Abstract
Osteosarcoma is an aggressive malignancy and represents the most frequent primary bone malignancy of dogs and humans. Prognostic factors reported for osteosarcoma include tumor size, presence of metastatic disease, and serum alkaline phosphatase (ALP) concentration at the time of diagnosis. To date, there have been no studies to determine whether the behavior of osteosarcoma cells differ based on serum ALP concentration. Here we report on the generation of six canine osteosarcoma cell lines from osteosarcoma-bearing dogs with differences in serum ALP concentration. To determine whether in vitro behavior differs between primary osteosarcoma cell lines generated from patients with normal or increased serum ALP assays were performed to evaluate proliferation, migration, invasion, and chemosensitivity. There were no significant differences in cell proliferation, migration, invasion, or chemosensitivity between cell lines associated normal or increased serum ALP concentration.
Introduction
Osteosarcoma is the most frequent primary bone malignancy in dogs and humans.1,2 In both species the disease is associated with a high metastatic rate and most disease-related mortalities are due to pulmonary metastatic disease. Given the disease similarities between the two species including skeletal sites affected, biological behavior of the tumor, prognostic factors and overlapping gene expression profiles, the dog is a relevant, spontaneously occurring model for human osteosarcoma.3–5 In dogs, osteosarcoma most frequently affects large breed dogs and arises in the metaphyseal region of long bones, specifically the proximal humerus, proximal tibia and distal femur, similar to sites in humans. Prognostic factors that have been identified for osteosarcoma include tumor location, presence of metastatic disease at diagnosis, response to chemotherapy, various genetic alterations, and serum alkaline phosphatase concentration.3,6–9
The alkaline phosphatase (ALP) enzyme catalyzes the hydrolysis of phosphate esters at an alkaline pH.10 In the dog two genes encode two distinct ALP isoenzymes: intestinal and tissue non-specific. The tissue non-specific isoenzyme gives rise to three isoforms: bone, liver, and kidney.11 The ALP enzymes are linked to the outer surface of the cell membrane via a glycophosphytidylinositol (GPI) linkage protein. The bone-specific ALP (bALP) isoform is frequently used to identify cells of the osteoblastic lineage and is a marker of osteoblastic activity. Though the exact function of bALP is unknown, it is thought to be involved with skeletal mineralization.12 Additionally, immunocytochemical or immunohistochemical staining for the presence of ALP activity and expression is used to differentiate osteosarcomas from other sarcoma types during cytologic and histopathologic examination, respectively.13
As previously noted, a subset of osteosarcoma patients have an increased serum ALP concentration at the time of diagnosis, which is associated with a worse prognosis.6–9,14–16 Relatively little work has been done to determine the relevance of increased serum ALP concentration as a negative prognostic factor in osteosarcoma. Studies attempting to correlate ALP concentration with tumor size or volume have been performed and the results are mixed, with some studies finding an association while others have not.6,17,18 It is conceivable that the increase in serum ALP concentration serves as a biomarker for a biologically distinct, more aggressive form of osteosarcoma. However, studies to address this hypothesis have yet to be performed. We believed questions regarding the biological relevance of increased serum ALP concentration could be addressed using the spontaneously occurring tumor in the dog as a model for the human disease given the numerous clinical, histologic, and genetic similarities between human and canine osteosarcoma. The advantages of using canine patients to investigate the utility of ALP as a marker for aggressive osteosarcoma behavior were threefold: first, samples could be accrued more quickly as the incidence of osteosarcoma in the dog is approximately 8–12 times higher than in humans19; second, clinicopathologic data could be matched at the time of tissue collection; and finally, the amount of chemotherapy or radiation therapy-naïve tumor tissue available to start a cell line was likely to be much greater in the canine patient as most dogs undergo limb amputation, or limb-sparing procedures, prior to the administration of chemotherapy.
The overall aim of this study was to establish primary canine osteosarcoma cell lines from osteosarcoma-bearing dogs having different serum ALP concentrations and to determine whether biological behavioral differences existed between cell lines from normal and increased serum ALP populations. Differences in the biological behavior detected between these cell line populations may aid in improving our understanding of the negative prognostic value of an increased serum ALP concentration. Biological behaviors assessed in this study included cell proliferation, migration, invasion, and chemosensitivity. We report here on six canine primary osteosarcoma cell lines (UWKOS1, UWKOS2, UWKOS3, UWKOS6, UWKOS7, UWKOS8) generated in our laboratory from the tumor of six dogs with osteosarcoma. Three cell lines (UWKOS1, UWKOS2, UWKOS3) were started from samples collected from patients with normal serum ALP concentration at the time of collection, and three cell lines (UWKOS6, UWKOS7, and UWKOS8) were started from the tumor of patients with increased serum ALP concentration.
