Abstract
Obesity disproportionately affects women, especially those of African descent, and is associated with increases in both fat and muscle masses. Although increased extremity muscle mass may be compensatory to fat mass load, we propose that elevated insulin levels resulting from diminished insulin sensitivity may additionally contribute to extremity muscle mass in overweight or obese women. The following measurements were performed in 197 non-diabetic women (57% black, 35% white; age 46±11 years [mean±SD], BMI range 25.0 to 57.7 kg/m2): dual-energy X-ray absorptiometry for fat and extremity muscle masses; exercise performance by duration and peak oxygen consumption (VO2 peak) during graded treadmill exercise; fasting insulin and in 183 subjects insulin sensitivity index (SI) calculated from the minimal model. SI (range 0.5 to 14.1 liter/mU−1•min−1) was negatively, and fasting insulin (range 1.9 to 35.6 μU/mL) positively, associated with extremity muscle mass (both P<0.001), independent of age and height. Sixty-seven percent of women completed 6 months of participation in a weight loss and exercise program: We found a significant association between reduction in fasting insulin and a decrease in extremity muscle mass (P=0.038), independent of reduction in fat mass or improvement in exercise performance by VO2 peak and exercise duration, and without association with change in SI or interaction by race. Thus, hyperinsulinemia in overweight or obese women is associated with increased extremity muscle mass, which is partially reversible with reduction in fasting insulin concentration, consistent with stimulatory effects of insulin on skeletal muscle.
INTRODUCTION
Technological advances in industrial societies have led to sedentary lifestyles at home and at work. Inactivity and the excess intake of calorie-rich foods are the main culprits in the twin epidemics of obesity and type 2 diabetes-associated cardiovascular disease, disproportionately affecting women, especially those of African descent.1–4 Skeletal muscle, the major site of glucose disposal, increases with body weight with steeper slopes of the regression lines in black women compared with white women and is associated with decreased insulin sensitivity.5
On the other hand, chronic elevation in insulin levels as a result of deceased insulin sensitivity may have direct effects on skeletal muscle mass by stimulating contractile protein synthesis, as has been shown in animal models6,7, possibly due to structural homology with insulin-like growth factor.8 Increased muscle mass--especially in the lower extremities--might be adaptive for accommodating an increased body mass during ambulation. Increased muscle mass however, may be maladaptive if muscle function is compromised by lipid-induced interference with mitochondrial oxidative metabolism, with greater imbalance between myofibrillar contractile units and energy supply for activation.9 Our hypothesis was that hyperinsulinemia has stimulatory effects on skeletal muscle mass. We addressed this hypothesis by: 1) analyzing cross-sectional insulin and muscle mass data from overweight women entering a worksite wellness program, and 2) determining changes in insulin and body composition following 6 months of participation in the diet and exercise program. As black women are reported to have greater skeletal muscle mass than white women in relation to body size5,10, as a secondary analysis we examined racial differences in associations between extremity muscle mass and insulin.
METHODS AND PROCEDURES
Overweight (body mass index [BMI] 25 to 30 kg/m2) and obese (BMI ≥ 30 kg/m2) non-diabetic (fasting glucose < 126 mg/dL) female employees of the National Institutes of Health, who self-identified as healthy and not active participants in exercise or weight-reduction programs, were enrolled. No evidence of anemia, liver, kidney or thyroid disease was detected by screening blood work. Each participant was provided internet-based nutrition and exercise information created for NHLBI employees (http://apps.nhlbi.nih.gov/keepthebeat/default.aspx) that included recommendations from the Department of Health and Human Services and the US Department of Agriculture11,12 and advised by the research team to reduce their daily caloric intake by 500 kcal. Participants were provided access to exercise equipment (treadmill, elliptical trainer, recumbent bicycle) near their work areas, informed of walking paths within the Clinical Center and around the Bethesda campus, and instructed by the research team to increase daily activity by 5,000 steps above baseline readings on pedometers issued at study entry. Aerobic exercise rather than strength training was emphasized in this program. Measurements described below were repeated in those subjects who completed 6 months of participation in the weight loss and exercise program. The protocol was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NCT00666172). All subjects provided informed, written consent. All testing was performed after an overnight fast, and for the 116 premenopausal women, within the first 10 days of their menstrual cycle.
