Abstract
Background
The Detection of Ischemia in Asymptomatic Diabetics (DIAD) study demonstrated a low 5-year hard cardiac event rate. We hypothesized that a blunted heart rate response (HRR, maximum percent change) to adenosine, a simple marker of cardiac autonomic neuropathy, will identify a cohort at higher cardiac risk.
Methods
In DIAD, 518 participants were randomized to screening adenosine myocardial perfusion imaging (MPI) and had available data. HRR <20% was considered abnormal. The primary endpoint was a composite of nonfatal myocardial infarction and cardiac death.
Results
During 4.7±0.9 years of follow-up 15(3%) participants experienced the primary outcome. Participants with lower HRR experienced more events than those with higher HRR (8%, 3%, 1%, for HRR <20%(n=79), 20-39%(n=182) and ≥40%(n=257), respectively, p=0.01). In a Cox proportional regression model that included MPI abnormalities and HRR, both were independently associated with cardiac events (p for model <0.001). HRR <20% was associated with 9-fold increased risk (p=0.007) and moderate/large abnormal MPI was associated with 6-fold increased risk (p=0.004). Participants with both abnormal MPI and HRR (n=8) were at highest risk for cardiac events (38%) whereas those with HRR ≥40%, irrespective of MPI abnormalities (n=234), were at extremely low risk (≤1%, log-rank p<0.001).
Conclusions
In DIAD, abnormal HRR to adenosine infusion is an independent predictor of cardiac events. This easily obtained marker of cardiac autonomic neuropathy identifies asymptomatic patients with type 2 diabetes mellitus at increased risk, particularly when associated with abnormal MPI, who may warrant further testing and more aggressive cardiovascular risk factor management.
Keywords: Type 2 diabetes mellitus, heart rate, adenosine, myocardial perfusion imaging, autonomic function
Introduction
Screening for coronary artery disease (CAD) in asymptomatic individuals with type 2 diabetes is controversial [1]. Appropriateness criteria published by the American College of Cardiology Foundation in collaboration with American Heart Association, American Society of Nuclear Cardiology and other societies indicate that screening for CAD with stress myocardial perfusion imaging (MPI) in the absence of symptoms is appropriate in individuals at high CAD risk, including in those with type 2 diabetes [2]. The Detection of Ischemia in Asymptomatic Diabetics (DIAD) study tested the hypothesis that systematic screening with MPI will identify higher risk type 2 diabetes individuals and this knowledge would eventually benefit their long-term cardiac outcomes. [3]. At the conclusion of the study, however, there was no difference in outcomes (cardiac death or non-fatal myocardial infarction) over a 5-year follow-up period between participants who underwent vs. did not undergo screening MPI, despite the finding of abnormal stress tests in 22% of patients. It is important to note that the overall incidence of events in DIAD was impressively low at ~3% cumulative event rate or 0.6%/year. Whether asymptomatic diabetics at higher baseline cardiac risk could derive benefit from MPI screening is unknown.
The change in heart rate (HR) seen with activation of the adenosine receptor A2A during vasodilator stress testing is a reflection of cardiac autonomic function [4-5]. We have previously demonstrated that patients with diabetes mellitus have a lower HR response (HRR) to adenosine [5], and that a lower HRR is an independent predictor of poor outcome in large cohorts of patients undergoing vasodilator MPI for clinical indications [6-7]. We therefore hypothesized that a blunted HRR to adenosine infusion, an easily obtainable marker of cardiac autonomic neuropathy (CAN), will identify within DIAD a cohort at higher cardiac risk. The ultimate aim is to use the overall findings from adenosine MPI (perfusion data and HRR) to categorize asymptomatic type 2 diabetes individuals into very low risk group that is unlikely to benefit from further interventions and a higher-risk group that should be targeted for intensive treatment and potentially screening.
