Abstract
Specific subsets of biochemical reactions in eukaryotic cells are restricted to individual membrane compartments, or organelles. Cells, therefore, face the monumental task of moving the products of those reactions between individual organelles. Because of the high density of the cytoplasm and the large size of membrane organelles, simple diffusion is grossly insufficient for this task. Proper trafficking between membrane organelles thus relies on cytoskeletal elements and the activity of motor proteins, that act both in transport of membrane compartments and as tethering agents to ensure their proper distribution and to facilitate organelle interactions.
INTRODUCTION
Organelles and other membranous vesicles depend on the activities of molecular motor proteins to determine their distribution within a cell. Three classes of motors - kinesins, dyneins, and myosins - utilize two types of polar cytoskeletal filaments to transport cargoes. Microtubules are long filaments that are typically arranged in a radial array with the plus ends near the cell periphery and the minus ends anchored near the cell center. Kinesins and dyneins move along microtubules and are responsible for most long-range movements of organelles and membranes. Actin filaments, in contrast, are shorter, and while the filaments themselves are polarized, they typically form a randomly oriented meshwork. Myosin motors that move along actin filaments mostly contribute to more localized, short-range movements of cargoes. By utilizing two distinct types of transport networks, delivery of cargoes within a cell can be both efficient and precise. As a given cargo is often attached to multiple motors of various classes, the precise mechanisms which control motor activity and directional bias can be complex. Further, while instrumental in the delivery of cargo by molecular motors, cytoskeletal filaments also act as compartments themselves, by restricting three-dimensional diffusion of membrane organelles to movement in one dimension. This facilitates the interactions between organelles necessary for the transfer of molecules between different membrane compartments. Here we highlight recent studies which aim to understand how motor proteins are regulated and how they work collectively in order to contribute to the organization of the cytoplasm.
INTERACTION BETWEEN MULTIPLE MOTORS
Individual cargoes are transported through the cell by multiple motors of several classes, which can work together or interfere with one another. Motors influence one another even in the relatively simple case where multiple copies of the same motor carry a cargo. In the past year several groups have used optical trapping to analyze forces and interactions between motors during cargo transport in live cells [1-3].
Probably the best example of cooperation between multiple copies of the same motor is provided by cytoplasmic dynein (hereafter called dynein). Optical trap measurements by Rai et al. [1], taken both in vitro and in live cells, show that multiple dynein motors cooperate in force production against the optical trap in a linear manner. That is, the force applied to cargo, under load, increases with the number of dyneins attached. An impressive, in-depth set of experiments, this work demonstrates that dynein motors undergo a load-induced shortening of step size, and that dynein motors in a team may take steps of varying sizes. The authors propose that by taking steps of different sizes, the lead dynein in a team (which initially encounters a higher load) can effectively slow down, allowing trailing dyneins to take larger steps to catch up. In this way, the load may be distributed equally among multiple motors. The authors found this to be in stark contrast to the behavior of multiple kinesin-1 molecules (referred to as kinesin in the remainder of this review), which were unable to cooperatively generate force against the applied load. Unable to vary from an 8nm step, the lead kinesin in a team cannot slow down to allow followers to catch up, thus making multiple kinesins inefficient at distributing loads among the group.
Consistent with this idea, in vitro measurement of single kinesins, combined with theoretical modeling of motor interactions, has shown that a kinesin attached to a cargo is less able to adjust its geometry -- the angle between the stalk and the microtubule -- than dynein [4]. This flexibility of dynein allows multiple motors to be attached to both cargo and the microtubule at an angle that allows them to effectively apply force. Because kinesin is less flexible, only one kinesin will be in a geometry to apply substantial force when multiple kinesins are attached to the same cargo, and thus multiple kinesins are less able to equally share the load. In agreement, optical trap measurements of two-kinesin behavior under constant and variable loads demonstrate that under most conditions two kinesins are usually not able to generate more force than a single kinesin [1,2,5].
