Abstract
Objectives
Older adults with dementia experience progressive functional decline, which contributes to caregiver burden and nursing home placement. The goal of this systematic review was to determine if any non-pharmacologic interventions have delayed functional decline among community-dwelling dementia patients.
Method
We completed a systematic literature review to identify controlled clinical trials reporting the impact of non-pharmacologic interventions on any measure of functional impairment or disability among community-dwelling dementia patients. We included studies that reported any proxy-respondent, self-reported, or performance-based standardized assessments.
Results
We identified 18 published clinical trials that met inclusion criteria and found that study interventions fell into three different groups: occupational therapy, exercise, and multi-faceted (“other”) interventions. The three groups of studies tended to vary systematically regarding the conceptual framework for the disabling process, target of intervention, and type of outcome measure. Approximately half the studies were conducted in the US with mean sample size of 99 (from 27 to 1131) and follow-up periods between three months and two years. Instruments used to measure functional impairment or disability varied widely with 55 instruments across 18 studies. Nine studies reported a statistically significant improvement in functional decline in the intervention group.
Conclusion
The current literature provides clinical trial evidence that non-pharmacologic interventions can delay progression of functional impairment or disability among community-dwelling dementia patients. The clinical significance of this early evidence is uncertain. These early studies provide rationale for larger and longer-term studies to determine if these interventions are sufficiently potent to delay institutionalization.
Keywords: occupational therapy, physical function, aged
Introduction
Approximately 3.8 million Americans suffer from dementia and 60–80% of these cases are believed to be due to Alzheimer’s disease (Plassman, 2007). According to the Alzheimer’s Association, over 15 million Americans currently provide unpaid care for family members with dementia. These informal or family caregivers often become “secondary patients” due to the stress of caregiving (Vitaliano, 2003). An NIH Consensus Conference concluded that there are no treatments demonstrated to alter the pathophysiology of Alzheimer’s disease and related dementias and there are no known effective preventive strategies (http://consensus.nih.gov/2010/alzstatement.htm). Although cholinesterase inhibitors may improve some aspects of cognitive and behavioral function for some patients, the clinical impact of these medications remains both modest and controversial (Raina, 2008). In addition, recent systematic reviews suggest that several classes of medications previously recommended for the treatment of behavioral and psychological symptoms of dementia have either unacceptable adverse effects and/or uncertain efficacy (Banerjee, 2011; Fox, 2012; Schneider, Dagerman, Insel, 2005; Schneider, Tariot, Dagerman, Davis, Hsiao, et al., 2006; Sink, 2005; Weintraub et al., 2010). The limited efficacy of pharmacologic interventions is placing renewed emphasis on the role of informal caregivers and non-pharmacologic strategies to care for a growing population of older adults with dementia. The term “non-pharmacologic” encompasses a broad range of services delivered to the patient, the caregiver, or the patient-caregiver dyad- the term encompasses essentially all interventions that are not captured in a pharmacopeia.
A central premise of both pharmacologic and non-pharmacologic interventions is that, even if the underlying pathophysiology cannot be altered, patients and their formal and informal caregivers can help the patient adapt to functional limitations and thereby delay disability. In the classical depiction of the disabling process, patients develop pathology that leads to impairments which may then lead to functional limitations and disability (Verbrugge, 1994). In this model, impairments are at the level of tissues and organs while functional limitations are at the level of the whole person. Disability implies a social or environmental context in that the functional limitations must limit social roles (e.g. self-care) before they are regarded as a disability (Guralnik, 2003). The central nervous system pathology due to the dementing illness is posited to lead to disability through impairments in the central nervous system that lead to functional limitations, such as loss of mobility. Functional decline in the form of increasing functional limitations may lead to disability. Disability may reach a level that leads to loss of independence and eventually placement in long-term care for persons with advanced dementia. Permanent placement in long-term care is often termed institutionalization in the extant literature. The US Census defines an “institutionalized person” as “persons under formally authorized, supervised care or custody in institutions at the time of enumeration.”
Within the concept of “functional limitations,” researchers may include a variety of subdomains including mobility, balance, muscle strength, motor processing, cognition, nutrition, endurance, and physical performance (Ferruci, 2004). Within the concept of “disability,” researchers may include subdomains such as self-care, activities of daily living, and other recreational, vocational, or social roles (Gill, 2010). A wide variety of instruments is available to measure each of the constructs in this disability framework among older adults (Ferruci, 2004). Published studies vary in their use of disability frameworks and in their definitions of subdomains. Interventions to prevent or delay disability among older adults with dementia may attempt to improve a functional limitation directly (e.g. strength training for lower extremity weakness) or alter the environment through assistive devices (e.g. bath chair) or by improving the skills of the caregiver (e.g. education in wheelchair to bath chair transfers). For simplicity of presentation, we use the term “functional decline” to encompass the movement of patients along this disability pathway.
In a prior clinical trial of collaborative care for older adults with Alzheimer’s disease, we demonstrated that a combination of pharmacologic and non-pharmacologic interventions could improve behavioral and psychological symptoms of dementia and reduce caregiver stress (Callahan et al., 2006). However, the intervention did not delay disability as assessed by the Alzheimer’s Disease Cooperative Study Group Activities of Daily Living scale. Prior studies have shown that functional limitations defined by the loss of mobility among nursing home patients can be slowed by physical exercise (Koroknay, Werner, Cohen-Mansfield, & Braun, 1995; Rolland et al., 2007; Tappen, Roach, Applegate, & Stowell, 2000). Such outcomes are less certain among older adults with dementia and those living in the community. Smits et al. published a systematic review of combined interventions for people living at home with dementia and their caregivers (Smits, 2007). The research team identified 22 programs meeting the study criteria but only four of the studies reported outcomes relevant to functional limitations or disability and only one reported significant improvements in some aspect of functional limitations. Other reviews of this literature suggest that any impact on the patient’s functional limitations is domain-specific (Ayalon, 2006; Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010b; Pinquart & Sorensen, 2006; Thompson, 2007).