Methods
The DIAD study (ClinicalTrials.gov Identifier: NCT00769275) recruited asymptomatic participants with type 2 diabetes from mainly outpatient diabetes practices at 14 centers in the U.S. and Canada between July 2000 and August 2002. Institutional review boards at each participating center approved the study protocol. Participants who agreed to participate provided written informed consent and were randomized to either screening with adenosine MPI or no screening [8]. Methods of recruitment and randomization, demographics, prevalence of perfusion abnormalities, and outcomes of the DIAD study have been published [3, 8-10]. Briefly, inclusion criteria were type 2 diabetes with no history of ketoacidosis, age range 50–75 years, and no symptoms or clinical signs suggestive of CAD. Exclusion criteria included angina pectoris or angina-equivalent symptoms, stress test or coronary angiography within the previous 3 years, history of myocardial infarction, heart failure, or coronary revascularization, ischemic changes on rest electrocardiogram, any current clinical indication for stress testing, active bronchospasm, and limited life expectancy due to cancer or end-stage renal or liver disease. The research protocol was approved by the institutional review boards at all participating sites. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [11].
Of 1,123 DIAD participants, 562 were randomized to the screening arm, of whom 522 underwent adenosine MPI. Hemodynamic data during adenosine infusion were missing on 4 participants. Thus, 518 participants were included in this analysis. Details of stress testing, image analysis and interpretation have been described [3, 8-9]. Image results were categorized based on quantitative analysis as normal, small (0 to <5% of left ventricle), moderate (≥5% and <10%) or large (≥10%) defects [3, 9].
A 12-lead electrocardiogram was recorded at every minute during vasodilator stress. The baseline HR was recorded prior to infusing adenosine. The peak HR was defined as the maximum achieved HR during adenosine infusion. The HRR was calculated as the maximum percent change from baseline as previously described [5-6, 12].
CAN was assessed by recording HR changes during the change of body position, deep breathing, and the Valsalva maneuver, as previously described [8]. Briefly, with a Holter monitor attached, the participant was asked to stand up from lying position. The change in HR (HR standing/lying ratio) was calculated as the ratio of the maximum RR interval around beat 30th to the minimum RR interval around beat 15th after standing-up. While in the sitting position, participants were asked to breathe deeply at a rate of 6 breaths/minute. The change in HR in response to deep breathing (HR deep breathing ratio) was calculated as the ratio between the minimum R-R interval during expiration to the maximum R-R interval during inspiration. Participants were then asked to perform the Valsalva maneuver and the change in HR that occurred (HR Valsalva ratio) was calculated the ratio of the longest R-R interval around the 20th beat after release of a Valsalva to the shortest R-R interval during the maneuver. The obtained ratios were categorized into quartiles and the highest quartile was used as the referent.
The primary end point of the study was a composite of nonfatal myocardial infarction and cardiac death [3]. Cardiac death was defined as death due to myocardial infarction (within 30 days), heart failure, arrhythmia, or sudden cardiac death [3].
Analysis
Because DIAD participants with small MPI defects had similar outcomes as patients with normal results [3], for the present analysis these groups were combined and compared to those with moderate or large MPI defects. Participants with normal myocardial perfusion and ischemic ST changes (n=21), transient ischemic dilation (n=4), or depressed systolic left ventricular function (left ventricular ejection fraction < 45%, n=5) were categorized as having non-perfusion abnormalities and were analyzed separately since the outcome of these participants was distinct from those having normal perfusion [3].
For this analysis, the DIAD participants with adenosine MPI were divided into 3 HRR groups, based on the increase from baseline HR: 1. HRR <20%, the lower limit of normal response to adenosine [6, 13]; 2. HRR ≥20% and less than the median response of the cohort; 3. HRR greater than the median HRR response of the cohort.
All statistical analyses were carried out using SPSS version 17 for Windows (SPSS Inc., Chicago, Illinois). Continuous variables are presented as mean±SD and discrete variables as frequencies and percentages. The chi-square test or an analysis of variance (ANOVA) was used for the comparison of categorical variables across the different groups. Continuous variables were compared using the unpaired student t test or the Mann-Whitney U test, as appropriate. Follow-up time was calculated from the time of evaluation to occurrence of primary endpoint or to end of follow-up. Event-free survival curves were constructed using the product-limit method (Kaplan-Meier) and differences among survival curves were estimated by the log-rank test. Cox proportional hazard analysis was used to estimate un-adjusted, age- and gender-adjusted, baseline HR-adjusted, and beta-blocker use-adjusted risks. A separate Cox model was constructed to include HRR and MPI. We introduced an interaction term to test for the interaction between HRR and MPI. Estimated risks were reported as hazard ratios with correspondent 95% confidence intervals (CI). All tests were 2-sided, and a p value of ≤0.05 was considered statistically significant.