Total fat mass and combined upper and lower extremity muscle masses as a measure of muscle mass were determined by dual-energy X-ray absorptiometry (DXA; iDXA Software Encore 11.10, GE Lunar Medical Systems, Madison, WI). If an extremity could not be included in the field of view because of the degree of obesity, the mass determination in the imaged extremity was doubled, as validated by Rothney et al.13 who compared DXA half body analysis with whole body analyses for lean mass in healthy obese subjects. Exercise performance was measured using graded treadmill exercise (Bruce Protocol) by duration of exercise and by peak oxygen consumption (VO2 peak) using a SensorMedics Vmax Spectra 229c metabolic cart (CareFusion, San Diego, CA) for the analysis of oxygen consumption averaged over the final 20 seconds of exercise.
Insulin levels were measured in plasma following overnight fasting in all 197 subjects by an electrochemiluminescence immunoassay (Roche Cobas e601 analyzer, Indianapolis, IN). Values below the lower limit of detectability for the assay (2 μU/mL) were assigned a value of 1.9 μU/mL. Insulin sensitivity testing was performed in 183 subjects, with the insulin sensitivity index (SI) derived from the reduced sample insulin-modified frequently sampled intravenous glucose tolerance test from the minimal model (MinMOD Millenium v6.02, Los Angeles, CA).14,15 Testing was performed at 8 AM following a 12 hour fast after placement of intravenous lines in both antecubital veins. At baseline, dextrose (0.3 g/kg) was injected over 1 minute. Insulin (0.03 units/kg) was injected as a bolus at 20 minutes. Blood samples were taken for determination of glucose and insulin concentrations at 0, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, 180 minutes. Insulin sensitivity testing was not performed in the remaining 14 subjects because of inability to place intravenous catheters in both antecubital veins or maintain patency of catheters over the three hour study. Insulin-like growth factor 1 (IGF-1) was measured in serum from 83 subjects by enzyme-labeled chemiluminescent immunometric assay with a coefficient of variance <6% (Immulite 2500, Siemens Healthcare Diagnostics, Tarrytown, NY).
Data analysis
Data are reported as mean ± standard deviation. Univariate associations among body composition measurements, insulin sensitivity index, fasting insulin and IGF-I were performed using either Pearson’s correlation or Spearman’s rank correlation, as appropriate. Skewed data were log-transformed. Step-wise multiple regression models were used to assess the independent effect of covariates on lower and upper extremity muscle mass. As prespecified in the protocol design, race was introduced into the model to test for interaction. If significant, associations were tested separately for the two largest racial groups—blacks and whites—using multivariable regression analysis. Categorical data were analyzed by Pearson’s χ2 (goodness-of-fit) test. To consider the confounding effects of hormonal status and hormonal treatments on insulin and muscle mass, premenopausal subjects taking no contraceptive hormonal therapy (n=103) were analyzed separately. All analyses were performed using the SAS statistical analysis package, utilizing the STEPWISE, SQUARE, GLM, and MEANS procedures (SAS User’s Guide: Statistics, Version 9 Edition: SAS Institute Inc, Cary, NC). A P-value ≤ 0.05 was considered significant.
RESULTS
One hundred, ninety-seven non-diabetic women were consented to the protocol, age 46±11 years with BMI ranging from 25.0 to 57.7 kg/m2 (67% with BMI ≥ 30 kg/m2), representing a consecutive series of subjects who underwent baseline testing. Racial and ethnic demographics by self report were as follows: black 112 (57%), white 69 (35%), Asian 4 (2%), Hispanic (black or white) 11 (5%), other (1%). Menopausal status of the participants were: 116 premenopausal (13 on hormonal contraception therapy [11%]), 81 peri or postmenopausal (8 on hormonal therapy [10%]). Fasting insulin levels averaged 7.5±6.2 μU/mL (range 1.9 to 35.6 μU/mL). SI averaged 3.8±2.4 liter/mU−1•min−1 (range 0.5 to 14.1 liter/mU−1•min−1) for all subjects who underwent testing and, as expected, was inversely associated with fasting insulin (r= −0.617, P<0.001). IGF-1 averaged 166.2±76.7 ng/mL (range 56 to 475 ng/mL) for all subjects who underwent testing, and was inversely associated with age (r= −0.554, P<0.001), trended towards an inverse association with fat mass (r= −0.182, P=0.100), but was unrelated to fasting insulin (r= −0.080 P=0.471) or SI (r= 0.0468, P=0.684). Exercise duration during treadmill exercise averaged 405±102 seconds (range 135 to 745 seconds) and VO2 peak averaged 23.4±5.3 mL O2/kg/min (range 10.9 to 44.3 mL O2/kg/min). Fat mass averaged 40.2±12.2 kg (range 20.9 to 89.8 kg) and, as expected, was associated with both lower SI (r= −0.547, P<0.001) and higher fasting insulin (r= 0.457, P<0.001).