Materials & Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the UW-Madison’s School of Veterinary Medicine Animal Care and Use Committee (Protocol: V01391-0-09-08). Owner consent was obtained prior to the collection of tumor tissue and generation of cell lines.
Clinical Sample Selection
Patient requirements for tumor tissue samples to be collected for this study included a histopathologic diagnosis of osteosarcoma and no previous treatment with any cytotoxic chemotherapy agent. The determination of bone-specific ALP isoform concentration was not performed, though attempts were made to be as stringent as possible regarding patient selection for the collection of tumor samples associated with increased serum ALP concentration. Patients could not have received any corticosteroids for a period of at least two weeks preceding the identification of an increased serum ALP concentration and tissue collection. In addition, with the exception of serum alkaline phosphatase, all renal and hepatic enzyme values were required to be within normal limits of the reporting clinical pathology laboratory. The determination of serum ALP concentration for patient samples utilized in this study occurred at multiple clinical pathology laboratories and at different times, resulting in differences in the reference range for ALP. For this reason each patient was classified as having normal or increased serum ALP according to the normal reference range for the reporting clinical pathology laboratory. Canine osteosarcoma tissue was collected at the time of diagnostic biopsy, surgical amputation or necropsy at the University of Wisconsin-Madison Veterinary Medical Teaching Hospital. A portion of the collected canine osteosarcoma tissue from each patient was placed into phosphate-buffered saline (PBS) for generation of canine primary osteosarcoma cell lines and another portion was snap frozen in liquid nitrogen.
Cell culture
Primary cell lines were generated from clinical tissue samples using previously described procedures.20 Briefly, the tumor tissue collected in PBS with PenStrepFungizone (Invitrogen, CA) underwent enzymatic and mechanical digestion using collagenase I (200 units/ml)(Worthington Biochemical, Lakewood NJ) and DNase I (100 units/ml)(Sigma Aldrich, St. Louis MO) in association with scalpel and scissors mincing, followed by filtration through a 40 mesh sieve until a single cell suspension was created. The resulting cell suspension was centrifuged for 7 min at 1400rpm. The pellet was washed with sterile saline and re-centrifuged with the same conditions. To the pellet, 3mL of complete modified eagle media (CMEM) was added, and the mixture was incubated in a flask with an additional 9mL of CMEM. All cells were maintained in CMEM supplemented with 10% heat-inactivated cosmic calf serum (Thermo Scientific, Waltham MA), sodium pyruvate (Corning, Manassas VA), l-glutamine (Corning, Manassas VA), modified eagle medium (MEM; Corning, Manassas VA) vitamins, non-essential amino acids, and 1% Pen/Strep (Corning, Manassas VA) at 37°C in a humidified incubator with 5% CO2. All cell lines used in this experiment were beyond the 15th passage.
Alkaline phosphatase activity in vitro
All cell lines were stained with the ALP substrate 5′-bromo-4′-chloro-3′-indol phosphate (BCIP) and nitro blue tetrazolium chloride (NBT) (Sigma Aldrich, B1026-100MG) to produce a colorimetric change in the cells, ensuring cell lines were not contaminant fibroblasts. Cells were plated in a 96-well plate (Thermo Fisher Scientific, Waltham, MA) with CMEM and grown to approximately 80% confluence. Following the removal of CMEM, cells were washed briefly in PBS, fixed in an ice-cold 1:1 methanol/acetone mix, and incubated for 10 minutes at −20°C. 150–200μL of BCIP/NBT was added to each well, plates were covered with microfilm and incubated overnight at room temperature. Wells were assessed grossly for a colorimetric change in color and examined using an inverted, phase-contrast microscope equipped with a Micron Imaging System for image-capture (Fisher Scientific, Waltham MA).
