed multiple adaptation mechanisms to the host. Several virulence factors (capsule and melanin) have been known for many years. However, this yeast also possesses a morphogenetic program that is still not well characterized. Cryptococcus neoformans has the ability to dramatically enlarge its size during infection to form “titan cells” that can reach up to 100 microns in cell body diameter, in contrast to typical size cells of 5-7 microns. These titan cells pose a problem for the host because they contribute to fungal survival, dissemination to the central nervous system, and possibly even latency. In this review, we will provide an overview of these cells, covering current knowledge about their phenotypic features, mechanism of formation, and their significance during infection.
Introduction
The interaction between microbial pathogens and their hosts is very complex, and the outcome depends on both host and pathogen responses. In the case of fungal pathogens, alterations in morphology are common. Dimorphic fungi, such as Histoplasma capsulatum and Blastomyces dermititidis, have a yeast morphology in vivo even though they grow filamentously in nature (1, 2). In contrast, Candida albicans produces yeasts, pseudohyphae and hyphae in vivo and the ability to change morphology is critical for pathogenesis (reviewed in (3)). These morphological changes play an important role in disease. They are involved in immune avoidance, adherence, dissemination, and penetration of biological barriers. Yet the role of morphogenesis in virulence is still poorly understood. For example, Candida glabrata, a common cause of candidemia worldwide, does not produce hyphae during infection even though hyphal formation is integral to C. albicans pathogenesis.
Perhaps most intriguing is the newly characterized morphological transition observed in Cryptococcus neoformans – titan cell formation. Cryptococcus neoformans is a basidiomyce yeast well known for its production of a polysaccharide capsule that surrounds the cell body (4-7). Cryptococcus neoformans does not form hyphae during infection. Some strains with the ability to form pseudohyphae have been occasionally described and this transition has been characterized at the molecular level (8, 9). But these pseudohyphal forms are rare during infection, and are associated with reduced virulence (10, 11). Instead, C. neoformans is typically found as a spherical budding yeast both in nature and during infection. In contrast to the typical hyphal morphological transitions observed in other fungal pathogens, C. neoformans possesses a complex “non-conventional” morphogenetic program that results in the appearance of multiple cellular size morphologies (12, 13). It has long been known that C. neoformans can modulate the size and structure of its capsule, which has profound consequences during interactions with the host (reviewed in (6, 14)). But more striking, this yeast also produces cells with dramatic differences in the cell body size, particularly during the initial pulmonary infection (12). The present review will focus on a recently characterized enlarged cell type produced by C. neoformans – titan cells (15, 16). These cells have been referred to in the literature as both “titan” and “giant” cells. However this review will use the titan cell nomenclature as the term “giant cell” can be confused with mammalian giant cells that can also be present in the lungs. We will summarize the known characteristics of titan cells, with special emphasis on their morphological features, formation, and importance during interactions with the host.
Morphological features of titan cells
Titan cells are significantly different from typical cryptococcal cells grown in vitro. The most striking feature of titan cells is their gigantic size. The titan cell body can reach up to 100 microns in diameter, significantly larger than the 5-7 micron diameter of typical cells grown in vitro (Figure 1)(15). As an arbitrary size, titan cells are defined as those with a cell body diameter greater than 15 microns. Titan cells can bud both in vivo and in vitro (15, 16). Interestingly, titan cells bud to produce typical size daughter cells. This feature is of great interest because, as will be discussed below, titan cells enhance dissemination in the host through this production of typical size progeny.