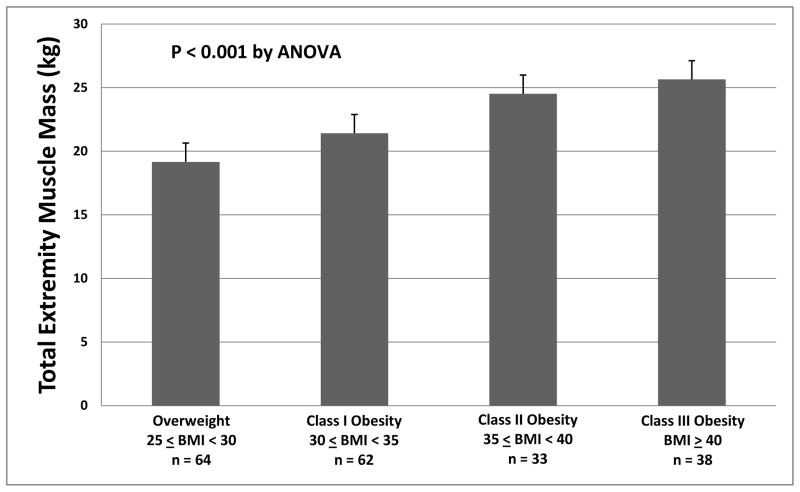
Total extremity muscle mass averaged 22.0±3.8 kg (range 13.8 to 31.9 kg), and was significantly greater in black women compared to white women (23.5±3.5 versus 20.0±3.2 kg, P<0.001), independent of age and height to allow comparison among subjects of different size (P<0.001). For the cohort, total extremity muscle mass increased with greater severity of obesity (Figure 1). This relationship was present both for load-bearing lower extremities and for non-load-bearing upper extremity muscle masses analyzed separately (both P< 0.001). Blacks and whites showed similar strength of associations between weight and extremity muscle mass (both r> 0.790, p<0.001). Despite increased total extremity muscle mass with greater obesity, both exercise duration (r= −0.360, P<0.001) and VO2 peak (r= −0.382, P<0.001) were inversely associated with total extremity muscle mass.
Figure 1.
Total extremity muscle mass is increased with greater severity of obesity.
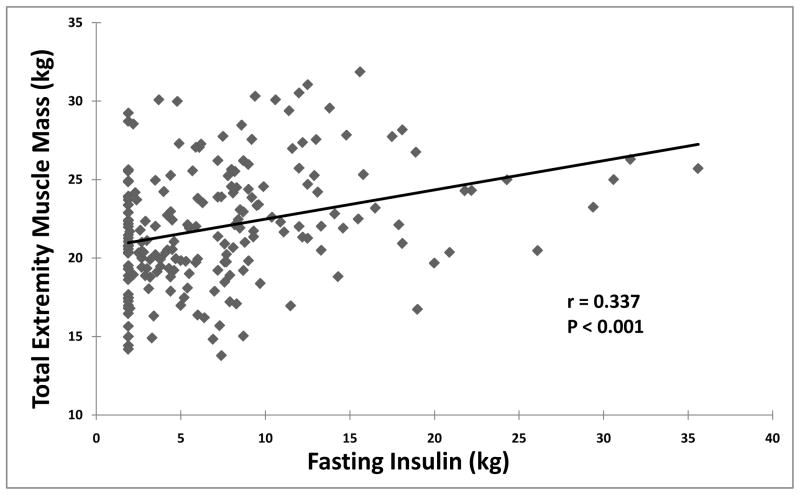
Total extremity muscle masses correlated positively with fasting insulin (Figure 2) independent of age and height. Significant correlations were also noted for lower (r= 0.328, P< 0.001) and upper (r=0.310, P< 0.001) extremity muscle masses analyzed separately. When fat mass was introduced into the multivariable regression model, the independence of insulin as a predictor of total extremity muscle mass was maintained (P= 0.032). For those with IGF-1 measurements, there were no significant associations between IGF-1 and total extremity muscle mass (r= 0.016, P=0.883).