In contrast to the results obtained in optical trapping experiments, recent in vivo work by Reis et al. [6] suggests that multiple kinesins can indeed share load and improve performance compared to a single kinesin. Membrane vesicle motility was carefully analyzed in Drosophila segmental nerve axons, and the authors observe that the distribution of anterograde velocities displays multiple peaks. These findings suggest that multiple populations of vesicles exist with varying numbers of kinesin motors attached, and that multiple copies of kinesin can move organelles faster than a single copy. Consistent with this interpretation, a decrease in the amount of kinesin in animals carrying only one copy of the kinesin heavy chain gene results in the shift of velocity distributions toward slower movements.
The discrepancy between the works described above may be explained by the fact that compliance of the membrane vesicles (as opposed to the rigidity of latex beads) can compensate for the lack of flexibility of the kinesin molecule, allowing multiple kinesins to effectively engage in transport of the same vesicle. Thus although the optical trapping studies noted above indicate that teams of kinesins struggle to share load, kinesin motors may indeed be able to work together in vivo to increase transport velocity.
The situation is even more complicated in the case of motors of opposite polarity attached to the same cargo. There are two main possibilities as to how such a cargo can be translocated in one direction: i) either only one population of motors is active at a time, and this defines the direction of movement, or ii) both motors are active simultaneously, pulling in opposite directions until one motor “wins”. In its simplest form, this latter model, known as tug-of-war, predicts that the net direction of movement will depend on the total force generated by each team of opposing motors, with the stronger team defining the direction of movement.
A unique in vitro study using DNA origami scaffolds as synthetic cargo supports the tug-of-war model. Derr et al. [7] observe that DNA cargoes with multiple kinesins and dyneins attached frequently stall in an immobile state. Detachment of one motor species from the cargo, via induction of a photocleavable handle, results in immediate motility in the direction of the remaining motor species, effectively resolving the tug-of-war. This work provides evidence that both teams of motors are attempting to engage in movement simultaneously. In addition, the authors found that directionality of the cargo was influenced by altering the ratio of dynein to kinesin motors present. Similar results were found for the in vitro motility of vesicles purified from neurons [8].
As one might predict, the in vivo situation is more complex. Numerous studies have shown that kinesin is required for dynein transport in vivo, and vice versa. Motility-deficient kinesin mutants are unable to stimulate dynein-driven transport in live cells, suggesting that a mechanical interaction between active motors is required [9]. In support of this idea, a recent study by Blehm et al. [3], using both in vivo and in vitro optical trapping, suggests that dynein remains attached to microtubules and active even when cargo is moving toward the plus ends of microtubules by kinesin. This result again argues that both dynein and kinesin are active at the same time, consistent with the tug-of-war model.
There are examples of transport which do not fit the tug-of-war model. Lipid droplet transport in Drosophila embryos does not correspond to mathematical descriptions of the tug-of-war model [10], which predict more frequent pauses and changes of direction than are observed experimentally. Leidel et al. [11] observed that lipid droplets that are detached from microtubules by an external force during transport are more likely to resume motion in the same direction they were traveling before detachment than to switch direction. These results imply that at any given time only certain motors are active and prepared to attach to microtubules; however, no explanation for how motors are activated or inactivated is currently available.
The studies discussed above generally support the idea that motors are capable of working in teams with little or no help from external factors. Interactions between like-polarity motors appear to be dependent on their physical properties, while interactions between opposite polarity motors appears mostly to depend on the ratio of motors moving in each direction. Even with the abundance of recent studies aimed at deciphering the underlying mechanisms of bidirectional transport, we cannot yet declare whether coordination of opposite polarity motors or a stochastic tug-of-war exists in vivo. However, one common theme that has emerged from the optical trapping of cargo both in vivo and in vitro is that due to its unique geometry, dynein can be better adapted to work in teams. The unique mechanical properties of the dynein molecule may explain why it is the only known minus-end microtubule motor that carries cargo in a cell opposing different types of more rigid and less-adaptable members of the kinesin family. In addition, the ability of dyneins to work in teams may also account for the breadth of cellular functions performed by this motor, from organelle transport to cell division.
