Abstract
Background
Current evidence does not clearly identify the contribution of kidney function decline and mortality to racial disparities in ESRD incidence. We used observed eGFR to project the time of onset of kidney failure and examined mortality to better understand these racial disparities.
Study Design
Retrospective cohort.
Setting & Participants
Adult members of Kaiser Permanente Southern California from 2003–2009 with >2 serum creatinine tests and >180 days between tests: 526,498 whites, 350,919 Hispanics, 136,923 blacks, and 105,476 Asians.
Predictor
Race/ethnicity.
Outcomes
ESRD (dialysis, transplantation); mortality.
Measurements
eGFR decline was modeled using linear regression. Kidney failure was projected based on predicted eGFR <15 mL/min/1.73m2 at specified times. Racial differences in projected kidney failure and mortality among those with projected kidney failure were estimated with adjustment for age, sex, and entry eGFR.
Results
Blacks had more extreme rates of eGFR decline (1st percentile, −23.6 mL/min/1.73m2 per year), followed by Hispanics (−20.9 mL/min/1.73m2 per year), whites (−20.1 mL/min/1.73m2 per year), and Asians (−17.6 mL/min/1.73m2 per year; P<0.001). There were 25,065 white, 11,368 Hispanic, 6,785 black, and 3,176 Asians with projected kidney failure during the study period. The ORs for projected kidney failure vs. whites during CKD stages 3 and 4 were 1.54 (95% CI, 1.46–1.62) in blacks, 1.49 (95% CI, 1.42–1.56) in Hispanics, and 1.41 (95% CI, 1.32–1.51) in Asians. Among those with projected kidney failure, the HRs of death vs. whites during CKD stages 3 and 4 were 0.82 (95% CI, 0.77–0.88) in blacks, 0.67 (95% CI, 0.63–0.72) in Hispanics, and 0.58 (95% CI, 0.52–0.65) in Asians.
Limitations
Results may not generalize to the uninsured or subgroups within a race. Projected kidney failure was based on linear trends from clinically obtained eGFR.
Conclusions
We found more extreme rates of eGFR decline in blacks. Projected kidney failure during CKD stages 3 and 4 was high in blacks, Hispanics, and Asians relative to whites. Mortality among those with projected kidney failure was highest in whites. Differences in eGFR decline and mortality contributed to racial disparities in ESRD incidence.
Index Words: chronic kidney disease, health disparities, epidemiology
In the United States, blacks have by far the greatest risk of end-stage renal disease (ESRD).1 Increased rates of ESRD also exist in Native Americans and Asians (ancestry from the Far East, Southeast Asia, or the Indian subcontinent2) compared to whites, and in Hispanics compared to non-Hispanics.1 While progress has been made, previous studies have not clearly identified the epidemiologic forces driving these racial disparities in ESRD incidence. To date, there has been little evidence that racial differences in kidney function decline or in mortality are of sufficient magnitude to explain the disparity. The difficulty in demonstrating differences in kidney function over time between groups is due, in part, to the wide variation in progression of kidney disease.3,4 Even among those with chronic kidney disease (CKD), only about 20%–25% of patients experience progression of disease, and only a few percent reach ESRD within several years of follow-up.3,5,6 Moreover, in patients with CKD, death prior to ESRD is more common than ESRD,5–8 and ESRD incidence rates are affected by the competing risk of death.9 Whether a patient reaches ESRD within any given period thus depends on race, the initial kidney function, rate of change in kidney function, and survival.
In this study, we aimed to untangle kidney function decline and survival as epidemiologic forces driving the disparity in ESRD incidence across a range of kidney function. To this end, we examined racial differences in projected kidney failure based on linear decline in estimated glomerular filtration rate (eGFR). We then examined survival among the subgroup of patients with projected kidney failure during the study period. Given that blacks have the highest ESRD incidence, we hypothesized that blacks would have a higher risk of projected kidney failure, and that among those with projected kidney failure, blacks would have lower mortality.