The purpose of the current study is to systematically review the literature to identify randomized clinical trial evidence demonstrating the impact of non-pharmacologic interventions on functional decline among community-dwelling older adults with dementia. We sought to identify studies that assessed functional limitations (e.g. muscle strength) or disability (e.g. activities of daily living) or combinations of these constructs. We also sought to delineate between studies measuring actual patient performance from those measuring self-reports from patients or caregivers.
METHODS
Search Terms
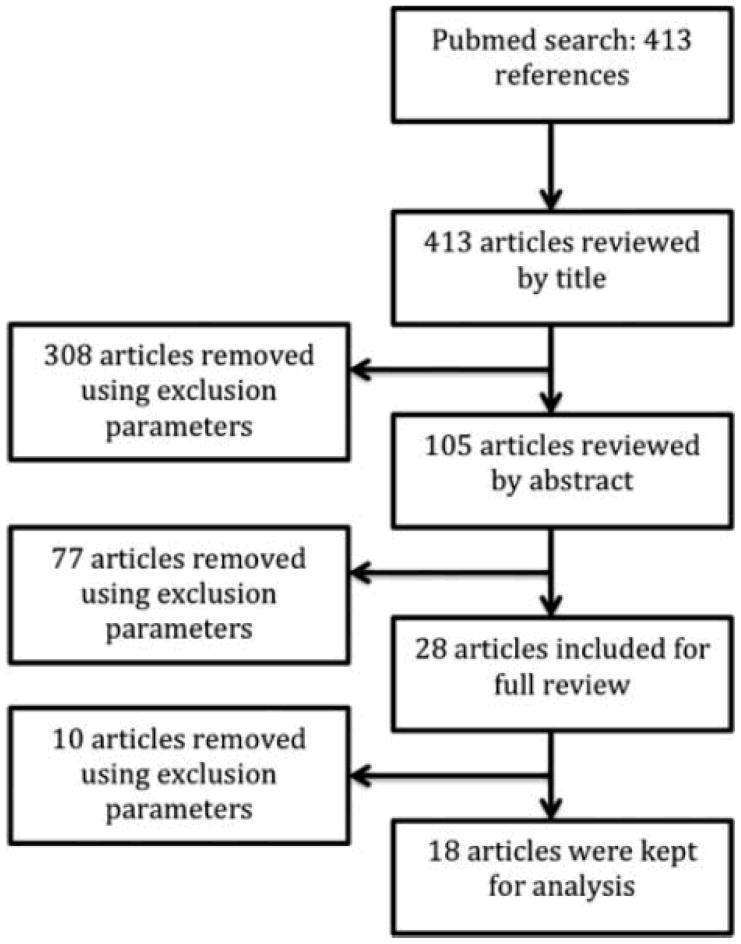
The Pubmed database was used to search for randomized controlled trials in English, eliminating studies with no human subjects. The search terms for population included “dementia” [MeSH] or “cognitive impairment” or “Alzheimer*.” These were combined with the intervention terms “motor activity” [MeSH] or “rehabilitation” [MeSH] or “physical therapy modalities” [MeSH] or “nonpharmacologic*” or “non pharmacologic*.” A research librarian assisted with the development of search terms. We used the PICOT framework for designating the inclusion and exclusion criteria for reviewed studies (Rios, 2010). This framework requires the naming of the Population, Intervention, Comparison groups, Outcomes, and Time frame for articles that would be potentially acceptable for inclusion in the review. Articles were initially reviewed by title and 308 were excluded because they met the PICOT exclusion criteria as shown in Table 1. The remaining 105 articles were then reviewed by abstract. Twenty-eight studies fit the inclusion criteria and underwent full review. Ten articles were eliminated after full review, leaving 18 that fit all the inclusion criteria (Table 1).
Table 1.
PICOT Criteria for study inclusion
| Inclusion: | Exclusion: | |
|---|---|---|
| Population | Community dwelling dementia patients with a primary caregiver | Nursing home patients, elderly but not demented, any other neurological disease |
| Intervention | Non-pharmacologic | Drug related therapy |
| Comparison | Any | |
| Outcome | Any functional performance outcome related to physical function, ADL ability scale, QOL scale, Short Form | Measures only mood, depression, cost effectiveness, informed consent, wandering, or doesn’t measure any patient functional abilities, protocol (no outcomes) |
| Time Frame | Any |
Notes: PICOT, Population, Intervention, Comparison, Outcome, Time frame; ADL, activities of daily living; QOL, quality of life.
Study Selection
Only English-language studies focusing on subjects with dementia were included. Studies that included other neurodegenerative diseases were eliminated, including participants with mild cognitive impairment, vascular cognitive impairment, and Parkinson’s disease. If an identified study was limited to subjects with vascular dementia, stroke, Parkinson’s disease, Huntington’s disease, or other progressive neurologic conditions, we excluded that study because these patients are considered to have distinctly different conditions. If the study included subjects with Alzheimer’s disease and those with mixed dementia, we included the study. Participants had to be community-dwelling with access to a primary (informal) caregiver. Studies focusing on nursing home or hospital settings were excluded. Any non-pharmacological intervention that had at least one primary outcome measuring any domain of functional limitations or disability was included. We included studies using self-reported measures as well as those using performance-based assessments. Any drug-based interventions were eliminated, but applicable studies including patients with stable medication dosages or studies that included multi-faceted interventions were not eliminated. All included trials were randomized controlled trials.