MOTOR REGULATION
Many molecular factors contribute to precisely regulating the delivery and distribution of organelles and vesicles in live cells. There are two steps in which motor-based transport may be regulated: i) attachment of molecular motors to cargo, or ii) attachment of molecular motors to their tracks.
Many motors utilize autoregulatory mechanisms to inhibit their catalytic activity when not transporting cargo. Binding to cargo is thought to allow motors to overcome autoinhibitory interactions. During bud formation in Saccharomyces cerevisiae, for example, the primary transport motor myosin V localizes to sites of cell growth only when bound to secretory vesicles [12]. This result indicates that the motor is only capable of walking along actin cables when it is bound to cargo.
Because of the importance of motor-cargo interactions in stimulating motor activity, significant effort has been invested in identifying motor-binding adaptor molecules and many such proteins have been described (see [13] for a review). Interaction between motors and adaptor proteins can be dynamic and tightly regulated. Recent work has identified golgin160 as an Arf1 effector protein required for dynein targeting to Golgi membranes [14]. Golgin160 was found to dissociate from the Golgi during mitosis. This cell-cycle specific regulation of the motor-cargo interaction thus prevents dynein-mediated membrane retention near the microtubule organizing center and allows dispersal of Golgi membranes to ensure their proper segregation between daughter cells.
One cargo adaptor that functions both in motor-cargo as well as motor-track interactions is the Rho-like GTPase Miro. Kinesin activity is regulated by Miro and milton, both mitochondria-associated proteins, which act in concert to recruit the motor to mitochondria [15]. In a meticulous study by Wang and Schwarz [16], Miro was found to possess additional regulatory activity mediated by a direct, calcium-dependent interaction with the kinesin motor domain. Motor-binding by Miro induces dissociation of kinesin from the microtubule track; thus mitochondria transport is interrupted in response to increased calcium. The authors further extended this work to determine that the Parkinson’s disease-related proteins PINK1 and Parkin, which become heavily recruited to depolarized mitochondria in neurons, act together to specifically target Miro for degradation, thus preventing interaction with kinesin and the motility of damaged mitochondria [17].
Recent comprehensive in vitro work by two groups has determined that the dynein-binding protein Lis1 enhances dynein’s motility by ultimately influencing motor attachment to the microtubule. McKenney et al. [18] found that in the presence of Lis1, single dynein molecules exhibited substantially decreased motor velocity. Optical trapping experiments revealed that Lis1-dynein complexes were capable of exhibiting higher force production, and remained attached to microtubules under higher load than dynein alone. These findings could not be explained by Lis1-mediated anchoring of dynein molecules to microtubules, as Lis1 did not exhibit microtubule-binding activity of its own. Through direct examination of the Lis1-dynein interaction by electron microscopy, an elegant study by Huang et al. [19] determined that Lis1 interacts with dynein between two of the motor’s catalytic domains, AAA3 and AAA4. This interaction does not alter the rate of ATP hydrolysis, but rather inhibits dynein from releasing microtubules upon binding ATP, the point in mechanochemical cycle in which it has the lowest affinity for the microtubule track. Lis1 thus allows dynein to remain bound to the microtubule longer between steps, acting as a molecular clutch to enhance the motor’s processivity and, presumably, increase force production. Because Lis1 was not observed to travel with moving dynein cargoes [20], it appears that Lis1 acts to initiate dynein motility events. This initiation step could aid dynein in transporting especially large organelles, such as the nucleus, which require high force production and thus a team of cooperating dynein molecules (see above). The Lis1-dynein complex may allow a single motor to effectively tether a cargo to the microtubule until other cargo-bound dynein molecules are able to engage the track and proceed in multi-motor-driven transport.