METHODS
Design, Setting, and Participants
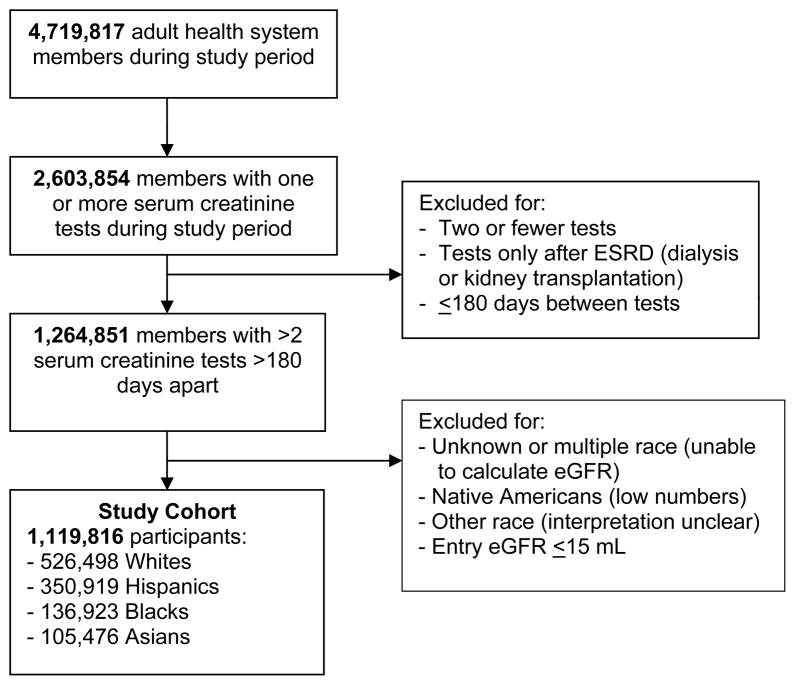
We conducted a retrospective cohort study of members of Kaiser Permanente Southern California, an integrated health system with 3.4 million members at thirteen medical centers and 197 medical offices throughout Southern California. All members have very similar coverage benefits and a limited range of co-payments for services and medications. Participants (Figure 1) include those who were enrolled in the health system for >1 month from 1st Jan 2003 to 31st Dec 2009 and had >2 serum creatinine tests not obtained during ESRD therapy. Participant age was restricted to >17 years at the time of the study entry serum creatinine test. The study protocol was reviewed and approved by the Institutional Review Board of Kaiser Permanente Southern California; a waiver was granted for informed consent.
Figure 1.
Flow diagram of study cohort assembly.
Outcomes and Covariates
Outcomes included (1) death without experiencing ESRD, and (2) ESRD, defined by the date of initiation of hemodialysis, peritoneal dialysis, or kidney transplantation. Mortality data came from California death certificates and the Social Security Death Index. All members undergoing dialysis are tracked by the health system’s Renal Program. Information for this tracking comes from required referral forms, which are regularly reconciled with internal dialysis unit census and outside claims.10 Participants given temporary dialysis, in which dialysis was no longer necessary and was stopped, are tracked and were not considered to have ESRD. A dialysis start within one day of death was counted as a death. Health system disenrollment was defined as a lapse in membership for two or more months regardless of future re-enrollment.
Race and ethnicity (hereafter referred to as race) data were available from health system records for 91% of those with >2 serum creatinine tests. We included members who were Hispanic, non-Hispanic Asian (including Pacific Islanders), non-Hispanic black, and non-Hispanic white. We did not include three additional groups for the following reasons: the number of Native Americans was too small to generate stable estimates; people of multiple races were excluded because GFR estimation was not clearly defined; and persons of “other” race did not have interpretable results.
Kidney function was estimated using the CKD Epidemiology Collaboration (CKD-EPI) creatinine (2009) equation, which calculates eGFR from serum creatinine concentration, age, sex, and black vs. non-black race.11 We excluded tests taken on the day of death or ESRD. When a series of tests were linked by dates within 7 days of any other, we took the median value and date of the series. The entry eGFR during the study period was required to be >15 mL/min/1.73m2. The time between the entry and last eGFR was required to be >180 days.
The overall rate of change in eGFR (mL/min/1.73m2 per year) was determined by the ordinary least squares regression line for each participant. Using the regression line, we stratified participants into higher and lower risk of ESRD. Higher risk participants were those with projected kidney failure based on a predicted eGFR <15 mL/min/1.73m2 (CKD stage 5) by a specified time point. We examined time frames of one, three, and five years after the participant’s entry eGFR. We also examined the time frame of the study period, which ended on 31st Dec 2009. By examining mortality in the subgroup with projected kidney failure within the study period, we gained insight into the influence of the competing risk of death on disparities in ESRD incidence.12
Statistical Analyses
Our goal was to estimate the contribution of eGFR decline and survival on the age- and sex-adjusted disparity in ESRD incidence rates. We also report unadjusted rates because these represent actual population differences and the full burden of disease. Racial differences were examined in all analyses. Participant characteristics were compared across race groups with the chi-square test and the Kruskal-Wallis test. The distributions of entry and last eGFR, and eGFR slopes, were compared using the Kolmogorov-Smirnov equality-of-distributions test.
The risk of having projected kidney failure within one, three, and five years, and within the study period, was determined using logistic regressions with adjustment for age, age-squared, sex, and entry eGFR as a categorical variable (15 mL/min/1.73 m2 per year increments, from 15 to ≥90). These risks were estimated for participants with entry eGFR above and below 60 mL/min/1.73m2 with the introduction of interaction terms in the model.
Likelihood ratios were used to assess how well projected kidney failure within the study period differentiated those who started ESRD therapy during the study period. ESRD rate ratios for each race group vs. whites, adjusted for age, sex, and entry eGFR, were estimated using the Mantel-Haenszel method. Rates of ESRD and death were determined in those with and without projected kidney failure within the study period.
The risk of death by race in those with projected kidney failure within the study period was estimated with competing risks proportional hazards regression, with ESRD as the competing risk, and adjusted for age, age-squared, sex, and entry eGFR.13 The risk of death by entry eGFR above and below 60 mL/min/1.73m2 was determined through the introduction of interaction terms into the model. The proportional hazards assumption was confirmed for race groups using plots of survival function vs. time.