Data Extraction and Quality Assessment
The sample size, location of the study, inclusion and exclusion criteria, intervention, outcome measures, and results were abstracted using a standardized form. Two reviewers graded the included articles using the Cochrane rating approach to determine the strength of evidence and risk of bias. We describe this approach briefly here. Articles are graded “A” if they are randomized, placebo-controlled with allocation-concealed. Articles are graded “B” if they a randomized, placebo-controlled and did not describe allocation concealment. The risk of bias was determined by analyzing the articles across seven criteria: type of controls, blinding methods, outcome reporting, well-defined inclusion criteria, rate of attrition, risk of confounding bias, and intention to treat analysis. These were based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for determining strength of evidence and Agency for Healthcare Research and Quality (AHRQ) methods for grading strength of evidence when comparing medical interventions (Guyatt, 2010; Owens, 2010).
RESULTS
Eighteen studies met all inclusion criteria, and study characteristics are shown in Table 2. After reviewing study characteristics, we found that studies fell into three major groups: (a) seven studies focusing on occupational therapy interventions; (b) six studies focusing on exercise; and (c) five studies of multi-faceted interventions. As shown in Table 2, most studies enrolled fewer than 250 subjects and had follow-up periods of less than twelve months. Half of the eighteen studies were completed outside of the US; half of the studies from within the US were from the Philadelphia REACH program.
Table 2.
Characteristics of Included Studies
| n | Sample | Exclusion/inclusion | Measures | Interventions | Outcomes | |
|---|---|---|---|---|---|---|
| OT | ||||||
| Gitlin 2001 | 171 | Social services/medical centers in Philadelphia | I: primary caregiver, dependent on 2+ ADLs; E: not bed ridden | Occurrence of behavior issues, level of ADL/IADL dependency, caregiver self efficacy and upset | Competence environmental press framework (caregiver education), OT training with ADLs and behavior | Less decline in IADL, trend of less decline in ADL/behavior (non sig) |
| Gitlin 2005 | 127 | Resources for Enhancing Alzheimer Caregiver Health (Philadelphia) | I: caregiver 21+ and lives with patient, provides 4+ hours a day of care, MMSE<24 | Days caregiver receives ADL help for patient, memory related, caregiver upset | 6 OT sessions to modify environment and support daily function | Improved skill, less help needed, fewer behavior occurrences |
| Gitlin 2008 | 60 | Media/mailing by social service agency in Philadelphia | I: English speaking, MMSE<24, can do 2+ ADLs; E: schizoid/bipolar, MMSE=0, bedridden | Agitated behavior in dementia scale, CSDD, QOL-AD, activity engagement self report | 8 OT sessions, with neuropsych testing and function testing (customized) (Tailored Activity Program) | 4 months: less behavioral occurrences, less agitation, higher QOL; increased caregiver self efficacy, decreased burden |
| Gitlin 2003 | 255 | Philadelphia REACH initiative | I: 1 limitation of ADL, caregiving 6+ months, caregiver 4 hrs/day; E: cancer, 3+ hospitalizations, going to NH in <6 months | Occurrence of behavior problems, dependence of ADLs/IADLS | 5 home, 1 phone contact with OT: education, problem solving, adaptive equipment, modify home environment | Less upset with memory, less need for assistance, better affect, no change in help w/ADLs |
| Gitlin 2010 | 237 | Media/mailing targeting caregivers by social service agency in Philadelphia | I: dementia diagnosis, MMSE<24, 21+ caregiver, English speaking, need help with daily activities, live within 5 miles of caregiver, not moving to institution for at least 9 months; E: terminal illnesses, cancer treatment, >3 acute hospitalizations in year, involved in another trial, bipolar/schizoid, dementia from trauma, MMSE=0, bedbound | Functional Independence Measure including 8 IADLS and 7 ADLs), QOL-AD, activity engagement, agitated behavior in dementia, caregiver well being | COPE: assessments, caregiver education/training, training in problem solving, communication, activities, simplifying tasks; 10 OT sessions, 2 nurse lessons, cognitive/functional testing | I group improved in IADLS (moderate effect), COPE improved ADLs but non-significant effect; 4 months: significant improvements in all except activities engagement, 9 months: no significant difference between COPE and control (perceived benefits favored intervention |
| Graff 2006 | 135 | Memory clinic and day clinic at UMCN (Amsterdam) | I: BCRS between 9–40; E: BDS>12, BPSD, severe illness | AMPS, IDDD | 5 home, 1 OT contact on phone; modify home to optimize ADL performance | Improved daily functioning, decreased caregiver burden |
| Nobili 2004 | 39 | Milan, Italy (Federazione Alzheimer Italia) | I: clinical diagnosis, 2+ on problem behavior, live near Milan, have a primary caregiver | RRS, SBI-C, ADL score (Katz) and IADL, help required (hrs/day) | 60 minute psychologist and 90 minute OT visit | Patients had improved cognitive function, fewer behavior occurrences, fewer drugs (sicker patients died or were institutionalized) **nonsig but trend: both groups lost ADLs and required more help after 12 months, but intervention group lost less |
| Exercise | ||||||
| Hauer 2012 | 122 | Bethanien Hospital and University of Heidelberg | I: dementia, no diseases, can walk 10 m | One rep max-leg press, 5 chair stand test (SPPB), GDS | Resistance and functional group training, including ADL performance like walking up stairs and advanced tasks | Training improved primary and secondary outcomes, improved motor performances |
| Kwak 2008 | 30 | Community wide advertising Korea, women providing care | I: postmenopausal women caregivers, 60+ years, MMSE between 10 and 26, living with AD relative, 10+ hours unpaid care/work, not moving in 12 months, mobile, doesn’t exercise regularly | MMSE, ADL, cardiopulmonary function, muscle strength | 12 months of regular exercise program, 30–60 min/day 2–3 times/week | Enhanced MMSE scores, ADL scores in exercise group |