Motor proteins may also be regulated directly by proteins that bind their track. Tropomyosin has previously been shown to inhibit type I myosins from binding actin filaments [21,22]. However, Hodges et al. [23] found tropomyosin to have a positive influence on yeast class V myosin (Myo2p) motility in vitro. The authors observed that Myo2p, which has previously been shown to possess non-processive behavior in vitro [24], became a processive motor when tropomyosin was present on actin cables. Through careful examination of the ATPase kinetics, the authors showed that tropomyosin slows the rate of ATP binding and ADP release by Myo2p. This leads to a reduction of the rate of Myo2p dissociation from actin cables.
Another example of motor regulation by proteins attached to the cytoskeletal track is provided by a conserved microtubule-binding protein, ensconsin. This protein was recently shown to be an essential cofactor for kinesin activity in live cells [25]. Interestingly, the ensconsin molecule contains two functional domains: an N-terminal domain responsible for binding to microtubules and a C-terminal domain that is sufficient to activate kinesin. This work suggests that ensconsin activates kinesin only in the vicinity of microtubules where it can efficiently be utilized for organelle transport.
THE CYTOSKELETON AS A COMPARTMENT
The regulation of molecular motors provides one way to specify when and where a cargo is transported or delivered. The arrangement of cytoskeletal filaments themselves is also critical for specific delivery of cargoes, as well as for providing dynamic scaffolding where organelle interactions can take place. Cytoskeletal filaments are continually undergoing reorganization and redistribution throughout the cell, and two recent studies beautifully demonstrate that membrane organelles not only utilize these dynamics, but can also directly influence them.
In a rare single author study, Melina Schuh [26] observed nucleation of actin filaments directly from the surface of vesicles to support long-range, directed transport in mouse oocytes. Two classes of actin nucleators, formin and Spire proteins, colocalized with vesicles destined for the plasma membrane, and cytochalasin washout experiments demonstrated localized formation of actin filaments at the vesicle surface. Schuh observed the convergence of vesicles toward one another along the series of short, interconnected actin filaments, before they were ultimately transported toward the plasma membrane. Interestingly, in the absence of actin filaments the number of vesicles increased, and their size decreased, suggesting that vesicle fusion events rely on their interactions along filaments.
The Golgi apparatus offers another example of an organelle capable of dynamically directing cytoskeletal organization. After fragmentation during mitosis, the Golgi reforms its ribbon-like structure in two stages. First, fragmented Golgi membranes cluster at the cell periphery, forming ministacks. The ministacks are then further organized into ribbons in the perinuclear region. An elegant study by Miller et al. [27] has shown that the initial clustering requires microtubules that are nucleated not at the centrosome, but at the surface of Golgi membranes themselves. Nucleation of Golgi-derived microtubules requires both gamma-tubulin and CLASPs, microtubule plus-end binding proteins which localize to the Golgi [28]. In cells depleted of CLASP proteins, Golgi-derived microtubules are not formed. Although Golgi membranes are still able to localize to the perinuclear region by centrosomal microtubules, the Golgi in these cells does not display classical ribbon morphology. FRAP experiments demonstrate a striking reduction of protein dynamics in Golgi in CLASP-depleted cells, consistent with the idea that Golgi-derived microtubules facilitate membrane fusion of ministacks [27].
A pair of complementary works highlights the importance of the cytoskeleton as a scaffolding that spatially confines organelles, increasing the opportunities for fusion and other interaction events. Friedman et al. [29] found that contacts between endoplasmic reticulum (ER) and mitochondria preferentially occur on acetylated microtubules and that these contacts are important for determining the sites of mitochondrial division [30]. Both ER and mitochondria are known to be moved by kinesin, which has been shown to preferentially bind acetylated microtubules [31,32]. Thus, ER and mitochondria interactions occur on a small subset of cytoplasmic microtubules that are modified by tubulin acetylation, further increasing the frequency of their interactions. These works provide important evidence for motor proteins and microtubules not just in cargo distribution, but also in establishment and maintenance of organelle morphology. Further support of this idea comes from a study in fission yeast, where the microtubule-binding protein mmb1p was shown to provide a link between mitochondria and dynamic microtubules to maintain mitochondria morphology and distribution [33]. In the absence of mmb1p, cells are more likely to display fragmented mitochondria as well as defects in mitochondria inheritance following cell division.