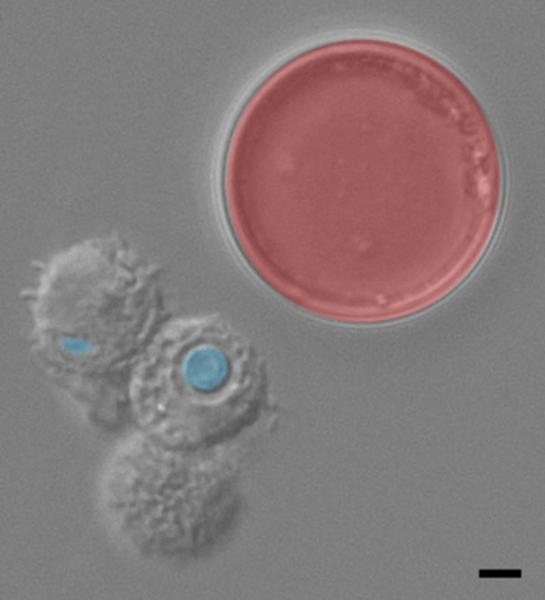
Figure 1. Cell enlargement in Cryptococcus neoformans.
Bronchoalveolar lavage from an infected mouse. Typical size cells, indicated in blue, are readily phagocytosed by host mononuclear cells (gray cells). Titan cells, indicated in red, are unable to be phagocytosed by host cells. Bar = 5 μm. Image courtesy of Laura Okagaki.
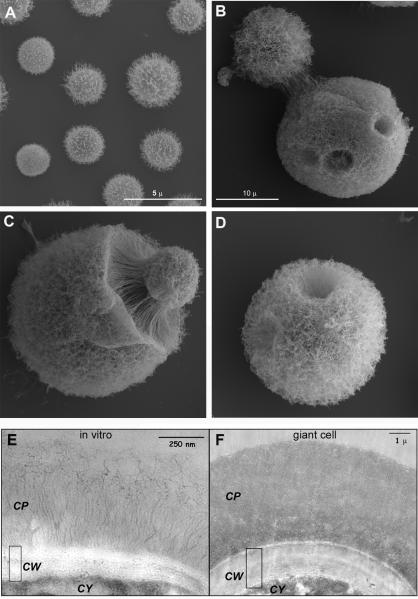
The capsule, which is the main virulence factor of C. neoformans, shows structural differences in titan cells. The capsule can change in density and structure depending on environmental condition (7). While typical size cells have diffuse capsules (Figure 2A), the titan cell capsule is significantly denser and more crosslinked (Figure 2, B-D) (16). This feature affects the permeability of the capsule to different compounds, such as complement proteins. Holes or dimples are also observed in the titan cell capsule (Figure 2B and D), which suggest locations where buds may have emerged from the titan cell. Titan cells also show an abnormally thick cell wall (16). While the cell wall in typical cells is around 150-200 nm thick (Figure 2E), in titan cells it can reach up to 2-3 microns (Figure 2F).
Figure 2. Morphological features of titan cells shown by electron microscopy.
A-D) Scanning electron microscopy of typical cells grown in Sabouraud medium (A) or of titan cells isolated from the lungs of infected mice (B-D). Scale bar in B applies to panels C and D. E-F) Transmission electron microscopy of cells grown in vitro (E) or of titan cells (F). CP, capsule; CW, cell wall; CY, cytoplasm. The pictures shown in this figure are reproduced from reference (16).
In addition to changes in surface characteristics, titan cells also have alterations in their internal organelles. Cell size is often associated with increased DNA content and vacuole formation (17, 18). Titan cells have extremely large vacuoles that appear to be involved in extracellular vesicle production (15, 19). Whether the contents of this vacuole and the vesicles it generates differ from typical cells remains unknown. Consistent with their large size, titan cells are also polyploid (15, 16). While typical cryptococcal cells are haploid, the titan cells are often tetraploid or octoploid with some of the larger titan cells containing 16, 32, 64, or more copies of the genome. Yet titan cells contain a single nucleus and their progeny are haploid (15). These data suggest titan cells likely form by endoreduplication (20), but C. neoformans may have a distinct cell cycle regulation that allows the titan cells to produce haploid progeny.
These morphological changes indicate that titan cell production involves a massive and coordinated alteration of multiple cellular structures, including cell body, internal organelles, cell wall and capsule. These changes indicate that titan cell production is not a stochastic event, but rather a distinct developmental transition that C. neoformans uses to adapt to its environment.