| Netz 2007 | 29 | Day care attendees in Israel | I: perform 2/3 tests, attendance 2 days/week, consent to MMSE | PR, TGUG, STS, FR | Group physical vs. social activity, twice weekly, 12–15 per group | Moderate intensity exercise improved TGUG, including severe dementia patients |
| Schwenk 2010 | 61 | Rehab from geriatric hospital in Bethanien (Heidelberg) | None listed | Motor performance (gait speed, cadence, stride length, stride time, single support) and cognitive performance (serial 2 forward calculation [S2], serial 3 backward calculation [S3] | Dual task based exercise (motor and cognitive performance) vs. control group with unspecific low intensity exercise | IG: reduced DTC =effective; DTC before intervention was 42%, DTC after intervention was half that (and comparable to healthy elderly) [other gait variables: 8.7% to 41.1% IG, 0.9% to 8.1% CG, p<0.001 to 0.056; combined motor/cognitive: 20.6% IG, 2.2% CG, p< 0.026 |
| Steinberg 2009 | 27 | From John Hopkins Comprehensive program in psych department | I: Alzheimer’s diagnosis, MMSE>10, community residing, stable health, ambulatory, caregiver 10+ hours; E: disease | YPAS, JTT, timed 8 foot walk, chair sit to stand, diaries of activities | 1. Aerobic fitness - walking, 2. Strength training, 3. Balance/flexibility | Significant improved performance on hand function test, secondary outcomes: slightly worse depression, decreased QOL |
| Teri 2003 | 153 | University of Washington Alzheimer disease patient registry | I: community dwelling, ambulatory, have a caregiver | SF-36, SIP | 12 hour-long sessions of aerobic/endurance, strength, balance, flexibility; 30 min/day of moderate exercise | RDAD had improved scores in physical function |
| OTHER | ||||||
| Burgener 1998 | 54 | None listed | None listed | MMSE, IADL/SCS, DBD, caregiver stress | Educational program to increase caregiver knowledge/understanding; behavior intervention to calm functional patients and increase patient self care, 1: both, 2: educational, 3: behavioral, 4: control | grps 1+2: = decrease difficult behaviors, groups 3+4: decrease difficult behaviors, group 3: most increase in self care ability; all interventions were rated “effective” |
| Callahan 2006 | 153 | Urban health care setting in Indianapolis | E: nursing home, non- English speaking, no telephone access, no caregiver to give consent | Memory and behavior problems checklist, NPI, ADL, health care resources use, CSDD, PHQ-9 | 12 months of collaborative care management, including advice on communication, caregiver coping, legal/financial, and patient exercise video | Intervention patients had fewer behavioral/psychological symptoms (based on NPI), less caregiver distress, no change in ADL or depression or nursing home placement time |
| Martin Cook 2005 | 49 | From community retirement and assisted living facilities in Dallas | I: community dwelling, mildly to moderately cognitively impaired, and had a consistent caregiver | Texas functional living scale (IADL), independent living scale, ADCS-MCI | 4 caregiver training sessions | No significant difference in MMSE, NPI, FMTCS; “The results of this study suggest that although caregivers report satisfaction with participation, there is no significant lasting benefit of short term-focused caregiver interventions” |
| Nourhashemi 2010 | 1131 | 50 memory clinics in France | I: community living dementia patients with MMSE between 12 and 26 | ADCS, rate of nursing home admission | Standardized twice yearly consult, advice on management of problems | Rate of functional decline at two years didn’t change |
| Olazaran 2004 | 84 | 12 neurologic, geriatric, or psychiatric clinics and 5 behavioral neurology units of the central and eastern metropolitan area of Madrid | I: community dwelling patients, a clinical diagnosis of either mild cognitive impairment or probable AD, a stage 3, 4, or 5 in the Global Deterioration Scale, current use of a daily dose of 5 to 10 mg of donepezil or 6 to 12 mg of rivastigmine for more than 1 month, patient’s and caregiver’s willingness to receive cognitive intervention; E: illiteracy, any physical condition that would prevent attendance/participation in the intervention | ADAS-COG, MMSE, FAQ (IADLS), GDS | 1 year of 103 sessions of cognitive exercises and social and psychomotor activities | Transient cognitive stabilization and long term mood benefit; psychomotor yielded benefits on cognitive and functional tests; physical activity and socialization might give nonspecific benefit to mood and behavior |
Notes: OT, occupational therapy; ADL, activities of daily living; IADL, instrumental activities of daily living; MMSE, mini mental status exam; CSDD, Cornell Scale for Depression in Dementia; QOL-AD, Quality of Life-Alzheimer’s Disease; NH, nursing home; COPE, Care of Persons with Dementia in their Environment; BCRS, brief cognitive rating scale; BDS, Blessed Dementia Scale; BPSD, Behavioral and Psychological Symptoms of Dementia; CES-D, Center for Epidemiologic Studies depression scale; AMPS, Assessment of Motor and Process Skills; IDDD, Interview for Deterioration in Daily Living Activities; RRS, Relative’s Stress Scale; SBI-C, Spontaneous Behavior Interview (9); GDS, Global Deterioration Scale; PR, performance rate; TGUG, Timed up and go test; STS, sit to stand test; FR, functional reach; DTC, dual-task costs; YPAS, Yale Physical Activity Survey; JTT, Jebsen-Taylor total time test; SF-36, Short-form (36) Health Survey; SIP, Sickness Impact Profile, RDAD, Reducing Disability in Alzheimer’s Disease; DBD, Dementia Behavior Disturbance Scale; SCS, social competence scale; SRQ-20, Self-reporting scale (20); NPI-Q, Neuropsychiatric Inventory Questionnaire; PHQ-9, Patient Health Questionnaire (9); WHO-QOL-BREF, World Health Organization Quality of Life questionnaire; ADCS, Alzheimer’s Disease Cooperative Study; ADSC-COG, Alzheimer’s Disease Cooperative Study -Cognitive subscale; FAQ, Functional Activities Questionnaire.