To quantify bone-specific ALP activity of the cell lines, a commercially-available ELISA kit validated for canines was utilized (MicroVue BAP EIA kit, Quidel, San Diego, CA). Briefly, cells were seeded in a 96-well plate at a density of 2 × 104 cells/well and allowed to adhere for 18 hours in complete media. Following 18 hours of incubation, the supernatant was collected and secreted bALP activities were determined by a colorimetric change quantified by a plate reader (BioTek ELx800, Winooski, VT). Secreted bALP activities were normalized against the metabolic activity of adhered cells assessed by an XTT assay (Promega, Madison, WI), and expressed as U/L/metabolic activity.
Proliferation Assays
To ensure all cells would be undergoing logarithmic growth and to optimize cell numbers plated for the chemosensitivity assays, proliferation assays were performed at increasing cell concentrations. Cells at 70% confluence were harvested using trypsin-ethylenediaminetetraacetic acid (EDTA) and brought to a concentration of 1×104 cells/mL of media with 10% FBS. A total of 1×103, 2×103, 4×103, 8×103, and 1×104 cells were plated in 96-well plates and incubated at 37°C in 5% CO2. At 24 hours post-plating, medium was aspirated off cells and replaced with fresh medium. Seventy-two hours following medium replacement, cell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium-based (MTS) assay. Briefly, MTS assay solution (Promega, Madison, WI) was added to each well, incubated at 37°C for 1 hour, and absorbance measured at 490 nm using a microplate reader. To generate growth curves, a total of 2×103 cells were plated in 96-well plates and incubated at 37°C in 5% CO2. At 24 hours post-plating, medium was aspirated off cells and replaced with fresh medium. MTS assays were performed on cells at 12, 24, 48, 72, and 96 hours as described above.
Migration & Invasion Assay
Invasion assays were performed on all cell lines using Matrigel-coated transwell chambers (BD Biosciences, Bedford, MA). Cells at 70% confluence in adherent flasks were starved with media containing 0.1% FBS for two hours prior to harvesting using trypsin-EDTA. 2.5×104 cells were plated in media containing 0.1% FBS in growth factor-reduced Matrigel-coated 24-well transwell chambers in triplicate, with one uncoated transwell chamber being used as a control. Media containing 10% FBS was used as a chemoattractant. Plates were incubated for 24 hours at 37°C in 5% CO2. Invading cells were then stained with Diff-Quik Stain Set (Jorgensen Laboratories, Loveland, CO) and mounted onto microscope slides. Cells were imaged on an inverted microscope at 100x and then counted in four fields. A migration index was established by counting the total number of cells present within the uncoated transwell chambers in four 100x fields, dividing by 2.5×104 and multiplying by 100. The percentage invasion for each cell line was calculated by dividing the number of cells present in each matrigel-coated insert by the number of cells present in the uncoated control insert. Assays were repeated in triplicate for each cell line.
Chemotoxicity Assay
Cells at 70% confluence were harvested using trypsin-EDTA and brought to a concentration of 1×104 cells/mL of media with 10% FBS. For each cell line a total of 2×103 cells were plated in 96-well plates (Thermo Fisher Scientific, Waltham, MA) and incubated at 37°C in 5% CO2. Following 24 hours of incubation, media was aspirated off and replaced with media containing either 1% or 10% FBS and either treated or not treated with carboplatin (Hospira, Inc., Lake Forest, IL) or doxorubicin (Pfizer Labs, New York, NY) chemotherapy. Carboplatin was added at concentrations of 0, 0.1, 1.0, 10, 100, and 1000 μM. Doxorubicin was added at concentrations of 0, 0.01, 0.05, 0.1, 0.5, and 1.0 μM. In vivo concentrations of carboplatin for dogs treated at 250mg/m2 have been reported to range from approximately 27 to 270 μM.21 In vivo concentrations of doxorubicin in dogs treated at 30mg/m2 may range from 0.3μM to 5μM.22 Seventy-two hours following the addition of chemotherapy-containing media cell viability was assessed using an MTS assay (Promega, Madison, WI) as previously described. Survival curves were generated by dividing the absorbance reading for treated cells at each concentration by the absorbance for the non-treated cells and multiplied by 100 from each individual cell line.