Figure 2.
Correlation between total extremity muscle mass and insulin levels determined in plasma following overnight fasting.
To evaluate the relationship between insulin and muscle mass prospectively, data were analyzed from 132 subjects in this cohort who completed the 6 month weight loss and exercise program. The group that completed the program did not differ from those who did not complete the program (n=65) in racial distribution (P=0.547). However, the group that did not complete the program tended to be younger (44±10 vs. 47±11 years, P=0.079) and were significantly more obese when entering the study (BMI 35.4±6.7 vs. 33.2±6.0 kg/m2, P=0.016). Additionally, those who did not complete the program trended to have higher fasting insulin values (8.9±7.3 vs. 6.8±5.5 μU/mL, P=0.069).
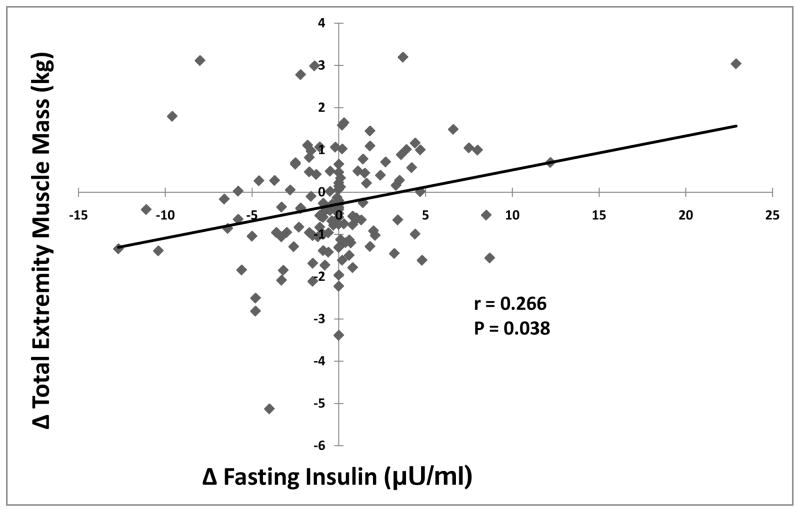
Significant reductions in weight (−2.5±4.3 kg, P<0.001) and fat mass (−1.9±3.5 kg, P<0.001), and improvement in exercise duration (+46±65 seconds, P<0.001) and VO2 peak (+1.5±3.2 mL O2/kg/min, P<0.001) were determined for subjects completing the program, although the range of measurements indicated increases in weight and fat mass in some subjects (Table 1). Reduction in fasting insulin from baseline to completion of the program was significantly associated with reductions in total extremity muscle mass (Figure 3), largely due to correlation for change in lower extremity mass analyzed separately (r =0.194, P=0.026). Correlation between change in insulin and change in upper extremity mass was not significant (P=0.452). To consider the confounding effects of hormonal status and hormonal treatments on insulin and muscle mass, we analyzed primary outcomes data separately on 103 premenopausal subjects who were not on hormonal therapy. At baseline, higher level of fasting insulin was associated with greater total extremity muscle mass (r= 0.304, p=0.002). Similarly, reduction in fasting insulin for those who completed the 6 month weight loss and exercise program was significantly associated with a reduction in total extremity muscle mass (r= 0.297, p=0.021). Thus, baseline and outcomes with respect to insulin and muscle mass for premenopausal women not on hormonal treatment were similar to findings in the entire cohort.
Table 1.
Changes in study parameters for 132 women who completed the 6 month diet and exercise program. Data are shown as mean±SD. Insulin sensitivity (liter/mU−1•min−1) was determined by the minimal model.
| Baseline | 6 Month Follow-Up | Range of Changes | P-value | |
|---|---|---|---|---|
| Weight (kg) | 88.7 ± 17.9 | 86.2 ± 18.2 | −19.3 – +6.6 | < 0.001 |
| Fat Mass (kg) | 38.7 ± 11.7 | 36.8 ± 11.8 | −15.7 – +4.8 | < 0.001 |
| Total Extremity Muscle Mass (kg) | 21.6 ± 3.8 | 21.3 ± 4.1 | −5.1 – +3.2 | 0.002 |
| Exercise Duration (sec) | 411 ± 104 | 457 ± 116 | +199 – −81 | < 0.001 |
| Peak VO2 (ml/kg/min) | 23.9 ± 5.3 | 25.5 ± 5.8 | +10.0 – −4.3 | < 0.001 |
| Insulin Sensitivity Index | 3.9 ± 2.4 | 3.8 ± 2.1 | −5.8 – +5.3 | 0.987 |
| Fasting Insulin (μU/ml) | 6.8 ± 3.8 | 6.7 ± 6.3 | −12.7 – +22.9 | 0.231 |
Figure 3.