Studies of Occupational Therapies
Seven randomized controlled trials were identified that examined an occupational therapy intervention to maintain physical function in dementia patients. These interventions were evaluated using various self-report scales, including activities of daily living. All interventions were performed for five weeks or fewer, and all reported positive, significant increases in abilities or quality of life, except for one in a study by Gitlin in 2003 (Gitlin et al., 2003).
Quality of Studies of Occupational Therapies
Two high quality RCTs (grade A) were identified. Both were evaluated to have a low risk of bias. One high quality article referred to study, in which an occupational therapist used five home visits and one phone call with the patient and caregiver to optimize the home environment for ADL performance (Graff et al., 2007; Graff et al., 2006). The article reported improved daily functioning based on the Assessment of Motor and Process Skills test (Graff et al., 2006). The other study performed a customized activity program based on functional testing and reported a trend towards increased quality of life (Gitlin et al., 2008).
Five trials were given Grade B, four with a medium risk for bias and one with a low risk. The study with low risk for bias showed fewer declines in IADLs following an intervention with a competence environmental press framework (improving the environment to aid patient’s functional ability and behavior) and OT training for ADLs and behavior (Gitlin, Corcoran, Winter, Boyce, & Hauck, 2001). In a 2010 study by Gitlin, the intervention group showed a moderate effect of IADL improvement using a modification of the Functional Independence Measure (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010a). It used a Care of Persons with Dementia in their Environments (COPE) intervention, which included 10 OT sessions, two nurse lessons, assessments, caregiver training, and other information on caring for dementia patients (Gitlin et al., 2010a). In another study by Gitlin in 2005, the intervention group had improved skill and needed fewer days of ADL help after being trained in six OT sessions designed to support function and modify home environment (Gitlin, Hauck, Dennis, & Winter, 2005). In another study at medium risk of bias, an intervention of one OT visit and one psychologist visit reported that twelve months later, the intervention group’s activities of daily living function had decreased less and the control group’s caregivers required more help from others (Nobili et al., 2004). A study by Gitlin in 2003 reported an insignificant change in ADL help requirements, but showed less caregiver need for assistance and less behavioral upset in patients (Gitlin et al., 2003).
Studies of Exercise Interventions
Six randomized controlled trials were identified that examined an exercise intervention in community-dwelling dementia patients. These interventions included a variety of training methods, such as aerobic exercise (usually walking), resistance training or weightlifting, and balance and flexibility training. Multiple functional outcome measurements were used, such as chair stand tests (STS), timed up and go (TGUG), and timed walking tests. All exercise interventions were for three months, except for the Kwak study, which ran for 1 year (Kwak, Um, Son, & Kim, 2008). All exercise interventions reported positive significant results in at least one primary outcome for functional performance.
Quality of Studies of Exercise Interventions
Of the six RCTS identified, four gave grade A evidence. One study used resistance and functional ability training in a group setting, and showed improved max-leg press ability and improved speed on the 5-chair stand test (Hauer et al., 2012). Another study used an intervention of walking, strength training, balance and flexibility, and reported significant improvement on the Jebsen total time measure, which is a hand function test that correlates to ADL ability (Steinberg, Leoutsakos, Podewils, & Lyketsos, 2009). The third study used a dual task based (DTC) exercise intervention, which had patients perform a mental and a physical task at the same time (Schwenk, Zieschang, Oster, & Hauer, 2010). With specific training, the intervention group improved their DTC performance under complex S3 conditions, showing an increase in gait speed (Schwenk et al., 2010). The fourth study showed that aerobic and endurance training improved scores in physical functioning, based on SF-36 health survey (Teri et al., 2003).
The other two articles were grade B studies with medium risk of bias. One study showed that a year of participation in a regular exercise program enhanced ADL scores (Kwak et al., 2008). The other study showed that group moderate physical activity improved TGUG scores compared to just social activity, even in severely demented patients (Netz, Axelrad, & Argov, 2007). A trend for improved STS and functional reach (FR) was seen as well, although some of the severely demented patients were unable to perform the FR in the last measurement because they could not understand the directions for the test (Netz et al., 2007).
Studies of Other Interventions
Five randomized controlled trials were identified that instituted “other” types of intervention to maintain physical function in dementia patients. These articles included two caregiver-training programs, one program that provided collaborative care, one that provided advice on problem management, and one that provided sessions with cognitive exercise and psychomotor activities. Two studies were rated grade A and three grade B. These interventions yielded mostly non-significant results with regards to physical function; however, one of these articles reported positive results in non-physical outcomes (Callahan et al., 2006; Gavrilova et al., 2009).
Quality of Studies of Other Interventions
Two high quality articles were identified from the other interventions category. A collaborative care management program, which gave caregivers advice on handling problems they might face, resulted in fewer behavioral problems and less caregiver stress, but no change in ADL ability or nursing home placement time (Callahan et al., 2006). The second high quality article, which reported on the use of cognitive exercises and psychomotor activities, indicated both cognitive and functional benefits on ADAS-Cog and Functional Activities Questionnaire, although the functional benefits were not significant (Olazaran et al., 2004).
The three remaining articles were classified as grade B evidence. One article did not report on attrition and had poorly described randomization, outcomes, and inclusion criteria, so the risk of bias is high on its report of increased self-care ability on an IADL scale following a caregiver education and behavior intervention program (Burgener, Bakas, Murray, Dunahee, & Tossey, 1998). One study used a caregiver training intervention and reported no significant difference in IADL ability or independent living (Martin-Cook, Davis, Hynan, & Weiner, 2005). The third grade B study’s intervention provided a comprehensive consultation twice a year, which delivered advice on problem management (Nourhashemi et al., 2010). This study reported no change in the rate of functional decline after two years (Nourhashemi et al., 2010).