Evidence for a healthy survivor effect that might influence racial disparities in ESRD incidence was sought by examining the probability of dying in each eGFR strata among those with projected kidney failure within the study period. Among those who died, we used multinomial (categorical) logistic regression models with adjustment for age, sex, and entry eGFR to estimate the marginal probability of death in each eGFR stratum, with covariates fixed at their mean values. Thus, the probability of death across all eGFR strata added to one.
In sensitivity analyses, we performed weighted regressions to compensate for uncertainty in the prediction of eGFR due to variation in the number of serum creatinine tests per subject. When a patient’s predicted eGFR at a specific time was <15 or >15 mL/min/1.73m2, the assigned weight was the probability of an eGFR <15 or >15, respectively. The probability was calculated using the standard t-score derived from the estimate, a sample mean of 15, and the standard error of prediction. The coefficients of weighted regression varied only slightly (maximum, 7%) from the unweighted regressions with no change in interpretation; thus, only unweighted results are reported to give equal importance to subjects who may utilize less health care.
We did not adjust for kidney disease type or health status covariates that vary by racial group (e.g., diabetes, hypertension, proteinuria) since these factors are in the causal pathway and adjustment for them would attenuate our estimates of racial differences that may contribute to disparities in ESRD incidence. All analyses were conducted by the research team using SAS EG 4.3 (SAS Institute Inc, Cary NC) and Stata 11.2 (StataCorp LP, College Station, TX).
RESULTS
Among the 1,119,816 members of the study cohort, there were 526,498 whites, 350,919 Hispanics, 136,923 blacks, and 105,476 Asians. The race distribution of the source population and the study cohort are shown in Table 1. Characteristics of participants by race are listed in Table 2. Given the large population sizes, statistically significant differences were observed in one or more race groups for all variables. For example, Whites tended to be older (mean age, 58 years) and Hispanics younger (mean age, 48 years). There were proportionately more females among blacks (63%) and fewer among whites (55%). Blacks had the longest membership enrollment time (mean, 10.1 years) while Hispanics had the shortest (mean, 6.1 years). The number of serum creatinine tests was lowest in Hispanics (mean, 8) and highest in blacks and whites (mean, 10).
Table 1.
Race and ethnicity in source population and in study cohort.
| Race & Ethnicity | Source Population | Study Cohort |
|---|---|---|
| (N = 4,719,817) | (n = 1,119,816) | |
| White | 1,180,131 (25.00) | 526,498 (47.02) |
| Hispanic | 1,199,181 (25.41) | 350,919 (31.34) |
| Black | 313,666 (6.65) | 136,923 (12.23) |
| Asian & Pacific Islander | 272,510 (5.77) | 105,476 (9.42) |
| Native American | 5,591 (0.12) | NA |
| Multiple | 8,157 (0.17) | NA |
| Other | 70,152 (1.49) | NA |
| Unknown | 1,670,429 (35.39) | NA |
Note: The source population was adult health system members from 1st January 2003 to 31st Dec 2009. The percent of adults with >2 serum creatinine tests whose race was unknown was 9.2%. The study cohort consisted of those with >2 serum creatinine tests, an entry estimated glomerular filtration rate >15 mL/min/1.73m2, and >180 days between the first and last serum creatinine test. Values are given as number (percentage).
NA, not applicable because excluded from analysis.
Table 2.
Characteristics of study participants by race and ethnicity.
| Characteristic | Black | White | Hispanic | Asian |
|---|---|---|---|---|
| No. | 136,923 | 526,498 | 350,919 | 105,476 |
| Age1 | ||||
| Mean (y) | 53 ± 15 | 58 ± 15 | 48 ± 15 | 53 ± 14 |
| Median (y) | 53 [42–64] | 58 [48–69] | 48 [38–58] | 53 [44–62] |
| Female sex | 85,999 (62.8) | 289,077 (54.9) | 208,330 (59.4) | 61,617 (58.4) |
| No. of serum creatinine tests | ||||
| Mean | 10 ± 8 | 10 ± 7 | 8 ± 6 | 9 ± 7 |
| Median | 8 [5–12] | 7 [5–12] | 6 [5–10] | 7 [5–11] |
| Observation period2 | ||||