Results
The baseline characteristics of the 518 DIAD participants who underwent adenosine MPI and had HR data available are summarized in Table 1. The participants had type 2 diabetes for an average of 8 years and were relatively well controlled as reflected by the glycosylated hemoglobin value of 7.1% obtained at baseline. Of these participants, 78% did not have evidence of albuminuria (<30 μg/mg creatinine) and only 14% had retinopathy and 9% peripheral vascular disease. Less than one-quarter (23%) of all participants were on insulin at study entry.
Table 1.
Baseline characteristics according to the heart rate response (HRR) to adenosine.
| Characteristic | All patients (518) |
HRR <20% (79) |
HRR 20-39% (182) |
HRR ≥40% (257) |
P value |
|---|---|---|---|---|---|
| Age (years) | 60.7±6.8 | 62.1±7.7 | 60.8±6.5 | 60.2±6.7 | 0.08 |
| Male gender | 275 (53%) | 44 (56%) | 102 (56%) | 129 (50%) | 0.4 |
| Caucasian race | 417 (81%) | 66 (84%) | 149 (82%) | 202 (79%) | 0.8 |
| Body Mass index (kg/m2) | 31.4±6.7 | 31.2±6.7 | 31.7±6.8 | 31.3±6.6 | 0.8 |
| Current smoking | 51 (10%) | 8 (10%) | 23 (13%) | 20 (8%) | 0.2 |
| Hypertension | 288 (56%) | 52 (66%) | 98 (54%) | 138 (54%) | 0.1 |
| Dyslipidemia | 240 (46%) | 36 (46%) | 91 (50%) | 113 (44%) | 0.5 |
| Family History of premature CAD* | 109 (21%) | 14 (18%) | 43 (24%) | 52 (20%) | 0.5 |
| Hemoglobin A1c (%) | 7.1±1.5 | 7.1±1.5 | 7.1±1.5 | 7.1±1.5 | 0.9 |
| T2DM duration (years) | 8.1±7.1 | 9.9±8.1 | 8.6±7.7 | 7.3±6.2 | 0.01 |
| Systolic blood pressure (mmHg) | 134±20 | 142±25 | 135±20 | 131±18 | <0.001 |
| Diastolic blood pressure (mmHg) | 76±10 | 75±11 | 76±10 | 76±10 | 0.6 |
| Baseline heart rate (beats/min) | 74±12 | 80±14 | 76±12 | 70±10 | <0.001 |
| HRR (%) | 42±22 | 11±7 | 30±6 | 60±16 | <0.001 |
| Serum creatinine (mg/dl) | 0.95±0.29 | 1.02±0.35 | 0.94±0.34 | 0.93±0.22 | 0.04 |
| Albuminuria (μg/mg creatinine) | 0.001 | ||||
| <30 | 395 (78%) | 46 (61%) | 137 (78%) | 212 (83%) | |
| 30-299 | 95 (19%) | 25 (33%) | 30 (17%) | 40 (16%) | |
| ≥300 | 17 (3%) | 5 (7%) | 8 (5%) | 4 (2%) | |
| Missing data | 11 (2%) | 3 (4%) | 7 (4%) | 1 (0%) | |
| Retinopathy | 72 (14%) | 19 (24%) | 25 (14%) | 28 (11%) | 0.01 |
| Peripheral vascular disease | 47 (9%) | 11 (14%) | 15 (8%) | 21 (8%) | 0.3 |
| Medication use | |||||
| Insulin | 117 (23%) | 22 (28%) | 48 (26%) | 47 (18%) | 0.07 |
| Metformin | 285 (55%) | 42 (53%) | 96 (53%) | 147 (57%) | 0.6 |
| ACE-Inhibitor | 189 (37%) | 35 (44%) | 64 (35%) | 90 (35%) | 0.3 |
| Beta-blocker | 55 (11%) | 13 (17%) | 26 (14%) | 16 (6%) | 0.005 |
| Calcium channel blocker | 61 (12%) | 16 (20%) | 23 (13%) | 22 (9%) | 0.02 |
| Diuretic | 86 (17%) | 11 (14%) | 32 (18%) | 43 (17%) | 0.8 |
| Statin | 197 (38%) | 30 (38%) | 75 (41%) | 92 (36%) | 0.5 |
Diagnosis of CAD in parents or siblings before age 50 years
Heart rate response to adenosine
The mean HRR (increase) to adenosine of 518 participants was 42±23%. The median HRR was 40% (interquartile range 26-56%). Seventy-nine (15%) participants had an abnormal HRR of <20%. One hundred eighty-two (35%) had a HRR 20-39% and 257 (50%) had a HRR ≥40%. Participants with a lower HRR had longer duration of type 2 diabetes, were more likely to have albuminuria and retinopathy, had higher serum creatinine, systolic blood pressure and baseline HR, and were more likely to be on beta-blockers and calcium channel blockers (Table 1).