Clinical Significance of Interventions
Schulz et al defined clinical significance as “the practical value of the effects of an intervention, or the extent to which an intervention makes a real difference in the everyday life of an individual” (Schulz, 2002). Although many of the reviewed studies did demonstrate statistical significance between groups on self-reported or performance-based measures of physical functioning, the clinical significance of these outcomes is less certain. In the literature reviewed here, there are three concerns about clinical significance. First, none of the reviewed studies was designed to demonstrate whether improvements in physical function translated into delayed institutionalization. Delayed institutionalization is not the only distal outcome of interest, but because of the importance of delaying nursing home placement for patients, families, and payers, this outcome is often seen as a goal of interventions among older adults with dementia. Second, even among the more proximate outcome measures such as activities of daily living, researchers relied on self-reports or proxy-reports of function rather than direct measurement. Because these interventions cannot be double-blind, the potential for bias exists in the self-report measures. In addition, some interventions focus on specific muscle groups or domain-specific tasks believed to be important determinants of disability rather than focusing on actual function at the level of the individual. Third, although some studies report statistical significance, the standardized effect sizes are often small to modest. Table 3 displays the effect sizes that we were able to calculate based on data reported in the original study. The effect sizes vary widely among the reviewed studies but in general effect sizes are higher for those outcomes that would be considered domain-specific and weaker for those studies reporting disability outcomes such as activities of daily living. Costs and burdens of the intervention as well as risks and benefits of the intervention are also considerations in interpreting clinical significance (Cohen, 1988; Kraemer, 2006; Samsa, 1999). We did not calculate a weighted mean effect size across all studies or even among the three subgroups because the sample populations, interventions, and outcomes measures across these studies are heterogeneous.
Table 3.
Effect sizes of non-pharmacologic interventions
| Study | Outcome measurement | Effect size |
|---|---|---|
| OT studies (Disability measures) | ||
| Gitlin 2001 | ADL dependence | −0.222 |
| IADL dependence | −0.437 | |
| Gitlin 2005 | Days receiving caregiver help | -- |
| Gitlin 2008 | Activity engagement | 0.61 |
| Gitlin 2003 | IADL functional | 0.048 |
| Gitlin 2010 | IADL dependence | 0.43 |
| Graff 2006 | AMPS process | 2.5 |
| Nobili 2004 | -- | |
| Exercise studies (Functional Impairment measures) | ||
| Hauer 2012 | 1RM | 0.18 |
| TGUG | 0.07 | |
| Walking speed | 0.15 | |
| Kwak 2008 | Muscle strength | 1.107 |
| Netz 2007 | TGUG | 0.426 |
| Schwenk 2010 | Motor (gait speed) + cognitive performance | 0.982 |
| Steinberg 2009 | YPAS | -- |
| Teri 2003 | SF-36 | 0.591 |
| SIP Mobility | 0.047 | |
| Other studies (Disability measures) | ||
| Burgener 1998 | Total intervention | -- |
| Callahan 2006 | ADL | 0.231 |
| Martin Cook 2005 | ILS | 2.014 |
| Nourhashemi 2010 | ADCS-ADL | 0.055 |
| Olazaran 2004 | FAQ | -- |
Notes: ADL, activities of daily living; IADL, instrumental activities of daily living; D-QOL, Dementia-Quality of Life; AMPS, Assessment of Motor and Process Skills; 1RM, 1RM, 1 rep max test; TGUG, Timed up and go test; YPAS, Yale Physical Activity Survey; SF-36, Short-form (36); SIP, Sickness Impact Profile; DEM-QOL, Dementia Quality of Life scale, ILS, Independent Living Scale; Alzheimer’s Disease Cooperative Study-Activities of Daily Living; FAQ, Functional Activities Questionnaire.
CONCLUSIONS
This systematic review sought to identify clinical trial evidence of any interventions that improved, maintained, or slowed the rate of functional decline among community-dwelling patients with dementia. Studies from both the exercise and occupational therapy literature reported statistically significant differences between study groups and thus, at minimum, the literature supports a “proof of concept” that the functional decline associated with dementia can be delayed. The clinical significance of the reported differences in functional impairment or disability between treatment groups remains uncertain. Dementia is a progressive illness that results in disability and death typically over a period of 5–10 years. Because there are no current preventive measures or treatments to slow the pathologic process of dementia, interventions that might delay disability and loss of independence are of great importance.
Many different sample populations, interventions, and outcome measures were used among the studies identified in this review. For this reason, we adjudged that it was inappropriate to conduct a quantitative summary of results across all studies (Eysenck, 1994). We did identify several patterns among study designs and results. Exercise interventions tended to target training to large muscle groups to improve the ability to walk, transfer, and go up and down stairs. Exercise trials also tended to use performance-based measures of functional limitations, reported statistically significant results, and highlighted mobility as the key to maintain independence (Hauer et al., 2012; Steinberg et al., 2009). Occupational therapy interventions were typically aimed at improving both the home environment and the patient’s ability to perform. Occupational therapy and the “other” category interventions most often used self-report measures of activities of daily living and thus focused on assessing disability. Studies differ in their targeted interventions but also in outcomes measures and the conceptual frameworks by which they sought to maintain independence. In the future, it may be helpful to standardize the outcome measures used to measure functional limitations and disability across interventions, or at least use one or more standardized measures across all studies.