| Mean (y) | 5.0 ± 1.7 | 4.9 ± 1.7 | 4.7 ± 1.7 | 5.0 ± 1.6 |
| Median (y) | 5.5 [3.9–6.4] | 5.4 [3.7–6.3] | 5.1 [3.5–6.2] | 5.5 [3.8–6.4] |
| Duration of Enrollment3 | ||||
| Mean (y) | 10.1 ± 9.0 | 8.6 ± 7.9 | 6.1 ± 6.7 | 7.1 ± 7.1 |
| Median (y) | 8.0 [2.9–14.7] | 7.2 [2.7–11.9] | 4.3 [0.9–8.9] | 5.6 [1.2–10.4] |
| Disenrolled before end of study period* | 16,848 (12.3) | 64,103 (12.2) | 59,606 (17.0) | 11,074 (10.5) |
| Entry eGFR | ||||
| 15–29 mL/min/1.73 m2 | 1,397 (1.02) | 5,520 (1.05) | 1,958 (0.56) | 613 (0.58) |
| 30–44 mL/min/1.73 m2 | 3,998 (2.92) | 20,732 (3.94) | 5,172 (1.47) | 2,041 (1,94) |
| 45–59 mL/min/1.73 m2 | 10,108 (7.38) | 55,528 (10.55) | 13,255 (3.78) | 5,439 (5.16) |
| 60–74 mL/min/1.73 m2 | 21,240 (15.51) | 113,132 (21.49) | 34,127 (9.73) | 13,300 (12.61) |
| 75–89 mL/min/1.73 m2 | 31,321 (22.87) | 145,521 (27.64) | 67,973 (19.37) | 23,862 (22.62) |
| ≥90 mL/min/1.73 m2 | 68,859 (50.29) | 186,065 (35.34) | 228,434 (65.10) | 60,222 (57.09) |
| Last eGFR | ||||
| <15 mL/min/1.73 m2 | 1,637 (1.20) | 3,288 (0.62) | 2,189 (0.62) | 696 (0.66) |
| 15–29 mL/min/1.73 m2 | 2,640 (1.93) | 11,535 (2.19) | 3,720 (1.06) | 1,278 (1.21) |
| 30–44 mL/min/1.73 m2 | 5,710 (4.17) | 32,319 (6.14) | 8,411 (2.40) | 3,423 (3.25) |
| 45–59 mL/min/1.73 m2 | 12,505 (9.13) | 73,758 (14.01) | 19,957 (5.69) | 8,317 (7.89) |
| 60–74 mL/min/1.73 m2 | 24,442 (17.85) | 126,888 (24.10) | 43,952 (12.52) | 18,009 (17.07) |
| 75–89 mL/min/1.73 m2 | 31,493 (23.00) | 138,056 (26.22) | 75,319 (21.46) | 26,317 (24.95) |
| ≥90 mL/min/1.73 m2 | 58,496 (42.72) | 140,654 (26.72) | 197,371 (56.24) | 47,437 (44.97) |
| Annual change in eGFR (%) | −4.0 [−16.8 to 7.7] | −4.1 [−17.0 to 3.5] | −4.3 [−16.0 to 0.5] | −3.7 [−14.5 to 2.2] |
| eGFR (mL/min/1.73 m2 per y) slope5 distribution | ||||
| 1st percentile | −23.6 | −20.1 | −20.9 | −17.6 |
| 5th percentile | −9.9 | −8.5 | −8.8 | −7.8 |
| 10th percentile | −6.6 | −5.8 | −5.9 | −5.5 |
| 25th percentile | −3.4 | −3.1 | −3.1 | −3.1 |
| 50th percentile | −1.1 | −1.1 | −1.1 | −1.3 |
| 75th percentile | 0.9 | 0.5 | 0.5 | 0.1 |
| 90th percentile | 3.7 | 2.9 | 3.1 | 2.3 |
| 95th percentile | 6.6 | 5.3 | 5.8 | 4.5 |
| 99th percentile | 18.5 | 14.9 | 15.9 | 12.5 |
Note: Characteristics were compared using the chi-square test (gender) and the Kruskal-Wallis test (all variables except gender and eGFR strata) and the Kolmogorov-Smirnov test (eGFR strata and slopes). All comparisons showed differences between one or more race/ethnic groups (P<0.001). Unless otherwise indicated, values for categorical variables are given as number (percentage); values for continuous variables, as or mean +/− SD or median [IQR].
Age of the study cohort was defined at the time of the first available serum creatinine test during study period.
Observation time = event date - first serum creatinine date.
Enrollment time prior to study entry.
Ordinary least squares regression line slope calculated for each individual in separate regressions. Perccntiles within group are given.
Without experiencing end-stage renal disease or death.
SD, standard deviation; IQR, interquartile range; eGFR, estimated glomerular filtration rate
The distribution of both entry and last eGFR varied by race and ethnicity (P<0.001 for all combinations). For both entry and last eGFR, a larger proportion of whites had eGFRs in the 30–74 mL/min/1.73m2 range. Both blacks and whites more commonly had eGFRs below 30 mL/min/1.73m2 than Hispanics and Asians; Hispanics and Asians more commonly had eGFRs above 90 mL/min/1.73m2. The median percentage change in eGFR was similar in all race groups (range, −3.7% to −4.3% per year), although the distributions were not identical (P<0.001). The distribution of eGFR regression slopes (mL/min/1.73m2 per year) varied by race and ethnicity (P<0.001 for all combinations). Blacks had more extreme rates of eGFR decline (Table 2, P<0.001 for all comparisons). For example, the 1st percentile of eGFR decline was −23.6 mL/min/1.73m2 per year in blacks, followed by −20.9 mL/min/1.73m2 per year in Hispanics, −20.1 mL/min/1.73m2 per year in whites, and −17.6 mL/min/1.73m2 per year in Asians.