The population was followed for 4.7±0.9 years during which 15 (3%) participants experienced the primary outcome (7 nonfatal myocardial infarctions and 8 cardiac deaths). The mean HRR was lower in those who had a cardiac event vs. those that did not (27±23% vs. 42±22%, p=0.004). Participants with a lower HRR experienced more events than those with higher HRR (6 of 79 participants with HRR < 20% [8%], 6 of 182 participants with HRR 20-39% [3%], and 3 of 257 participants with HRR ≥40% [1%], p=0.008, Figure 1). Thus, the group with a HRR <20% had a 7-fold increased risk of the primary outcome (hazard ratio 7.0, 95%CI 1.7-27.8, p=0.006), which was only slightly attenuated by age and gender adjustment (hazard ratio 6.0, 95%CI 1.5-24.1, p=0.01), compared to the group with HRR ≥40%. Importantly, when both HRR and baseline HR were included in the model the association between HRR and outcomes was not attenuated (hazard ratio 7.1, 95%CI 1.7-30.0, p=0.008) and baseline HR was not associated with the occurrence of cardiac events (hazard ratio 0.999, 95%CI 0.959-1.039, p=0.9). Similar results were obtained for HRR (hazard ratio 7.0, 95%CI 1.7-28.0, p=0.006) and beta-blocker use (hazard ratio 1.0, 95%CI 0.2-4.3, p=0.97). When the HRR was included in the Cox model as a continuous variable it was significantly associated with the occurrence of events (hazard ratio per 1% increase in HRR 0.964, 95%CI 0.938-0.991, p=0.009).
Figure 1.
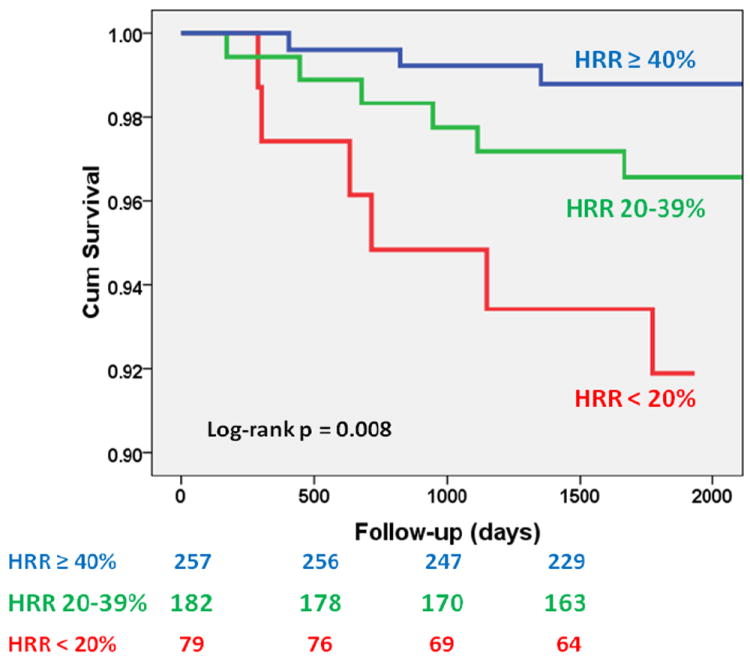
Event-free (nonfatal myocardial infarction and cardiac death) survival according to heart rate response (HRR) to adenosine infusion. HRR <20% constitutes an abnormal response. HRR≥ 40% is the median response of the cohort.