While several studies included in this review reported statistically significant findings, the clinical relevance of these findings is less clear. As reported in Table 3, the occupational therapy studies reported effect sizes ranging from −0.222 to 2.5; exercise interventions reported effect sizes ranging from 0.07 to 1.107; and the “other” category reported effect sizes from 0.055 to 2.014. In a recent review of pharmacological interventions for dementia, effects sizes for medications ranged from −1.25 (favors drug intervention) to 0.02 (favors placebo) (Trinh, 2003). In general, effect sizes of 0.2 are considered to have small clinical significance, while effect sizes of 0.5 have moderate clinical significance (Perera, 2006; Rockwood, 2001). With reference to clinical significance of the studies reviewed here, most studies were not designed to demonstrate that differences in functional decline translated into differences in rates of independent living or nursing facility utilization.
Due to the progressive nature of Alzheimer’s disease, the severity of illness in study participants must also be taken into account. The outcomes among studies enrolling more severely demented patients could be different than those enrolling subjects earlier in the course of their illness. Conversely, positive results found in mild to moderately impaired patients may not translate to severely demented patients (Hauer et al., 2012). Because patients are declining over time, an intervention may have a limited window of opportunity to slow functional impairment or disability. Most of the studies reviewed here are of short duration compared to the course of the illness, but the progression of the illness may also limit the subject’s ability to participate in the intervention. In one study, some participants were unable to perform the functional reach test in a fourth follow-up assessment due to the progression of their cognitive impairment (Netz et al., 2007). These circumstances can make it difficult to obtain results that generalize to the entire dementia population. Extending follow-up length would allow long-term prognoses to be studied to determine if both the interventions and results are maintainable.
Excepting one study with an enrollment of 1,131 (Nourhashemi et al., 2010), the average study size was small at approximately 100 subjects and most studies were single site trials. Most were also located in large cities and to centers with uncertain referral patterns. Larger trials with a recruitment process that targeted more representative populations might also provide more opportunity for subgroup analysis, where the effect of potential confounders like age, gender, and caregiver demographics can be studied. Further research could also include combining an exercise intervention with occupational therapy to target both the underlying functional limitation and the environment. Finally, an important area for future research would be to understand the biological basis of the impact of these interventions including a better understanding of whether the intervention alters the patient’s actual functional limitation or improves their adaptation to the environment.
Overall, both occupational therapies and exercise interventions were shown to slow functional decline, but the successes were measured with different theories about what is needed to maintain independence. While slowing functional decline is of primary importance to preventing institutionalization (ADL deterioration was found to be a better predictive measure than cognitive deterioration for nursing home placement) (Wattmo, Wallin, Londos, & Minthon, 2011), it is still only one of many factors that can complicate the decision for long-term care, including medication dosage, gender of patient and disease severity (Wattmo et al., 2011), and the resources of the home environment. For the trials analyzed in this systematic review, related outcomes demonstrated that interventions not only slow the rate of functional decline, but also have the potential to improve quality of life and decrease caregiver burden (Callahan et al., 2006; Gitlin et al., 2008; Gitlin et al., 2003; Graff et al., 2007). Although no direct link between delaying functional limitations or disability and delaying institutionalization was found in these studies, the success of interventions like exercise and occupational therapy in Alzheimer’s patients to delay functional decline merits further study.
Figure 1.
Flow chart of included articles.
References
- Ayalon L, Gum AM, Feliciano L, Arean PA. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182–2188. doi: 10.1001/archinte.166.20.2182. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378:403–411. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- Burgener SC, Bakas T, Murray C, Dunahee J, Tossey S. Effective caregiving approaches for patients with Alzheimer’s disease. Geriatric Nursing. 1998;19(3):121–126. doi: 10.1016/s0197-4572(98)90055-6. [DOI] [PubMed] [Google Scholar]
- Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, Hendrie HC. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, New Jersey: Lawrence Eribaum Associates; 1988. [Google Scholar]
- Eysenck H. Systematic Reviews: Meta-analysis and its problems. British Medical Journal. 1994;309:789. doi: 10.1136/bmj.309.6957.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruci L, MD, PhD, Guralnik Jack M, MD, PhD, Studenski Stephanie, MD, MPH, Fried Linda P, MD, MPH, Cutler Gordon B, Jr, MD, Walston Jeremy D., MD Designing Randomized, Controlled Trials Aimed at Preventing or Delaying Functional Decline and Disability in Frail, Older Persons: A Consensus Report. Journal of American Geriatrics Society. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- Fox CCM, Maidment I, Auestad BH, Coulton S, et al. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS One. 2012;7:e35185. doi: 10.1371/journal.pone.0035185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care--the 10/66 dementia research group’s randomized control trial of a caregiver intervention in Russia. Int J Geriatr Psychiatry. 2009;24(4):347–354. doi: 10.1002/gps.2126. [DOI] [PubMed] [Google Scholar]
- Gill TM. Assessment of Function and Disability in Longitudinal Studies. J Am Geriat Soc. 2010;58(2):308–312. doi: 10.1111/j.1532-5415.2010.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck WW. A randomized, controlled trial of a home environmental intervention: effect on efficacy and upset in caregivers and on daily function of persons with dementia. Gerontologist. 2001;41(1):4–14. doi: 10.1093/geront/41.1.4. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Hauck WW, Dennis MP, Winter L. Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2005;60(3):368–374. doi: 10.1093/gerona/60.3.368. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. American Journal of Geriatric Psychiatry. 2008;16(3):229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. Gerontologist. 2003;43(4):532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010a;304(9):983–991. doi: 10.1001/jama.2010.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society. 2010b;58(8):1465–1474. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Olderikkert MG. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2007;62(9):1002–1009. doi: 10.1093/gerona/62.9.1002. [DOI] [PubMed] [Google Scholar]