The proportion of subjects with a projected kidney failure within one, three, and five years was greater in blacks (0.25%, 1.39%, and 3.00%) followed by whites (0.13%, 1.14%, and 2.74%), Hispanics (0.13%, 0.90%, and 2.14%), and Asians (0.12%, 0.84%, and 1.98%). The adjusted (age, sex, entry eGFR) risk of projected kidney failure was highest in blacks and lowest in Asians (Table 3). Racial differences in risk were attenuated across increasingly greater time horizons for kidney failure of one, three, and five years. There were 25,065 white, 11,368 Hispanic, 6,785 black, and 3,176 Asian participants with projected kidney failure during the study period. The risk of projected kidney failure within the study period was substantially higher among non-whites when the entry eGFR was below 60 mL/min/1.73m2: the ORs of projected kidney failure by race vs. whites were 1.54 (95% confidence interval [CI], 1.46–1.62) in blacks, 1.49 (95% CI, 1.42–1.56) in Hispanics, and 1.41 (95% CI, 1.32–1.51) in Asians.
Table 3.
Risk of projected kidney failure by race compared to whites.
| Black | Hispanic | Asian | |
|---|---|---|---|
| Time after Entry eGFR | |||
| 1 y | 2.17 (1.89–2.49) | 1.78 (1.56–2.02) | 1.47 (1.20–1.80) |
| 3 y | 1.53 (1.45–1.61) | 1.30 (1.24–1.36) | 1.10 (1.03–1.19) |
| 5 y | 1.34 (1.29–1.39) | 1.15 (1.12–1.19) | 0.99 (0.94–1.04) |
| During Study Period1 | |||
| Any entry eGFR | 1.34 (1.30–1.38) | 1.08 (1.05–1.10) | 0.89 (0.85–0.92) |
| Entry eGFR ≥60 mL/min/1.73 m2 | 1.19 (1.47–1.23) | 0.92 (0.89–0.94) | 0.69 (0.66–0.73) |
| Entry eGFR <60 mL/min/1.73 m2 | 1.54 (1.46–1.62) | 1.49 (1.42–1.56) | 1.41 (1.32–1.51) |
Note: Values are shown as OR (95 % CI). Projected kidney failure was defined by an ordinary least squared regression line that would result in eGFR<15 mL/min/1.73m2 by one, three, or five years, or within the study period (by 31st Dec 2009). Logistic regression models were used to estimate the OR and 95% CI for each race/ethnic group in comparison to whites (OR = 1) within the same eGFR strata (n=1,119,816). Regression results were adjusted for age, age-squared, sex, and entry eGFR categorized from 15 to ≥90 mL/min/1.73m2 by units of 15 mL/min/1.73m2.
By 31st December 2009.
eGFR, estimated glomerular filtration rate; OR, odds ratio; CI, confidence interval
Projected kidney failure during the study period was useful to differentiate those who entered ESRD; the positive likelihood ratios by race were as follows: Asians, 37.3; blacks, 22.6; Hispanics, 34.4; and whites, 20.4. The age- and sex-adjusted ESRD incidence rate ratios by race vs. whites were 2.47 (95% CI, 2.29–2.66) in blacks, 2.23 (95% CI, 2.08–2.39) in Hispanics, and 2.03 (95% CI, 1.84–2.24) in Asians. Unadjusted rates of ESRD and death in the total cohort and by projected kidney failure within the study period are shown in Table 4. ESRD rates were highest in the black population (16.6/10,000) and lowest in the white population (6.7/10,000). Death without experiencing ESRD was more common in the white population (166.7/10,000) and least common in the Asian populations (52.6/10,000). Even in the group with projected kidney failure within the study period, death was seven times more common than ESRD in whites and about twice as common in Hispanics, Asians, and blacks.
Table 4.
Outcomes during study period by race and projected kidney failure within study period.
| Projected Kidney Failure | ESRD | Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Hispanic | Asian | Total | Black | White | Hispanic | Asian | Total | |
| Yes | 501.19 (472.18–531.97) | 213.91 (203.61–224.72) | 444.22 (422.61–466.94) | 468.48 (428.55–512.13) | 333.73 (324.31–343.42) | 1,068.68 (1,025.93–1,113.21) | 1,513.73 (1,485,93–1,524.06) | 785.73 (756.81–815.76) | 793.71 (741.20–849.94) | 1,212.32 (1,194.24–1,230.66) |
| No | 0.90 (0.70–1.16) | 0.60 (0.51–0.70) | 0.33 (0.25–0.43) | 0.43 (0.28–0.65) | 0.54 (0.48–0.60) | 89.39 (87.14–91.69) | 127.10 (125.71–128.50) | 45.05 (44.03–46.10) | 44.51 (42.72–46.36) | 89.47 (88.47–90.07) |
| Total | 16.62 (15.69–17.62) | 6.68 (6.38–7.01) | 9.60 (9.14–10.08) | 9.61 (8.81–10.49) | 9.10 (8.85–9.36) | 120.16 (117.59–122.78) | 166.67 (165.11–168.25) | 60.52 (59.35–61.72) | 52.64 (57.17–61.32) | 118.14 (117.23–119.05) |
Note: Values shown as event rate per 10,000 person-years (95% CI). Projected kidney failure was defined by an ordinary least squares regression line that would result in estimated glomerular filtration rate<15 mL/min/1.73m2 within the study period (by 31st Dec 2009). Ninety-four percent of all participants experienced neither death nor ESRD within the study period.