Correlation between change in total extremity muscle mass and change in insulin levels determined in plasma following overnight fasting.
Multivariable regression analysis with total extremity muscle mass as the dependent variable showed that change in insulin was a predictor of change in total extremity muscle mass (β= 0.053, P=0.038), independent of changes in fat mass, exercise duration or VO2 peak(Table 2). There was no interaction by race in this analysis. Reduction in extremity muscle masses was not associated with diminished exercise performance by exercise duration (r= −0.176, p=0.045) or by VO2 peak (r=−0.138, p=0.117).
Table 2.
Multivariable regression analysis with total extremity muscle mass as the dependent variable.
| Change in Total Extremity Muscle Mass as Dependent Variable: | ||
|---|---|---|
| Covariate: | β-value | P-value |
| Δ Fat Mass | +0.104 | 0.001 |
| Δ Fasting Insulin | +0.053 | 0.038 |
| Δ Exercise Duration | −0.002 | 0.171 |
| Δ VO2 Peak | +0.006 | 0.876 |
DISCUSSION
The principal finding of our study is that total extremity muscle mass was significantly associated with insulin levels in overweight or obese women, independent of age, body size or fat mass. This relation was present for load-bearing lower extremities as well as non-load-bearing upper extremities when analyzed separately. Correlations were similar for black women compared with white women, with too few subjects in other racial or ethnic groups for comparison. In order to determine an independent contribution of insulin to muscle mass prospectively, subjects participated in a 6-month weight loss and exercise program at our institution, with the expected reduction in weight and fat mass and improvement in exercise performance achieved by the majority of participants who completed the program. Reduction in fat and muscle masses achieved by our participants were not associated with improvement in insulin sensitivity by the minimal model, consistent with findings in women participating in the Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) trial despite reduction in thigh intermuscular adipose tissue.16 We found that reduction in insulin concentrations was significantly associated with reduction in total (primarily lower) extremity muscle mass, independent of fat mass loss and improvement in exercise performance and VO2 peak, and without interaction by race. Thus, our data support the contribution of chronic hyperinsulinemia to increased extremity muscle mass in overweight or obese non-diabetic women that is partially reversible with reduction in insulin concentrations associated with weight loss and improved exercise performance.
Increases in extremity muscle mass and muscle have been reported in diabetics compared with controls, especially notable in older individuals, who in the non-diabetic state commonly lose muscle mass with aging (sarcopenia).17,18 Thus, in the Health, Aging, and Body Composition (Health ABC) study17 485 type 2 diabetics (including 212 women) aged 70–79 years underwent DXA determination of extremity muscle mass, which was found to be 5–10% greater for both upper and lower extremities (for both men and women) compared with 2,132 non-diabetic controls of the same age range. Albu et al5 reported that skeletal muscle mass measured by magnetic resonance imaging increased with body weight, with steeper slopes of the regression lines in black women compared with white women.