Signals for titan cell production
The mechanism of titan cell formation remains unknown, but signaling pathways involved in titan cell production have begun to be elucidated. Recent studies show that titan cell production is regulated, in part, by the same core cyclic AMP (cAMP)/protein kinase A (PKA) pathway that regulates many of the other cryptococcal virulence factors (16). Titan cell production increases in mating type a cells upon signaling via the Ste3a pheromone receptor (15). Okagaki et al. (21) went on to show that an additional g-protein coupled receptor, Gpr5, also stimulates titan cell production. While Ste3a is postulated to interact with alpha pheromone, the ligand for Gpr5 remains unknown. Based on the requirement for Gpr5 to induce titan cell production in the lungs, the ligand is likely to be a compound prevalent in the pulmonary environment (21). Cryptococcal cell enlargement has been observed in response to the mammalian lung environment, macrophages, Galleria mellonella (greater wax moth), or purified phosphatidylcholine (15, 16, 22, 23). These data suggest titan cell formation might be a general response to phagocytic cells through sensing of phospholipids in their membranes (23). Whether Gpr5, Ste3a, or another uncharacterized pathway is involved in phospholipid sensing needs to be determined. Both Ste3a and Gpr5 interact either directly or indirectly with the Gpa1 g-protein. Gpa1 signals via the cAMP/PKA pathway and both adenylate cyclase and PKA mutants have aberrant titan cell production (16, 19). The transcription factor Rim101 acts downstream of the cAMP/PKA pathway (24). Deletion of Rim101 abolishs in vivo titan cell production, suggesting Rim101 is a major transcription factor promoting titan cell formation (21).
How Rim101 promotes titan cell formation remains unclear. A few downstream effectors required for titan cell production were identified in mutant screens, including the G1 cyclin Pcl103 and several GTPases (21). Whether these genes are direct targets of Rim101 is unknown. Detailed analyses of transcriptional and proteomic differences between typical cells and titan cells are needed to understand the molecular modifications that give rise to the size, capsule, cell wall, and ploidy changes present in titan cells.
Role in virulence and host-pathogen interactions
Cryptococcal cell enlargement occurs in response to both amoebae and wax moths (22, 23). It is necessary to determine whether these enlarged cells have other characteristics of titan cells (such as cross-linked capsule, thick cell wall, and increased ploidy). However, if these are titan cells, then the data suggest that titan cell formation could be important to protect against predation and promote survival in the environment. Analysis of the effect of titan cell formation during a murine model of cryptococcosis shows that titan cells also promote persistence in the host as well as promote disease through enhanced survival, dissemination, and ultimately virulence of C. neoformans (25).
Initial studies showed that the proportion of titan cells is high during asymptomatic pulmonary infections (16). Consistent with these findings, the otc1 (over-producer of titan cells 1) mutant is not cleared from the lungs by the host immune response (25). Titan cells are not phagocytosed, likely due to their large size (15, 16). Yet Okagaki et al (26) showed the presence of titan cells also reduces phagocytosis of typical size cryptococcal cells. This reduction in phagocytosis promotes survival of the entire cryptococcal population in the lungs. Taken together, these data suggest a role for titan cells in establishment and persistence of the pulmonary infection. Cryptococcus neoformans is known to persist within humans for years as a latent infection (27-29). Titan cells likely play a key role in preventing clearance by the host and establishment of this latent infection.
Titan cells themselves are unable to disseminate to the brain due to their large size (15, 25). However, titan cell production promotes dissemination of the typical size cryptococcal cells from the lungs to other tissues (25). Titan cell production results in a 300-fold increase in dissemination of C. neoformans to the central nervous system (CNS). Robust infection of G. mellonella with titan cells isolated from mice confirmed that titan cells also promote disease in non-mammalian hosts in a similar way as typical size cells (22). The G. mellonella infection was associated with the production of typical size progeny, further supporting the hypothesis that titan cells contribute to dissemination through production of typical size daughter cells.