CI, confidence interval; ESRD, end-stage renal disease;
The adjusted (age, sex, entry eGFR) risk of death among those with projected kidney failure within the study period is summarized in Table 5. With the exception of blacks who had an entry eGFR>60 mL/min/1.73m2, the risk of death was always clearly higher in whites. The HRs for death by race vs. whites were 0.93 (95% CI, 0.89–0.98) in blacks, 0.73 (95% CI, 0.70–0.77) in Hispanics, and 0.65 (95% CI, 0.60–0.70) in Asians. Differences between whites and other race groups increased when the entry eGFR was <60 mL/min/1.73m2.
Table 5.
Risk of death among those with projected kidney failure within study period by race compared with whites.
| Entry eGFR | Black | Hispanic | Asian |
|---|---|---|---|
| Any | 0.93 (0.89–0.98) | 0.73 (0.70–0.77) | 0.65 (0.60–0.70) |
| ≥60 mL/min/1.73 m2 | 1.05 (0.99–1.12) | 0.80 (0.76–0.84) | 0.73 (0.66–0.80) |
| <60 mL/min/1.73 m2 | 0.82 (0.77–0.88) | 0.67 (0.63–0.72) | 0.58 (0.52–0.65) |
Note: Values are shown as HR (95 % CI). Competing risks proportional hazards regression (competing risk of end-stage renal disease) was used to estimate the HR and 95% CI for each race/ethnic group in comparison to whites (HR = 1) within the same eGFR strata (n=46,393). Regression results were adjusted for age, age-squared, sex, and entry eGFR categorized from 15 to >90 mL/min/1.73m2 by units of 15 mL/min/1.73m2.
eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval
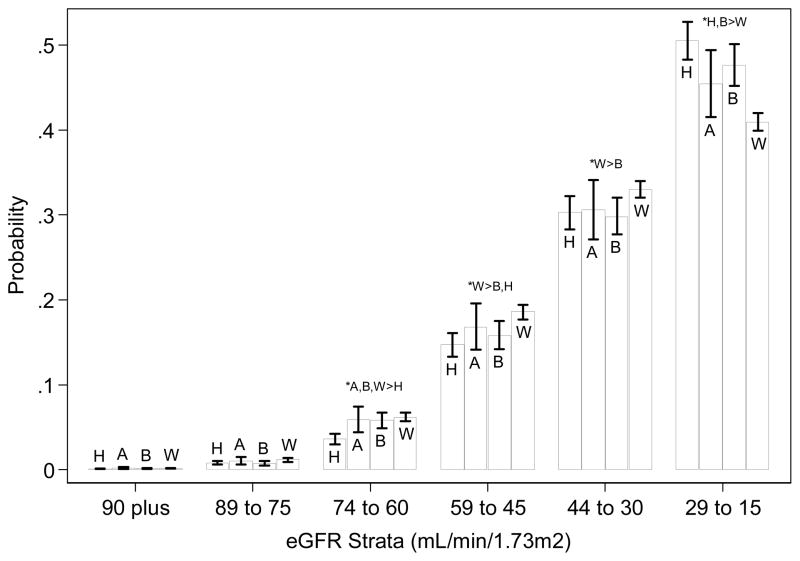
Racial differences were observed in the probability distribution of the eGFR strata in which death occurred (Figure 2). Relative to other race groups, deaths in Hispanics tended to occur more often at the lowest eGFR strata (15–29 mL/min/1.73m2). Deaths in whites tended to occur relatively less often at the lowest eGFR strata and more often at eGFR 30–59 mL/min/1.73m2.
Figure 2.
Probability of death by race while in each eGFR strata below 90 mL/min/1.73m2 in those with projected kidney failure during the study period.
Asians: A
Blacks: B
Hispanics: H
Whites: W
*P<0.05
Note: Deaths after the start of ESRD therapy were not included. Projected kidney failure was defined by an ordinary least squares regression line that would result in eGFR<15 mL/min/1.73m2 within the study period (by 31st Dec 2009). Multinomial logistic regression was used to estimate the marginal probability and 95% CI for each cell with covariates at their means (n=17,030). Covariates included age, sex, and entry eGFR. Significant differences (P<0.05) between race/ethnic groups are noted. Significant (P<0.05) differences between groups are shown.
DISCUSSION
In this cohort study we developed an approach which focused on patients at higher risk of ESRD to examine the forces driving racial disparities in ESRD incidence. Patients with projected kidney failure were defined by an eGFR decline sufficient to reach CKD stage 5 (eGFR <15 mL/min/1.73m2) during a specified period. We demonstrated that blacks were at highest risk of projected kidney failure. Hispanics and Asians were also more likely than whites to have projected kidney failure from CKD stages 3 and 4 (eGFR 15–59 mL/min/1.73m2). We then examined racial differences in mortality among those with projected kidney failure during the study period and demonstrated greater mortality among whites in CKD stages 3 and 4. These racial differences in eGFR decline and mortality among those at higher risk of ESRD appeared large enough to explain much of the disparities in ESRD incidence.