The current work clearly shows that motor proteins and cytoskeletal tracks are essential players in organization of membrane compartments in a cell. Both tracks and motors are regulated via multiple molecular mechanisms and we will likely uncover many more examples of regulation of individual motors. What still remains a major unanswered question in the field is whether there is a unified molecular mechanism that defines kinesin-dynein cross-talk and transport of many types of membrane organelles. More likely, behavior of individual organelles is defined by stochastic interaction between motors of the opposite polarity, and these motors are independently regulated by specific pathways unique for each motor-cargo combination. These pathways would provide the most efficient way for fine-tuning the distribution and trafficking of individual membrane compartments and would allow the cell to adapt to ever-changing environments.
Acknowledgements
We thank Nico Camargo for help in preparing Figure 1. Research in the Gelfand lab is supported by the National Institute of General Medical Science of the National Institutes of Health under awards R01GM052111 and P01GM096971. Molly Rossow is supported in part by NIH/NHLBI training grant T32HL076139.
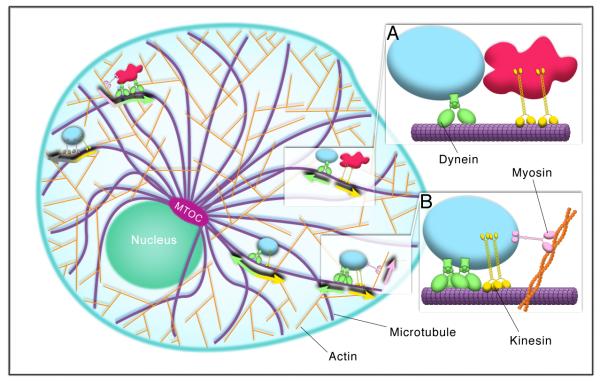
Figure 1.
Membrane organelles require multiple motors and cytoskeletal filaments for their distribution. Long distance transport generally occurs along microtubules (purple), via the molecular motors kinesin (yellow) and dynein (green). Myosin motors (pink, gray) move along actin filaments (orange), and contribute mostly to short-range transport of cargoes. Microtubules and actin also provide scaffolding where organelle interactions can take place (inset A), as attachment to a filament restricts three-dimensional diffusion of organelles to movement in one dimension. Organelles are often moved on filaments by multiple copies of motors, including motors of opposite polarity (inset B). The activity of these motors, and the way in which individual cargoes are transported, is likely regulated by specific factors on individual organelles, to allow for rapid changes in distribution and motility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
- ** 1.Rai AK, Rai A, Ramaiya AJ, Jha R, Mallik R. Molecular Adaptations in Dynein to Generate Large Collective Forces Inside Cells. Cell. 2013;152:172–182. doi: 10.1016/j.cell.2012.11.044. This study shows that groups of dynein molecules attached to a cargo can generate forces against an optical trap that scale linearly with the number of dynein molecules, something multiple kinesin molecules cannot do. An individual dynein motor takes steps of varying sizes, which scale inversely with load applied, which may contribute to load-sharing ability when multiple dynein molecules are on the same cargo.
- 2.Hendricks AG, Holzbaur ELF, Goldman YE. Force measurements on cargoes in living cells reveal collective dynamics of microtubule motors. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1215462109. doi:10.1073/pnas.1215462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blehm BH, Schroer TA, Trybus KM, Chemla YR, Selvin PR. In vivo optical trapping indicates kinesin’s stall force is reduced by dynein during intracellular transport. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1219961110. doi:10.1073/pnas.1219961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 4.Driver JW, Jamison DK, Uppulury K, Rogers AR, Kolomeisky AB, Diehl MR. Productive Cooperation among Processive Motors Depends Inversely on Their Mechanochemical Efficiency. Biophys J. 2011;101:386–395. doi: 10.1016/j.bpj.2011.05.067. Using theoretical modeling and optical trapping measurements this work shows that dynein is better able to adjust the angle between the stalk and the microtubule than kinesin is, and therefore multiple dynein molecules are better able to share loads equally.