Myocardial perfusion imaging
As previously reported, four hundred and six (78%) participants had normal, 49 (10%) small, 33 (6%) moderate or large and 30 (6%) non-perfusion abnormalities on MPI [8]. In a Cox proportional regression model that included MPI abnormalities and HRR, both were independently associated with cardiac events (p for model <0.001). HRR <20% was associated with 9-fold increased risk (hazard ratio 9.0, 95%CI 1.8-44.5, p=0.007) and abnormal perfusion was associated with a 6-fold increased risk (hazard ratio 5.7, 95%CI 1.7-18.9, p=0.004). There was no interaction between HRR and MPI abnormalities for the prediction of events (p for interaction =0.3).
When participants were divided into 6 subgroups according to their HRR and MPI findings, there was a gradation of risk with decreasing HRR and perfusion abnormalities (Figure 2, log-rank p<0.001). Participants with HRR ≥40% were at low risk irrespective of MPI abnormalities: cardiac event rates of 1% for those with normal MPI (normal or small MPI abnormality, n=216) and 0% for those with abnormal MPI (moderate or large perfusion defect, n=16). Participants with normal MPI and HRR of 20-39% (n=168) and <20% (n=69) had event rates of 2% and 4%, respectively, while those with abnormal MPI and HRR of 20-39% (n=9) and <20% (n=8) had event rates of 11% and 38%, respectively. The HRR to adenosine was not associated with events in participants with non-perfusion abnormalities on their MPI (n=30, 6%, log-rank p=0.4).
Figure 2.
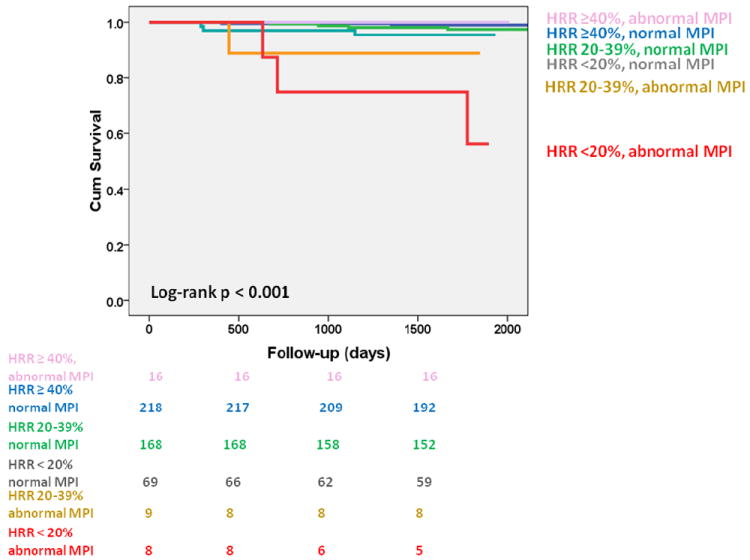
Event-free (nonfatal myocardial infarction and cardiac death) survival according to heart rate response (HRR) to adenosine infusion and myocardial perfusion imaging (MPI) results.
Cardiac autonomic neuropathy
The HRR to adenosine was associated with several assessments of CAN. There was a stepwise decrease in the HRR to adenosine with decreasing HR standing/lying ratio (Figure 3, p<0.001). The HRR was similarly associated with the HR Valsalva ratio (p=0.005) and the HR deep breathing ratio (p=0.006). A lower ratio with each of these maneuvers signifies CAN.
Figure 3.