National prevalence estimates show a relative reduction in the point-in-time prevalence of mild to moderate kidney disease among blacks14 that is consistent with racial differences in rates of kidney function decline.9,15,16 Our study cohort has a similarly lower proportion of blacks, but also Hispanics and Asians, with initial study eGFRs of 45–89 mL/min/1.73m2. Equally striking is the lower proportion of whites at eGFR >90 mL/min/1.73m2. The observed prevalence distribution makes sense if whites are less likely to be in rapid decline at lower eGFR, since the time spent in each CKD stage and thus the point prevalence would be inversely proportional to the rate of kidney function decline. This possibility is supported by the lower risk of projected kidney failure we observed among whites in CKD stages 3 and 4. The lower risk of projected kidney failure above eGFR 60 mL/min/1.73m2 among Asians, and to a lesser extent Hispanics, similarly suggests that rapid eGFR decline is less common in these race groups, prior to CKD stages 3 and 4.
Recent studies examining kidney function change and patient survival to explain racial disparities in ESRD incidence provided additional insights but also raised questions. In a study by Kovesdy, et al. of 1,243 male veterans with moderately to severely decreased kidney function (most had eGFR<45 mL/min/1.73m2), pre-dialysis mortality was lower in blacks (unadjusted HR, 0.75).17 Their data suggested that survival differences provided the best explanation for the racial disparity in ESRD, given minimal differences in kidney disease progression. In a study of 2,015,891 veterans, Choi, et al. found an increased mortality risk among blacks, after adjustment for age, gender, common comorbidities, and socioeconomic status indicators.18 These authors suggested that kidney function decline was primarily responsible for racial disparities in ESRD incidence, although the difference in rates was on average <1 mL/min per year in CKD stages 3 and 4. The differing results of these studies may be due to differences in cohorts, eGFR distribution, adjustment for covariates, and the use of analytic methods that focus on central tendency vs. individual variation. By approaching the problem differently, we found an increased risk of projected kidney failure among blacks, Asians, and Hispanics during CKD stages 3 and 4, affirming the importance of eGFR decline. Our findings also clearly point to higher mortality among whites with projected kidney failure during CKD stages 3 and 4.
Whether a survival advantage contributes to the increased incidence of ESRD in blacks has been a source of debate. Greater mortality across the stages of CKD prior to ESRD has been reported among blacks in our health system and in other cohorts.9,18–20 Among those with projected kidney failure within the study period, whites had the highest risk of death. Although any deaths in those at higher risk of ESRD may affect ESRD incidence, we did not determine what proportion of these deaths were attributable to CKD. The observation of reduced mortality among blacks as CKD progresses to ESRD has suggested a healthy survivor effect.9,17,20,21 In this scenario, early mortality due to CKD-related cardiovascular disease could create a culling effect that results in “healthier” patients in the later stages of CKD who survive longer during ESRD. A healthier black population in late stage CKD is supported by the observation that longer survival among blacks on dialysis is attenuated by case-mix adjustment.22 We examined the last eGFR among those with a projected kidney failure and found no evidence of increased mortality at higher eGFR levels and decreased mortality at lower eGFR levels in any group, with the possible exception of whites.
The increased prevalence of diabetes and hypertension (the two leading causes of ESRD in the U.S.) among blacks and Hispanics contributes to, but does not fully explain, at least in blacks, the increased rates of ESRD in these populations.1,20,23,24 The discovery of apolipoprotein L1 gene variants may explain, in part, progressive decline in kidney function in blacks with hypertension.25 This study suggests that there is another disparity – increased morality during CKD stage 3 and 4 – in which whites are at highest risk and Asians at lowest risk. One hypothesis is that diabetes and hypertension control helps prevent eGFR decline during CKD stages 3 and 4 in whites, while mortality due to CKD-related cardiovascular disease continues to progress further in whites relative to other races, especially Asians.
Some assumptions were necessary to use eGFR decline and projected kidney failure to assess the influence of the competing risk of death on ESRD disparities. For simplicity we modeled a linear eGFR trend and assumed that the trajectory would continue without change after the last serum creatinine test. The assumption of a linear trend was justified by additional analyses in which a quadratic term was found to be significant (P<0.05) in only 10% of participants, with no differences by race. We assumed that projected kidney failure would have similar accuracy to predict ESRD among those who died had death been avoided longer, and among those who disenrolled had they stayed in the health system longer. Projected kidney failure depended on sufficient serum creatinine tests. The utility of eGFR decline to separate individuals at higher risk of ESRD in a clinical setting could vary substantially from our results.
Sensitivity analyses with different restrictions on serum creatinine tests were conducted. Racial differences in eGFR trajectory did not substantially change when we used only outpatient serum creatinine tests or excluded tests within 30 days of death, when kidney function may be compromised by multiple organ system failure.