- 5.Jamison DK, Driver JW, Diehl MR. Cooperative Responses of Multiple Kinesins to Variable and Constant Loads. Journal of Biological Chemistry. 2012;287:3357–3365. doi: 10.1074/jbc.M111.296582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 6.Reis GF, Yang G, Szpankowski L, Weaver C, Shah SB, Robinson JT, Hays TS, Danuser G, Goldstein LSB. Molecular motor function in axonal transport in vivo probed by genetic and computational analysis in Drosophila. Mol Biol Cell. 2012;23:1700–1714. doi: 10.1091/mbc.E11-11-0938. This work utilizes the power of Drosophila genetics to alter expression levels of motor proteins and analyze vesicle motility in live larvae. The study shows that decreasing the expression of kinesin leads to slower vesicle movements. These findings suggest that kinesin may be able to combine forces effectively in vivo when transporting compliant cargo.
- ** 7.Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Tug-of-War in Motor Protein Ensembles Revealed with a Programmable DNA Origami Scaffold. Science. 2012 doi: 10.1126/science.1226734. doi:10.1126/science.1226734. This paper provides truly convincing evidence in support of a tug-of-war between motors of opposity polarity in vitro, using DNA origami scaffolds as cargo. The motors attached to these cargoes can be precisely controlled in terms of their number, orientation, and spacing, advantages that will likely lead to the increased use of DNA scaffolds in motility assays in vitro.
- * 8.Hendricks AG, Perlson E, Ross JL, Schroeder HW, Tokito M, Holzbaur ELF. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. This work reconstitutes, in vitro, the motility of vesicles purified from mouse neurons, and provides an analysis of motor numbers and types found on individual vesicles.
- 9.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. The Journal of Cell Biology. 2009;187:1071–1082. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunwar A, Tripathy SK, Xu J, Mattson MK, Anand P, Sigua R, Vershinin M, Mckenney RJ, Yu CC, Mogilner A, et al. Mechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. Proceedings of the National Academy of Sciences. 2011;108:18960–18965. doi: 10.1073/pnas.1107841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leidel C, Longoria RA, Gutierrez FM, Shubeita GT. Measuring Molecular Motor Forces In Vivo: Implications for Tug-of-War Models of Bidirectional Transport. Biophys J. 2012;103:492–500. doi: 10.1016/j.bpj.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan KW, Bretscher A. Myosin-V Is Activated by Binding Secretory Cargo and Released in Coordination with Rab/Exocyst Function. Developmental Cell. 2012;23:13. doi: 10.1016/j.devcel.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akhmanova A, Hammer JA., III Linking molecular motors to membrane cargo. Curr Opin Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav S, Puthenveedu MA, Linstedt AD. Golgin160 recruits the dynein motor to position the Golgi apparatus. Developmental Cell. 2012;23:153–165. doi: 10.1016/j.devcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. The Journal of Cell Biology. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 16.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. Through a combination of biochemistry and live cell imaging, this paper identifies the mitochondria-associated GTPase Miro as a calcium-dependent regulator of kinesin. In the presence of high concentrations of calcium, the kinesin motor is shown to remain attached the mitochondria but inactivated via direct interaction with Miro.
- * 17.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. This work cleanly shows that the Ser/Thr kinase PINK1 and the E3 ubiquitin ligase Parkin cooperate to degrade Miro specifically on mitochondria that have become depolarized, leading to dissociation of kinesin and an inhibition of mitochondria motility.