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ. 2006;333(7580):1196. doi: 10.1136/bmj.39001.688843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferruci Luigi. Assessing the building blocks of function: Utilizing measures of functional limitation. American Journal of Preventive Medicine. 2003;25(3):112–121. doi: 10.1016/s0749-3797(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman Andrew D, Vist Gunn, Kunz Regina, Brozek Jan, Alonso-Coello Pablo, Montori Victor, Akl Elie A, Djulbegovic Ben, Falck-Ytter Yngve, Norris Susan L, Williams John W, Jr, Atkins David, JoergMeerpohl Joerg, Schunemann Holger J. GRADE guidelines: 4. Rating the quality of evidenced study limitations (risk of bias) Journal of Clinical Epidemiology. 2010;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomized controlled trial. [Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Journal of the American Geriatrics Society. 2012;60(1):8–15. doi: 10.1111/j.1532-5415.2011.03778.x. doi: http://dx.doi.org/10.1111/j.1532-5415.2011.03778.x http://consensus.nih.gov/2010/alzstatement.htm. [DOI] [PubMed] [Google Scholar]
- Koroknay VJ, Werner P, Cohen-Mansfield J, Braun JV. Maintaining ambulation in the frail nursing home resident: a nursing administered walking program. Journal of Gerontological Nursing. 1995;21(11):18–24. doi: 10.3928/0098-9134-19951101-05. [DOI] [PubMed] [Google Scholar]
- Kraemer H, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med. 2008;29(6):471–474. doi: 10.1055/s-2007-964853. [DOI] [PubMed] [Google Scholar]
- Martin-Cook K, Davis BA, Hynan LS, Weiner MF. A randomized, controlled study of an Alzheimer’s caregiver skills training program. American Journal of Alzheimer’s Disease & Other Dementias. 2005;20(4):204–210. doi: 10.1177/153331750502000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz Y, Axelrad S, Argov E. Group physical activity for demented older adults feasibility and effectiveness. Clinical Rehabilitation. 2007;21(11):977–986. doi: 10.1177/0269215507078318. [DOI] [PubMed] [Google Scholar]
- Nobili A, Riva E, Tettamanti M, Lucca U, Liscio M, Petrucci B, Porro GS. The effect of a structured intervention on caregivers of patients with dementia and problem behaviors: a randomized controlled pilot study. Alzheimer Disease & Associated Disorders. 2004;18(2):75–82. doi: 10.1097/01.wad.0000126618.98867.fc. [DOI] [PubMed] [Google Scholar]
- Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Giraudeau B, Cantet C, Coley N, Vellas B. Effectiveness of a specific care plan in patients with Alzheimer’s disease: cluster randomised trial (PLASA study) BMJ. 2010;340:c2466. doi: 10.1136/bmj.c2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazaran J, Muniz R, Reisberg B, Pena-Casanova J, del Ser T, Cruz-Jentoft AJ, Sevilla C. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. 2004;63(12):2348–2353. doi: 10.1212/01.wnl.0000147478.03911.28. [DOI] [PubMed] [Google Scholar]
- Owens D, Lohr K, Atkins D, et al. AHRQ Series Paper 5: Grading the strength of a body of evidence when comparing medical interventions: AHRQ and the Effective Health Care Program. Journal of Clinical Epidemiology. 2010;63:513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful Change and Responsiveness in Common Physical Performnace Measures in Older Adults. Journal of the American Geriatrics Society. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? [Meta-Analysis Research Support, N.I.H., Extramural] International Psychogeriatrics. 2006;18(4):577–595. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]
- Plassman BL. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- Rios L, Ye C, Thabane L. Association between framing of the research question using the PICOT format and reporting quality of randomized controlled trials. BMC Med Res Methodol. 2010;10:11. doi: 10.1186/1471-2288-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, MacKnight C. Assessing the clinical importance of statistically significant improvement in anti-dementia drug trials. Neuroepidemiology. 2001;20:51–56. doi: 10.1159/000054761. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, Vellas B. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55(2):158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Samsa G, Edelman D, Rothman ML, Williams R, Lipscomb J, Matchar D. Determining clinically important differences in health status measures. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- Schneider L, Dagerman Insel. Risk of death with atypical antipsychotic drug treatment fordementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- Schneider L, Tariot Dagerman, Davis Hsiao, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien A, Czaja S. Dementia caregiver intervention research: in search of clinical significance. Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improvedin patients with dementia: a randomized controlled trial. Neurology. 2010;74(24):1961–1968. doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- Sink K, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- Smits CHM, et al. Effects of combined intervention programmes for people with dementia living at home and their caregivers: a systematic review. INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY. 2007;22:1181–1193. doi: 10.1002/gps.1805. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Leoutsakos JMS, Podewils LJ, Lyketsos CG. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the Maximizing Independence in Dementia (MIND) study. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Int J Geriatr Psychiatry. 2009;24(7):680–685. doi: 10.1002/gps.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappen RM, Roach KE, Applegate EB, Stowell P. Effect of a combined walking and conversation intervention on functional mobility of nursing home residents with Alzheimer disease. Alzheimer Disease & Associated Disorders. 2000;14(4):196–201. doi: 10.1097/00002093-200010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Larson EB. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Thompson C, et al. BMC Geriatrics. 2007 doi: 10.1186/1471-2318-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh N, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. Journal of the American Medical Association. 2003;289:210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- Verbrugge L, Jette AM. The disablement process. Social science & medicine. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Vitaliano P, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Journal of Gerontology: Psychological Sciences. 2003;57B(5):453–460. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Wattmo C, Wallin AK, Londos E, Minthon L. Risk factors for nursing home placement in Alzheimer’s disease: a longitudinal study of cognition, ADL, service utilization, and cholinesterase inhibitor treatment. Gerontologist. 2011;51(1):17–27. doi: 10.1093/geront/gnq050. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Lyketsos CG. Sertraline for the treatment of depression in Alzheimer disease: week-24 outcomes. American Journal of Geriatric Psychiatry. 2010;18(4):332–340. doi: 10.1097/JGP.0b013e3181cc0333. [DOI] [PMC free article] [PubMed] [Google Scholar]