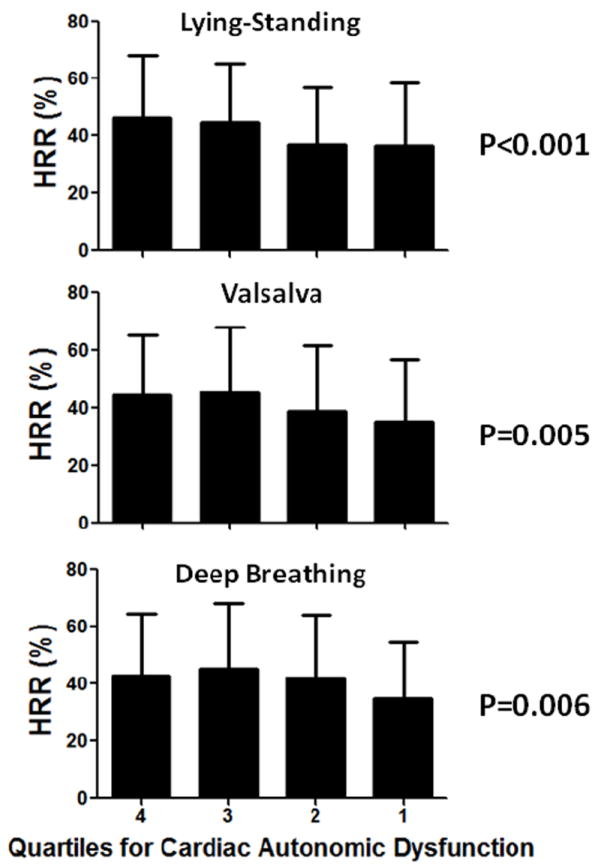
Relationship between heart rate response (HRR) to adenosine and quartiles of heart rate standing/lying ratio, Valsalva ratio, and deep breathing ratio. Lower quartiles signify worse cardiac autonomic dysfunction.
Discussion
Patients with type 2 diabetes are classically considered to be at high cardiac risk and singled out for aggressive interventions aimed at lowering this risk irrespective of the presence of symptoms of CAD [14]. Since myocardial ischemia in type 2 diabetes may result in little or no symptoms [15], it has been postulated that asymptomatic ischemia may account for the increased risk seen in type 2 diabetes. The DIAD study was unable to demonstrate benefit from systematic screening for myocardial ischemia on hard outcomes in asymptomatic participants who were randomized to no-screening vs. screening [3]. We have previously argued that the aggressive treatment of DIAD participants with medical interventions, proven to lower cardiac risk, may have resulted in the low event rate in both arms of the trial, even though more than one-half of participants were at intermediate or high risk according to accepted risk stratification tools [10]. Despite the overall low event rate, HRR identified a small subset of participants that is at an exceptionally high cardiac risk and may benefit from further investigation and therapy, i.e. those with both HRR <20% and moderate or large MPI abnormalities. An argument has recently been made by Roos et al. that screening may be of benefit in a subset of asymptomatic type 2 diabetes individuals who are at high-risk [16].
Nerve damage in patients with type 2 diabetes is known to cause sensory as well as autonomic neuropathy. Damage to the autonomic nerve fibers that innervate the heart causes cardiac CAN which has been associated with increased mortality [17-19]. Since the infusion of adenosine as a stress agent during MPI causes a rise in HR due to its direct effect on the sympathetic nervous system [4], the change in HR in response to adenosine can by theoretically used to gauge the integrity of the cardiac autonomic system. In the Adenosine versus Regadenoson Comparative Evaluation for Myocardial Perfusion Imaging (ADVANCE MPI) 1 and 2 trials (643 patients with diabetes and 1,357 with no diabetes), diabetes patients had a significantly lower HRR to adenosine consistent with the increased prevalence of CAN [5]. In addition to diabetes, other independent predictors of a lower HRR in that study included advanced age, male gender, worse renal function, and left ventricular systolic dysfunction. In a cohort of 879 symptomatic patients (40% with diabetes) who underwent adenosine MPI for clinical reasons at the University of Alabama at Birmingham: 1) there was a stepwise increase in mortality with decreasing HRR, 2) HRR provided incremental prognostic data on top of traditional MPI findings and clinical variables, and 3) HRR successfully risk-stratified diabetes patients into risk categories and was also strongly associated with adverse outcomes in non-diabetics [6].
In the asymptomatic DIAD participants, we saw a similar stepwise increase in cardiac event with decreasing HRR (Figure 1). This association persisted even after controlling for age and gender, for baseline HR, or for beta-blocker use. Similar to our previous observations in patients with indicated MPIs [6-7], HRR provided incremental prognostic information to myocardial perfusion. Importantly, we were able to identify a small asymptomatic population of DIAD participants (n=8) with abnormal myocardial perfusion and depressed HRR (<20%) who had an exceptionally high cardiac event rate (38% over a mean of 4.7 years, Figure 2). This group constituted a small subset of the entire cohort and therefore may not justify a recommendation for screening. We also labeled a large segment of the population (n=232) who had a normal HRR (≥40%) irrespective of myocardial perfusion as being at low risk (<1% over a mean of 4.7 years). If these observations prove to be reproducible by other investigators in asymptomatic populations at higher overall risk than DIAD, they will extend the previous findings in clinically indicated MPIs to a population of truly asymptomatic type 2 diabetes patients and will help in risk stratifying them into a population that does not need any further intervention (normal HRR) and a high-risk population (either low HRR or a combination of low HRR and abnormal perfusion) that may benefit from intensive interventions. These findings may eventually replace the current paradigm of treating all diabetics as CAD risk equivalent with a more reasonable risk stratification model that focuses interventions on individuals at elevated risk.