Statistical Analyses
All experiments were performed on triplicate samples at a minimum of three independent times. The results from individual cell lines were pooled according to serum ALP concentration (normal ALP vs. increased ALP). The average of triplicate experiments for each cell line was combined with cell lines of similar ALP status, thereby giving three values for normal ALP cell lines and three values for increased ALP cell lines to be compared. All data is presented as mean ± standard deviation (SD). Differences in ALP activity, migration, and invasion significance were estimated using a two-tailed unpaired t-test. Two-way ANOVA testing was performed to determine the impact of time and ALP status on cell proliferation as well as chemotherapy concentration and ALP status on chemosensitivity. Bonferroni post-testing was performed to determine which chemotherapy concentrations resulted in differences in chemosensitivity between the normal ALP and increased ALP cell lines. Values of P < 0.05 were considered significant. All graphs were generated and statistical analyses were performed using Prism 4.0 for Macintosh (GraphPad Software, Inc., San Diego, CA).
Results
Generation of canine primary osteosarcoma cell lines
Canine primary osteosarcoma cell lines were generated from the tumor tissue of dogs presenting for the diagnosis and/or treatment of osteosarcoma following owner consent. All dogs were considered to be large breed, with a median age of 10 years, and all had osteosarcoma associated with the appendicular skeleton, typical of most canine osteosarcoma cases (Table 1).
Table 1.
Patient characteristics associated with tumor tissue and cell lines.
| ID # | Breed | Age | Sex | Tumor Location | Serum ALP Concentration (U/L) |
|---|---|---|---|---|---|
| UWKOS1 | Rottweiler | 10yr | FS | Proximal humerus | 218 (13–289) |
| UWKOS2 | Irish Terrier | 9.5yr | M | Proximal humerus | 104 (20–157) |
| UWKOS3 | Mastiff | 10yr | MN | Proximal tibia | 124 (13–289) |
| UWKOS6 | Labrador retriever | 11yr | MN | Distal radius | 365 (13–289) |
| UWKOS7 | German shepherd mix | 9yr | FS | Distal radius | 388 (20–157) |
| UWKOS8 | Border collie mix | 9yr | MN | Distal radius | 248 (20–157) |
Normal reference ranges for serum ALP concentration provided by the reporting clinical pathology laboratory given in parentheses.
Abbreviations: female-spayed (FS) and male-neutered (MN).
Six canine primary osteosarcoma cell lines (UWKOS1, UWKOS2, UWKOS3, UWKOS6, UWKOS7, UWKOS8) were generated from tumor-bearing dogs with known serum ALP concentration. The specific anatomic location of the osteosarcoma and serum ALP concentration for all patients are provided in Table 1. All tumors were confirmed to be osteosarcoma on histopathologic evaluation by a veterinary pathologist at the University of Wisconsin-Madison’s School of Veterinary Medicine.
In vitro alkaline phosphatase activity
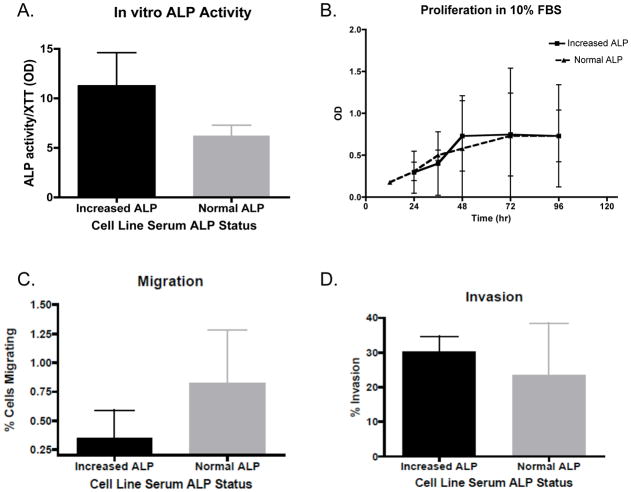
All primary cell lines were treated with 5′-bromo-4′-chloro-3′-indol phosphate/nitro blue tetrazolium chloride (BCIP/NBT) to detect the presence of alkaline phosphatase and all cell lines stained positive as indicated by the resulting blue coloration of cells. Interestingly, when bALP activity was quantified there was no difference noted between cell lines derived from patients with normal or increased serum ALP concentration (6.2 ± 1.0 U/L/metabolic activity vs. 11.3 ± 3.3 U/L/metabolic activity, respectively; p = 0.2)(Figure 1).