Increased skeletal muscle mass associated with obesity has been considered to be a result of generalized increase in body mass (including fat and bone).17 In addition, increased lower extremity skeletal muscle mass may compensate for increased fat mass in order to accommodate standing and ambulation. We considered possible effects of insulin on muscle as an additional contributor to muscle mass in our subjects, a hypothesis supported by animal studies. 6,7 In humans, an increase in myosin heavy-chain mRNA was noted after 3 hours of insulin infusion.19 In addition to stimulatory effects of insulin on glucose transport via IRS-1/PI3-K/Akt signaling, insulin also activates protein synthesis in skeletal muscle through signaling downstream of the mammalian target of rapamycin (mTOR) associated with IGF-1 receptor activation, especially following muscle loading in animal models. Framingham investigators recently reported inverse correlations of IGF-1 concentrations with age, BMI and insulin resistance by the homeostatic model assessment in men and women.20 We also found an inverse association of IGF-1 levels with age, with a trend to an inverse association with fat mass in our subjects who underwent this testing, but not with insulin sensitivity assessed by the minimal model. Thus, we find no evidence to suggest resistance to IGF-1 signaling analogous to resistance to insulin signaling in obesity with a compensatory rise in insulin concentrations, and no reason to suspect that IGF-1 is responsible for increases in extremity muscle mass measured in our subjects. Our data are consistent with the possibility that with increasing obesity-associated insulin resistance, higher chronic insulin levels may stimulate muscle hypertrophy through increased protein synthesis. Similar effects of insulin on cardiac mass have been shown experimentally.21 Animal data suggest, however, that with increasing severity of obesity, resistance to insulin may ultimately develop in the mTOR pathway, which may compromise further skeletal muscle hypertrophy in response to fat mass load, and even lead to muscle loss.22,23 Our data further suggest that increased extremity muscle mass in overweight or obese non-diabetic women is partially reversible with reduction in insulin concentrations.
The increase in muscle mass associated with obesity may not translate into improved exercise performance, in contrast to increases in muscle mass associated with strength and endurance training. Investigators in the Health ABC study reported muscle strength per unit cross sectional area in upper and lower extremities was lower in older men and women with diabetes than those without diabetes despite greater extremity muscle masses.24 Increased inter-and intramuscular fat--reported to be greater in women (especially of African descent) than men 4,16,24,25--may not only contribute to insulin resistance but also compromise muscle bioenergetics. Petersen et al9 reported that lipid content in the soleus muscle (measured by 1H magnetic resonance spectroscopy) was 80% higher and rates of mitochondrial phosphorylation (measured by 31P magnetic resonance spectroscopy) were 30% lower in insulin-resistant offspring of type 2 diabetic patients compared with insulin-sensitive control subjects. An additional effect of insulin that may contribute to poor exercise performance despite increased muscle mass is the experimental evidence in the rat model that insulin changes fiber composition in skeletal muscle to more fast-twitch, type IIb fibers26, which in humans is associated with low capillary and mitochondrial density and low oxidative capacity. Muscle biopsy studies in insulin-resistant first-degree relatives of patients with adult-onset diabetes and in obese women suggest the same phenomenon.27,28 A predominance of type IIb fibers may be deleterious to endurance-type activity necessary to improve exercise fitness with sufficient energy expenditure to achieve weight loss. In our study, there was a significant improvement in exercise performance for the total cohort. Whether reduced insulin in obese subjects following weight loss has favorable effects on muscle fiber type conducive to endurance exercise is unknown and deserves further study.
A limitation of this study is the cross-sectional design for some analyses that limits conclusions regarding cause and effect. In particular, the time-course of onset of increases in extremity muscle mass relative to increases in fat mass, development of insulin resistance and elevation in insulin levels cannot be determined from our study. Nonetheless, loss of muscle mass (including lower extremity muscle mass) with weight loss reported by others supports the compensatory nature of increased muscle mass.29–31 Our data suggest that reduction in insulin might also lead to a reduced muscle mass, independent of fat loss, but without compromise to exercise performance. Another potential limitation is that muscle mass determination in the extremities by DXA scanning might not accurately measure skeletal muscle. Muscle mass measured in the thigh by DXA strongly correlated with skeletal muscle measured in the thigh by multi-slice CT (r2=0.96) in a study by Levine et al.32, but with a systematic overestimate of thigh muscle mass averaging 12%, partly explained by inclusion of skin mass as muscle mass in DXA measurements but not in CT muscle measurements. We are not aware of correlations between DXA measures of extremity muscle mass and either CT or MRI measures of skeletal muscle in extreme obesity (BMI≥ 40 kg/m2), however, which comprised approximately 15% of our cohort.
In conclusion, fasting hyperinsulinemia in overweight women is associated with increased total extremity muscle mass, which is partially reversible with reduction in insulin independent of fat mass loss or improvement in exercise performance and peak VO2 or with significant increase in insulin sensitivity, and consistent with stimulatory effects of insulin on skeletal muscle mass.
Acknowledgments
We gratefully acknowledge the contributions of Janet Dejesus, MS, RD, Rachel Permuth-Levine, PhD, MSPH, Nancy Sebring, MEd, RD, Amber Courville, PhD, RD, Rita Lapointe, MBA, Diane Dellavalle, RD, Greg McMahon, Catherine Marinac, Rachel Perron, Chris Idelson and Megan Sabo to this study.