How titan cells, which are sequestered in the lungs, are able to alter dissemination of typical size cells to other tissues remains a mystery. Intracellular cryptococcal cells have increased dissemination to the CNS compared to extracellular cells (30), thus the observation that titan cells decrease phagocytosis yet increase dissemination is paradoxical. Titan cell production enhances the prevalence of eosinophils in the lungs (25). Eosinophils are classically produced during Th2-mediated responses to parasitic infections and during pulmonary allergic responses. Titan cell production might alter host recognition of the infection, inducing an aberrant Th2 immune response that is unable to control the infection. An alternate hypothesis is that the titan cell progeny are better able to survive within the host, that the titan cells confer traits to their daughters that promote virulence. Titan cell progeny have increased resistance to oxidative and nitrosative stresses similar to those utilized by phagocytic cells (15, 16). Thus, the titan progeny may be able to survive within phagocytes, resulting in enhanced intracellular dissemination. Ultimately, the increased survival and dissemination of C. neoformans due to titan cell production promotes overall virulence in the murine model (25).
Conclusions
Currently, abundant titan cell production has only been possible in vivo. While multiple groups have been able to generate enlarged cells in vitro, these cells are often a very small proportion of the population and/or do not have all the characteristics of titan cells (15, 22, 23). This limitation has inhibited our ability to study this unique cell type. In order to perform analytical studies of the capsule, cell wall, metabolite production, and replication/division of titan cells, a robust in vitro system that can generate abundant titan cells that exhibit all the titan cell characteristics is needed.
Yet recent studies have clearly shown that titan cell production is a distinct morphological transition in C. neoformans that promotes virulence of this important human fungal pathogen. Although titan cells are best known for their production during mammalian infections, similar cells have also been described during interactions with invertebrate hosts, such as amoebae and insects. These observations suggest that titan cell formation could be under selective pressure in the environment.
On the surface, titan cell formation may look radically different from the yeast/hyphal switch observed in other human fungal pathogens. Whether this assumption is valid remains to be determined. Similar to titan cell production, hyphal formation in C. albicans is regulated, in part, by both the cAMP pathway and the transcription factor Rim101 (reviewed in (31)). Transition from the filamentous to yeast phase in dimorphic fungal pathogens induces expression of yeast specific traits including alterations in the cell wall (reviewed in (32)). Alterations in the titan cell surface are also observed that may affect host recognition. It seems likely that titan cell production in C. neoformans is performing a similar function during pathogenesis to morphological transitions in other pathogens. Understanding how titan cells form and how they promote pathogenesis will identify why these cells play a key role in cryptococcal virulence, how this role is similar/different from morphological transitions in other pathogens, and how titan cell formation can be targeted in novel treatment strategies to limit cryptococcal infections and subsequent disease. Because titan cells appear to play a role in both latent infection and disseminated disease, treatment strategies targeting titan cells could have a profound impact on disease prevention and treatment in at-risk patient populations.
Highlights.
Cryptococcus has an unusual morphological transition in vivo to generate enlarged titan cells
Titan cells are greater than 15 microns in diameter, have a thick cell wall, and highly cross-linked capsule
Titan cells are produced in both mammalian and invertebrate infections
Titan cells are important for cryptococcal pathogenesis
Acknowledgements
The writing of this review was supported by National Institutes of Health grant AI080275 to K.N. and the Spanish Ministry of Economics and Competitivity grant SAF2011-25140 to O.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kane J. Conversion of Blastomyces dermatitidis to the yeast form at 37°C and 26°C. Journal of Clinical Mmicrobiology. 1984;20:594–596. doi: 10.1128/jcm.20.3.594-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maresca B, Kobayashi GS. Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiological Reviews. 1989;53:186–209. doi: 10.1128/mr.53.2.186-209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Annual Review of Microbiology. 2007;61:529–553. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.O'Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clinical Microbiology Reviews. 2012;25:387–408. doi: 10.1128/CMR.00001-12. [Recent review about the cryptococcal capsule.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annual Review of Microbiology. 2009;63:223–247. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Advances in Applied Microbiology. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. Cryptococcus : from human pathogen to model yeast. ASM Press; Washington, DC: 2011. [Recent book describing the current state of knowledge about the pathogenic Cryptococcus species.] [Google Scholar]