Figure 1. Characteristics of canine primary OSA cell lines associated with normal or increased serum ALP.
There were no differences between primary OSA cell lines associated with normal or increased serum ALP concentration in: (A) ALP activity, (B) cell proliferation, (C) migration, or (D) invasion. Comparisons made using a two-tailed unpaired t-test.
Proliferation
To determine if differences in growth existed between canine osteosarcoma cell lines generated from patients with normal and increased serum ALP concentration MTS assays were performed on all cell lines grown in 10% and 1% FBS-containing media. There were no differences in proliferation between cell lines derived from patients with normal or increased serum ALP concentrations in either the 10% and 1% FBS-containing media (data not shown).
Migration
A directional migration assay was performed to determine if differences in migration existed between osteosarcoma cells lines of differing serum ALP concentration. Cell line migration through the non-insert control wells within Transwell chambers was assessed. There was no difference in ability to migrate towards a chemoattractant between osteosarcoma cell lines associated with normal serum ALP and increased serum ALP (0.83% ± 0.45 vs. 0.35% ± 0.24, respectively; p = 0.18).
Invasion
Matrigel invasion assays were performed to determine whether differences in invasion existed between the primary canine osteosarcoma cell lines from patients with normal or increased serum ALP. There was no difference between normal and increased serum ALP cell lines in their ability to invade through a Matrigel-substrate (23.4% ± 15 vs. 30.3% ± 4.4, respectively; p = 0.49).
Chemosensitivity
To determine whether differences in chemosensitivity existed between osteosarcoma cell lines associated with normal or increased serum ALP cell lines were treated with an increasing concentration carboplatin and doxorubicin, two chemotherapeutics commonly used against osteosarcoma. Both groups of cell lines displayed a concentration dependent sensitivity to carboplatin and doxorubicin chemotherapy in 10% and 1% FBS-containing cell media (Figures 2 and 3). However, there was no difference in sensitivity to carboplatin treatment between normal serum ALP and increased serum ALP cell lines in 10% and 1% FBS-containing cell media (Figure 2A & B). Similarly, when cells were treated with doxorubicin in 10% or 1% FBS-containing media, there was no significant difference in sensitivity to doxorubicin between cell lines associated with normal or increased serum ALP (Figure 3A & B).
Figure 2. Evaluation of sensitivity of canine primary OSA cell lines associated with normal or increased serum ALP to carboplatin chemotherapy.
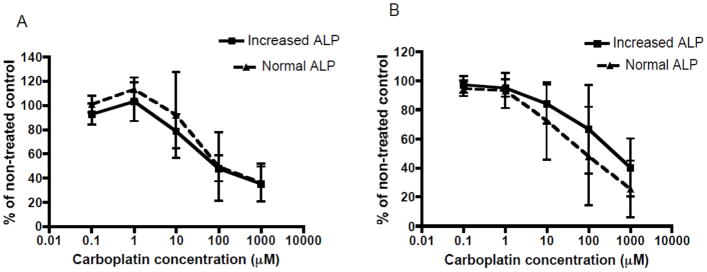
There is no difference in sensitivity to increasing carboplatin concentrations (0.1–1,000μM) between canine primary OSA cell lines associated with normal or increased serum ALP concentration under: (A) 10% FBS-containing media conditions, or (B) 1% FBS-containing media conditions. Comparisons made using two-way ANOVA testing.
Figure 3. Evaluation of sensitivity of canine primary OSA cell lines associated with normal or increased serum ALP to doxorubicin chemotherapy.
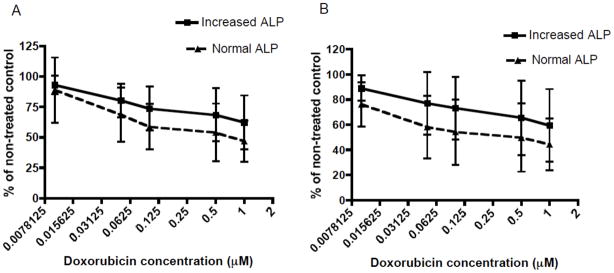
There is no difference in sensitivity to increasing doxorubicin concentrations (0.01–1μM) between canine primary OSA cell lines associated with normal or increased serum ALP concentration under: (A) 10% FBS-containing media conditions, or (B) 1% FBS-containing media conditions. Comparisons made using two-way ANOVA testing.