In this study, the source population was not systematically screened for CKD, which represents a potential limitation; however, serum creatinine tests done for clinical reasons seem to identify most individuals with decreased kidney function in our health system.9 Moreover, essentially all new ESRD patients in our health system have serum creatinine tests prior to the start of therapy. The GFR estimating equation may not apply well to some populations outside of the development and validation samples. The CKD-EPI equation performs well in Hispanics and appears to predict outcomes more accurately than the Modification of Diet in Renal Disease (MDRD) Study equation in Asians.26,27
This study included participants from a large, regional integrated health system and therefore may not be representative of the larger US population. Diversity in the Asian and Hispanic population between Southern California and the rest of the U.S. could limit generalizability to other Asian and Hispanic populations.28 Our comparison of Hispanics to non-Hispanic whites is not equivalent to a comparison of Hispanics to non-Hispanics, which would include many non-whites.29 Comparing persons with equivalent health insurance is expected to minimize disparities between race groups that are more or less insured, such as whites and blacks.30 While this may limit generalizability, it represents a particular strength of this study in that comparisons between races are much less confounded by health care access.
In this population with equivalent health insurance, ESRD incidence rates were higher in Asians, blacks, and Hispanics relative to whites. ESRD rates were influenced by increased eGFR decline among blacks, Hispanics, and Asians, augmented by increased mortality among whites. These racial differences were greatest during CKD stages 3 and 4. Elucidating the reasons for these differences, such as control of CKD and cardiovascular disease risk factors, may reveal ways to help reduce disparities in ESRD incidence and improve CKD outcomes, regardless of race.
Acknowledgments
We thank In-Lu A. Liu, MS, for her work in preparing our original CKD case-identification database and Portia Summers for her work in preparing this manuscript.
Support: This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK080097 to Dr Derose, and R01DK078106 to Dr Kalantar-Zadeh). Sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephen F. Derose, Department of Research & Evaluation, Kaiser Permanente Southern California, Pasadena, CA, United States.
Mark P. Rutkowski, Department of Medicine, Baldwin Park Medical Center, Kaiser Permanente Southern California, Baldwin Park, CA, United States.
Peter W. Crooks, Renal Business Group, Kaiser Permanente Southern California, Pasadena, CA, United States.
Jiaxiao M. Shi, Department of Research & Evaluation, Kaiser Permanente Southern California, Pasadena, CA, United States.
Jean Wang, Department of Research & Evaluation, Kaiser Permanente Southern California, Pasadena, CA, United States.
Kamyar Kalantar-Zadeh, Harold Simmons Center for Kidney Disease Research and Epidemiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA & David Geffen School of Medicine at UCLA, Los Angeles, CA, United States.
Csaba P. Kovesdy, Division of Nephrology, Salem Veterans Affairs Medical Center, Salem, VA & Division of Nephrology, University of Virginia, Charlottesville, VA, United States.
Nathan W. Levin, Renal Research Institute, New York, New York, United States.
Steven J. Jacobsen, Department of Research & Evaluation, Kaiser Permanente Southern California, Pasadena, CA, United States.
References
- 1.U. S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 2.United States Census Bureau. [Accessed 7/14/2012, 2012];Race Main: What is Race. 2012 http://www.census.gov/population/race/
- 3.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006 Jan;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 4.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51(6):1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 7.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 9.Derose SF, Rutkowski MP, Levin NW, et al. Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int. 2009 doi: 10.1038/ki.2009.209. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski M, Mann W, Derose S, et al. Implementing KDOQI CKD definition and staging guidelines in Southern California Kaiser Permanente. Am J Kidney Dis. 2009 Mar;53(3 Suppl 3):S86–99. doi: 10.1053/j.ajkd.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pintilie M. Competing Risks: A Practical Perspective. Padstow, Cornwall: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94 (446):496–509. [Google Scholar]
- 14.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 16.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17(6):1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes Associated with Race in Males with Nondialysis-Dependent Chronic Kidney Disease. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.06031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 20.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial Differences in Mortality Among Those with CKD. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(9):493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 23.Borrell LN, Crawford ND, Dallo FJ, Baquero MC. Self-reported diabetes in Hispanic subgroup, non-Hispanic black, and non-Hispanic white populations: National Health Interview Survey, 1997–2005. Public Health Rep. 2009 Sep-Oct;124(5):702–710. doi: 10.1177/003335490912400512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrell LN, Crawford N, Huynh M, Dumanovsky T. Self-reported hypertension and race among hispanic and non-hispanic adults: the New York City community Health Survey. Ethn Dis. 2008 Summer;18(3):299–305. [PubMed] [Google Scholar]
- 25.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010 Aug 13;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011 Mar;79(5):555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA: the journal of the American Medical Association. 2012 May 9;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau. [Accessed May 30, 2012];FactFinder2: Race and Ethnic Groups. 2012 http://factfinder2.census.gov/faces/nav/jsf/pages/searchresults.xhtml?ref=ci&refresh=t.
- 29.U.S. Census Bureau. [Accessed May 29, 2012, 2012];2010 Census Shows America’s Diversity. 2011 http://2010.census.gov/news/releases/operations/cb11-cn125.html.
- 30.The Office of Minority Health. [Accessed May 30, 2012];African American Profile: Insurance Coverage. 2012 http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=51.