- 18.Mckenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 19.Huang J, Roberts AJ, Leschziner AE, Reck-Peterson SL. Lis1 Acts as a “Clutch” between the ATPase and Microtubule-Binding Domains of the Dynein Motor. Cell. 2012;150:975–986. doi: 10.1016/j.cell.2012.07.022. This work illustrates, in part, a beautiful integration of structural biology with functional biological studies. The Lis1-dynein complex is purified and analyzed by electron microscopy, and structural insights from previous work lead the researchers to investigate an arginine finger motif in the dynein motor. Mutation of this motif yields a dynein motor with motile properties similar to those of the Lis1-dynein complex, and this leads the authors to propose that Lis1 interrupts structural transmission around the AAA+ ring.
- 20.Egan MJ, Tan K, Reck-Peterson SL. Lis1 is an initiation factor for dynein-driven organelle transport. The Journal of Cell Biology. 2012 doi: 10.1083/jcb.201112101. doi:10.1083/jcb.201112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang N, Ostap EM. Motor domain-dependent localization of myo1b (myr-1) Curr Biol. 2001;11:1131–1135. doi: 10.1016/s0960-9822(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 22.Clayton JE, Sammons MR, Stark BC, Hodges AR, Lord M. Differential Regulation of Unconventional Fission Yeast Myosins via the Actin Track. Current Biology. 2010;20:1423–1431. doi: 10.1016/j.cub.2010.07.026. [DOI] [PubMed] [Google Scholar]
- * 23.Hodges AR, Krementsova EB, Bookwalter CS, Fagnant PM, Sladewski TE, Trybus KM. Tropomyosin is essential for processive movement of a class v Myosin from budding yeast. Curr Biol. 2012;22:1410–1416. doi: 10.1016/j.cub.2012.05.035. Combining traditional in vitro motility assays with enzyme kinetics studies, the authors neatly unravel the molecular mechanism contributing to the effect of tropomyosin on myosin V activity.
- 24.Reck-Peterson SL, Tyska MJ, Novick PJ, Mooseker MS. The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. The Journal of Cell Biology. 2001;153:1121–1126. doi: 10.1083/jcb.153.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 25.Barlan K, Lu W, Gelfand VI. The Microtubule-Binding Protein Ensconsin Is an Essential Cofactor of Kinesin-1. Current Biology. 2013 doi: 10.1016/j.cub.2013.01.008. doi:10.1016/j.cub.2013.01.008. In this study, ensconsin is shown to be a cofactor for kinesin activity in vivo. Ensconsin has two domains that function independently of one another to either bind microtubules or activate kinesin. The findings presented here indicate that although kinesin is a fully functional, self-sufficient motor in vitro, kinesin activity in vivo requires an additional factor.
- ** 26.Schuh M. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol. 2011 doi: 10.1038/ncb2353. doi:10.1038/ncb2353. This study provides a striking example of long-range transport that depends not on microtubules, but on the actin cytoskeleton. The system analyzed here, vesicle trafficking in mouse oocytes, requires tracking the movements of vesicles in three dimensions and thus gives a unique perspective on cytoskeletal organization.
- 27.Miller PM, Folkmann AW, Maia ARR, Efimova N, Efimov A, Kaverina I. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nature Publishing Group. 2009;11:1069–1080. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia ARR, McLeod IX. Asymmetric CLASP-Dependent Nucleation of Noncentrosomal Microtubules at the trans-Golgi Network. Developmental Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. The Journal of Cell Biology. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 30.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. In addition to live cell imaging, electron microscopy and tomography are used to analyze the structure of ER-mitochondria contacts in budding yeast. This study gives a prime example of the types of cellular processes that require inter-organelle contacts.
- 31.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Current Biology. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Cai D, McEwen DP, Martens JR, Meyhofer E, Verhey KJ. Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 2009;7:e1000216. doi: 10.1371/journal.pbio.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu C, Jain D, Costa J, Velve-Casquillas G, Tran PT. mmb1p Binds Mitochondria to Dynamic Microtubules. Curr Biol. 2011;21:1431–1439. doi: 10.1016/j.cub.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]