Discussion
Osteosarcoma is the most common primary bone malignancy in both dogs and humans. Prognostic factors for both species are similar and include patient age, the presence of gross metastatic disease at diagnosis, tumor location, tumor size, and serum ALP concentration. An increase in serum ALP concentration has been associated with a worse prognosis in both canine and human osteosarcoma patients.6–9,14 While ALP is accepted as a negative prognostic factor, relatively little is known as to the biological implications of ALP as a negative prognostic factor.
Recently, a positive correlation was found between the serum concentration of the bone-specific ALP isoform and osteosarcoma tumor volume in humans.18 The study by Limmahakhun and colleagues did not indicate whether tumor size or ALP concentration held prognostic significance in their patient population. As increased tumor size is a known negative prognostic factor, the positive correlation between tumor size and serum ALP concentration could explain the negative prognosis for osteosarcoma patients with increased serum ALP. However, serum ALP concentration and tumor size have been found to be independent prognostic factors on multivariate analysis, suggesting this correlation does not entirely explain the relevance of ALP as a negative prognostic factor. Similarly, other studies have failed to identify any association between serum ALP concentration and tumor size or other markers of bone turnover in osteosarcoma.6,17
Other plausible explanations for increased serum ALP concentration having prognostic value may be split into two paradigms. The first posits that increased serum ALP is the result of intrinsic differences between the tumor cells, resulting in an increased production of ALP, production of a more stable ALP protein, or an increased release of ALP from the cells. The intrinsic differences between the tumor cells derived from patients with normal or increased serum ALP concentrations may also produce vastly different phenotypes giving rise to the more aggressive disease course observed in osteosarcoma patients with increased serum ALP concentrations. Alternatively, the second potential explanation is that the microenvironment of these tumors may harbor increased activity of enzymes capable of cleaving ALP from the membrane surface of osteoblasts, resulting in a subsequent increase in serum ALP concentration. Further, the enhanced enzymatic activity may also serve to free malignant cells from surrounding stroma, and thereby increase metastatic ability. The commonality between any of these hypotheses is that serum ALP concentrations serves as a biomarker denoting a biologically more aggressive form of osteosarcoma.
To address questions regarding differences in behavior based on serum ALP status, it is necessary to have knowledge of the serum ALP status of all cell lines being tested. This information, to our knowledge, does not exist for the majority of currently established canine osteosarcoma cell lines and it was necessary to establish primary cell lines for study. No difference was identified in the ALP activity of cell lines associated with differences in serum ALP concentration. This important finding highlights a key point, that the ALP activity of cell lines in vitro can not be used to predict a patient’s serum ALP concentration. Plausible explanations for the lack of differences may be the selection and expansion of a tumor cell from the primary tumor that was not producing increased amounts of ALP. Alternatively, the growth cells in vitro and passaging of tumor cell lines are both known to modify the behavior of cells and may result in changes in ALP activity.23
Despite the lack of differences in ALP activity between the cell lines associated with normal and increased serum ALP, we were interested in determining whether behavioral differences existed which may aid in explaining the negative prognosis for osteosarcoma associated with increased serum ALP concentration. An increased rate of cellular proliferation, as determined by Ki-67 staining on histopathology sections, has been associated with the level of malignancy in human bone tumors.16 In this study, there were no differences in proliferation, as determined by the MTS-assay, between cell lines from patients with a normal serum ALP and increased serum ALP cell lines.
The abilities to migrate and invade are two characteristics tumor cells must possess in order to metastasize. There were no differences between normal and increased serum ALP cell lines in respects to migration ability. In addition to the ability to migrate, cells must also have the potential for invasion in order to metastasize. There were no differences in invasion through an artificial substrate between normal and increased serum ALP cell lines. Characteristics including an enhanced ability to generate new vascular supply, immune system evasion, and extravasation are also of critical importance in dictating the ability of cells to metastasize but were not assessed in the current study.24,25 These questions will be important to address in future studies, as the majority of dogs with osteosarcoma are suspected to have micrometastatic disease at the time of diagnosis and it is plausible that dogs with increased serum ALP concentrations could have an increased micrometastatic disease burden.