Footnotes
DISCLOSURE
This research was supported by the intramural research programs of the National Heart, Lung, and Blood Institute and the National Institute of Diabetes, and Digestive and Kidney Diseases, National Institutes of Health. No author has any conflict of interest related to this study.
References
- 1.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MK, Chiuve SE, Rimm EB, et al. Obesity, behavioral lifestyle factors and risk of acute coronary events. Circulation. 2008:3062–9. doi: 10.1161/CIRCULATIONAHA.107.759951. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball SR, Farrell PA, Jefferson LS. Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–80. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- 7.Prod’homme M, Balage M, Debras E, et al. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol. 2005;563:235–48. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortiz O, Russell M, Daley TL, et al. Differences in skeletal muscle mass and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr. 1992;55:8–13. doi: 10.1093/ajcn/55.1.8. [DOI] [PubMed] [Google Scholar]
- 11.United States Department of Agriculture. USDA’s MyPlate. 2011 < http://www.choosemyplate.gov/>.
- 12.United States Department of Agriculture. Dietary Guidelines for Americans. 2011 < http://www.health.gov/dietaryguidelines/>.
- 13.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body Composition Measured by Dual-energy X-ray Absorptiometry Half-body Scans in Obese Adults. Obesity. 2009;17:1281–6. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumner AE, Luercio MF, Frempong BA, et al. Validity of the reduced-sample-insulin-modified-frequently sampled intravenous glucose tolerance test using the nonlinear regression approach. Metabolism. 2009;58:220–5. doi: 10.1016/j.metabol.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: A computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–15. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 16.Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab. 2008;295:E407–12. doi: 10.1152/ajpendo.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes. The Health, Aging, and Body Composition Study. Diabetes. 2006;55:1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender and ethnicity. J Appl Physiol. 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 19.Houmard JA, O’Neill DS, Zheng D, Hickey MS, Dohm GL. Impact of hyperinsulinemia on myosin heavy chain gene regulation. J Appl Physiol. 1999;86:1828–32. doi: 10.1152/jappl.1999.86.6.1828. [DOI] [PubMed] [Google Scholar]
- 20.Lam CS, Chen MH, Lacey SM, et al. Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. 2010;30:1479–84. doi: 10.1161/ATVBAHA.110.203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmang A, Yoshida N, Jennische E, Waldenstrom A, Bjorntorp P. The effects of hyperinsulinemia on myocardial mass, blood pressure regulation and central hemodynamics in rats. Eur J Clin Invest. 1996;26:973–8. doi: 10.1046/j.1365-2362.1996.2880577.x. [DOI] [PubMed] [Google Scholar]
- 22.Sitnick M, Bodine SC, Rutledge JC. Chronic high feeding attenuates load-induced hypertrophy in mice. J Physiol. 2009;587.23:5753–65. doi: 10.1113/jphysiol.2009.180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katta A, Kundla S, Kakarla SK, et al. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1666–75. doi: 10.1152/ajpregu.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10:336–44. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 25.Ingram KH, Lara-Castro C, Gower BA, et al. Intramyocellular lipid and insulin resistance: differential relationships in European and African Americans. Obesity. 2011;19:1469–75. doi: 10.1038/oby.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmang A, Brzezinska Z, Bjorntorp P. Effects of hyperinsulinemia on muscle fiber composition and capillarization in rats. Diabetes. 1993;42:1073–81. doi: 10.2337/diab.42.7.1073. [DOI] [PubMed] [Google Scholar]
- 27.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–6. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 28.Nyholm B, Qu Z, Kaal A, et al. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–8. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, You T, Lenchik L, Nicklas BJ. Resting energy expenditure changes with weight loss: racial differences. Obesity. 2010;18:86–91. doi: 10.1038/oby.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carty CL, Kooperberg C, Neuhouser ML, et al. Low-fat dietary pattern and change in body-composition traits in the Women’s Health Initiative Dietary Modification Trial. Am J Clin Nutr. 2011;93:516–24. doi: 10.3945/ajcn.110.006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol. 2000;88:452–6. doi: 10.1152/jappl.2000.88.2.452. [DOI] [PubMed] [Google Scholar]