- 8.Magditch DA, Liu TB, Xue C, Idnurm A. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathogens. 2012;8:e1002936. doi: 10.1371/journal.ppat.1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SC, Phadke S, Sun S, Heitman J. Pseudohyphal growth of Cryptococcus neoformans is a reversible dimorphic transition in response to ammonium that requires Amt1 and Amt2 ammonium permeases. Eukaryotic Cell. 2012;11:1391–1398. doi: 10.1128/EC.00242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neilson JB, Fromtling RA, Bulmer GS. Pseudohyphal forms of Cryptococcus neoformans: decreased survival in vivo. Mycopathologia. 1981;73:57–59. doi: 10.1007/BF00443015. [DOI] [PubMed] [Google Scholar]
- 11.Fromtling RA, Blackstock R, Hall NK, Bulmer GS. Immunization of mice with an avirulent pseudohyphal form of Cryptococcus neoformans. Mycopathologia. 1979;68:179–181. doi: 10.1007/BF00578527. [DOI] [PubMed] [Google Scholar]
- 12.Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank JG, Cavill R, Jelbert M. Cryptococcus neoformans of unusual morphology. Applied Microbiology. 1973;25:309–312. doi: 10.1128/am.25.2.309-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaragoza O. Multiple disguises for the same party: the concepts of morphogenesis and phenotypic variations in Cryptococcus neoformans. Frontiers in Microbiology. 2011;2:181. doi: 10.3389/fmicb.2011.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathogens. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [Seminal manuscript that identified and characterized the titan cell phenotype.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathogens. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. [Seminal manuscript that identified and characterized the titan cell phenotype.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harold FM. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiological Reviews. 1990;54:381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veses V, Richards A, Gow NA. Vacuoles and fungal biology. Current Opinion in Microbiology. 2008;11:503–510. doi: 10.1016/j.mib.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Vogl AW, Kronstad JW. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Molecular Microbiology. 2012;85:700–715. doi: 10.1111/j.1365-2958.2012.08134.x. [DOI] [PubMed] [Google Scholar]
- 20.Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M, Saikia S, Wang J. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nature Reviews Microbiology. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Okagaki LH, Wang Y, Ballou ER, O'Meara TR, Bahn YS, Alspaugh JA, Xue C, Nielsen K. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryotic Cell. 2011;10:1306–1316. doi: 10.1128/EC.05179-11. [Describes the signal transduction pathways involved in titan cell production.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Rodas R, Casadevall A, Rodriguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One. 2011;6:e24485. doi: 10.1371/journal.pone.0024485. [Evidence that titan cells are formed not only during interaction with mammals but also with invertibrate hosts.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathogens. 2011;7:e1002047. doi: 10.1371/journal.ppat.1002047. [Describes cell enlargement in response to amoebae and nematodes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathogens. 2010;6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infection & Immunity. 2012;80:3776–3785. doi: 10.1128/IAI.00507-12. [Analysis of the role of titan cells in cryptococcal virulence in a murine model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryotic Cell. 2012;11:820–826. doi: 10.1128/EC.00121-12. [Shows titan cell production blocks phagocytosis of typical size cryptococcal cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker RD. The primary pulmonary lymph node complex of cryptococcosis. American Journal of Clinical Pathology. 1976;65:83–92. doi: 10.1093/ajcp/65.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Dromer F, Ronin O, Dupont B. Isolation of Cryptococcus neoformans var. gattii from an Asian patient in France: evidence for dormant infection in healthy subjects. Journal of Medical and Veterinary Mycology. 1992;30:395–397. [PubMed] [Google Scholar]
- 29.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. Journal of Clinical Microbiology. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infection & Immunity. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudbery PE. Growth of Candida albicans hyphae. Nature Reviews Microbiology. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 32.Klein BS, Tebbets B. Dimorphism and virulence in fungi. Current Opinion in Microbiology. 2007;10:314–319. doi: 10.1016/j.mib.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]