Osteosarcoma is known to develop resistance to chemotherapeutics, which is a frequent cause of treatment failure in dogs and humans with osteosarcoma.26 Therefore, another potential explanation for the enhanced clinical aggressiveness of osteosarcoma associated with increased serum ALP concentrations may be the malignant cells in this population of patients are less sensitive to standard chemotherapeutics. We assessed the sensitivity of both cell populations to carboplatin and doxorubicin, two commonly used chemotherapeutics for the treatment of canine osteosarcoma, and found both cell populations to be sensitive to both chemotherapies in a dose dependent manner. However, no differences were noted between cell lines associated with normal and increased serum ALP in sensitivity to carboplatin or doxorubicin under 1% FBS or 10% FBS-containing media conditions.
The lack of behavioral differences between these cell populations is interesting, however, there are potential limitations to this study. Serum ALP concentration has not been uniformly identified as a prognostic factor in canine or human osteosarcoma studies. Similarly, not all patients with increased serum ALP will have worse outcomes relative to patients with normal serum ALP. However, in larger studies and a recent meta-analysis increased serum ALP in osteosarcoma patients is associated with poor prognosis.6–9,14,27,28 Unfortunately, the prognostic significance of serum ALP was not able to be assessed in the patients from which cell lines were developed as tumor tissue for one cell line was collected at the time of euthanasia and the remaining dogs were all treated differently. Additionally, in the current study a relatively small number of cell lines were utilized, therefore these results may not be representative of the larger population of osteosarcoma patients with differing serum ALP concentration. Another potential limitation regarding the lack of differences noted in the study include tumor location from where cell lines were harvested was different between the two populations (normal vs. increased ALP). Two of the normal ALP cell lines were generated from OSA of the proximal humerus, a site proposed to be associated with poorer outcomes. Finally, cell behavior and characteristics can be significantly different in vitro compared to in vivo and it is possible these changes may have contributed to the lack of differences noted in these studies. The tumor microenvironment is known to impact cancer behavior and the current study was unable to assess the impact of tumor microenvironment on these cell lines’ behavior.
In conclusion, we have successfully generated six new canine primary osteosarcoma cell lines from patients differing in serum ALP concentration. These new cell lines were used to begin addressing questions regarding the biological behavior of osteosarcoma cells from patients that differ in serum ALP concentration, a known prognostic factor. Importantly, there were no differences identified in cell growth, migration, invasion, sensitivity to carboplatin or doxorubicin between cell lines associated with normal or increased serum ALP.
Acknowledgments
The authors wish to acknowledge Nicholas Kuehler for his assistance with the performance of statistical analyses of our study. The authors also with to acknowledge the clients whom allowed for tissue to be collected from their pet. The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021 (TJS) and T32RR17503 (CMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Dernell WS, Ehrhart NP, Straw RC, Vail DM. Tumors of the skeletal system. In: Withrow SJ, Vail DM, editors. Withrow and MacEwen’s Small Animal Oncology. St. Louis, MO: Saunders, Elsevier; 2007. pp. 540–582. [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. International Journal of Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, Hewitt S, Triche T, Meltzer P, Khanna C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. 2009;10:625. doi: 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott MC, Sarver AL, Gavin KJ, Thyanithy V, Getzy DM, Newman RA, Cutter GR, Linblad-Toh K, Kisseberth WC, Hunter LE, Subramanian S, Breen M, Modiano JF. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;36:404–410. doi: 10.1016/j.bone.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angstadt AY, Motsinger-Reif A, Thomas R, Kisseberth WC, Guillermo Couto C, Duval DL, Nielsen DM, Modiano JF, Breen M. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosomes & Cancer. 2011;50:859–847. doi: 10.1002/gcc.20908. [DOI] [PubMed] [Google Scholar]
- 6.Ehrhart N, Dernell WS, Hoffmann WE, Weigel RM, Powers BE, Withrow SJ. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996) Journal of the American Veterinary Medical Association. 1998;213:1002–1006. [PubMed] [Google Scholar]
- 7.Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. Journal of Veterinary Internal Medicine. 2000;14:587–592. doi: 10.1892/0891-6640(2000)014<0587:psosap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, Vail DM. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. Journal of the American Animal Hospital Association. 2009;45:33–38. doi: 10.5326/0450033. [DOI] [PubMed] [Google Scholar]
- 9.Moore AS, Dernell WS, Ogilvie GK, Kristal O, Elmslie R, Kitchell B, Susaneck S, Rosenthal R, Klein MK, Obradovich J, Legendre A, Haddad T, Hahn K, Powers BE, Warren D. Doxorubicin and BAY 12-9566 for the treatment of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study. Journal of Veterinary Internal Medicine. 2007;21:783–790. doi: 10.1892/0891-6640(2007)21[783:dabftt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Harris HH. The human alkaline phosphatases: what we know and what we don’t know. Clinica Chimica Acta. 1989;186:133–150. doi: 10.1016/0009-8981(90)90031-m. [DOI] [PubMed] [Google Scholar]
- 11.Henthorn PS, Millán JL, Leboy P. Acid and Alkaline Phosphatases. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. San Diego, CA: Academic Press; 1999. pp. 130–134. [Google Scholar]
- 12.Rodan GA. Introduction to bone biology. Bone. 1992;13:S3–6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 13.Barger A, Graca R, Bailey K, Messick J, de Lorimer LP, Fan T, Hoffmann W. Use of alkaline phosphatase to differentiate canine osteosarcoma from other vimentin-positive tumors. Veterinary Pathology. 2005;42:161–165. doi: 10.1354/vp.42-2-161. [DOI] [PubMed] [Google Scholar]
- 14.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 15.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. Journal of Clinical Oncology. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 16.Bramer JAM, Abudu AA, Tillman RM, Carter SR, Sumathi VP, Grimer RJ. Pre- and post-chemotherapy alkaline phosphatase levels as prognostic indicators in adults with localized osteosarcoma. European Journal of Cancer. 2005;41:2846–2852. doi: 10.1016/j.ejca.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Lacoste H, Fan TM, de Lorimier LP, Charney SC. Urine N-telopeptide excretion in dogs with appendicular osteosarcoma. Journal of Veterinary Internal Medicine. 2006;20:335–341. doi: 10.1892/0891-6640(2006)20[335:uneidw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Limmahakhun S, Pothacharoen P, Theera-Umpon N, Arpornchayanon O, Leerapun T, Luevitoonvechkij S, Pruksakorn D. Relationships between serum biomarker levels and clinical presentation of human osteosarcoma. Asian Pacific Journal of Cancer Prevention. 2011;12:1717–1722. [PubMed] [Google Scholar]
- 19.Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Research. 2007;27:155–164. [PubMed] [Google Scholar]
- 20.Piskun CM, Muthuswamy A, Huelsmeyer MK, Thompson V, Stein TJ. Wnt/β-catenin expression does not correlate with serum alkaline phosphatase concentration in canine osteosarcoma patients. PLoS One. 2011;2011(6):e26106.2011. doi: 10.1371/journal.pone.0026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villarino N, Cox S, Yarbrough J, Matin-Jimenez T. Determination of carboplatin in canine plasma by high-performance liquid chromatography. Biomedical Chromatography. 2010;24:908–913. doi: 10.1002/bmc.1385. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson DL, Rastatter JC, Colombo T, Long ME. Doxorubicin pharmacokinetics: macromolecular binding, metabolism, and excretion in the context of a physiologic model. Journal of Pharmaceutical Sciences. 2002;91:1488–1501. doi: 10.1002/jps.10161. [DOI] [PubMed] [Google Scholar]
- 23.Reid YA. Characterization and authetication of cancer cell lines: an overview. Methods in Molecular Biology. 2011;731:35–43. doi: 10.1007/978-1-61779-080-5_4. [DOI] [PubMed] [Google Scholar]
- 24.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer. 2003;3:1–6. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 25.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 26.Walters DK, Steinmann P, Langsam B, Schmutz S, Born W, Fuchs B. Identification of potential chemoresistance genes in osteosarcoma. Anticancer Research. 2008;28:673–679. [PubMed] [Google Scholar]
- 27.Han J, Yong B, Luo C, Tan P, Peng T, Shen J. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World Journal of Surgical Oncology. 2012;10:37. doi: 10.1186/1477-7819-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boerman I, Selvarajah GT, Nielen M, Kirpenteijn J. Prognostic factors in canine appendicular osteosarcoma – a meta-analysis. BMC Veterinary Research. 2012;8:56. doi: 10.1186/1746-6148-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]