Participants with a lower HRR tended to be older and more likely to be male (although both observations did not reach statistical significance). Further, these participants had more nephropathy, retinopathy, higher systolic blood pressure and baseline HR, a longer duration of type 2 diabetes and a higher likelihood of being on beta-blocker and calcium channel blockers. The increasing rate of insulin use with decreasing HRR did not reach statistical significance (p=0.07). These observations are largely consistent with our previous observations in distinct cohorts [5-6]. In those previous studies, HRR did not only track with multiple cardiac risk factors but it also provided incremental prognostic information after controlling for these variables making it an ideal and easily obtainable risk marker.
CAN can be diagnosed using a battery of tests that evaluate the autonomic nervous system [18, 20]. These tests include a spectral analysis of HR variability as well as the change in HR in response to deep breathing, lying-to-standing, and Valsalva. Patients with CAN also have decreased myocardial uptake of the non-metabolized norepinephrine analogue I-123 metaiodobenzylguanidine (MIBG) on single-photon emission computed tomography which allows for direct imaging of CAN. Recently, CAN on MIBG imaging was associated with increased risk of cardiac events in heart failure patients in a large prospective trial [21]. DIAD included a detailed assessment of CAN using the HR changes on telemetry with various maneuvers as detailed in the methods section and elsewhere [8]. This allowed us to confirm for the first time in humans that the HRR to adenosine is indeed an assessment of cardiac autonomic function. In a small study (61 diabetes patients and 28 controls), Lee et al. found that the HRR to dipyridamole was lower in diabetes than controls and was associated with CAN in patients with diabetes [22].
In DIAD, CAN assessed using these maneuvers was associated with an abnormal MPI (odds ratio for lowest quartile HR change with Valsalva of 2.6, p<0.001) [8] and with the development of cardiac events (age and gender adjusted hazard ratio for lowest quartile HR during lying-to-standing test of 4.3, p<0.001) [3]. Despite the usefulness of these tests they are rather cumbersome to perform, require trained personnel and Holter monitoring and are seldom performed in busy clinical care. The advantages of the HRR to adenosine over these other assessments include the ease of obtaining this measurement and the fact that the data is already available within clinically performed adenosine MPIs. It also promises to be more reproducible than these other measurements since it requires minimal cooperation from the patient and no expertise from the provider. Clinicians will therefore not need to learn and invest in new tests but can obtain valuable prognostic information from tests they already order routinely.
Study Limitations
This study is limited by small number of cardiac events which prevented adjustment for multiple covariates that are known to influence both the HRR and cardiac outcomes. These data may not be generalizable to other type 2 diabetes populations who are at higher cardiac risk such as those that are not as aggressively treated. Further, this study represents a post-hoc analysis that was not pre-specified. It is important to note that we used the primary outcome of the trial that was prospectively collected and adjudicated by an independent committee in addition to a priori specified cut-offs of the HRR to adenosine.
Conclusions
In conclusion, abnormal HRR response to adenosine infusion in combination with moderate or large MPI abnormalities in asymptomatic patients with type 2 diabetes identifies a subgroup of patients at high cardiovascular risk who might benefit from further investigation and more aggressive management of cardiovascular risk factors.
Acknowledgments
The DIAD was supported by grants from Bristol Myers-Squibb Medical Imaging (North Billerica, Massachusetts, USA) and Astellas Pharma (Deerfield, Michigan, USA), who also provided Technetium-99m Sestamibi (Cardiolite®) and Adenosine (Adenoscan®) for study patients.
DIAD is an investigator-initiated study. The industrial sponsors had no role in the design or conduct of the study, in the collection, analysis or interpretation of data, or in the preparation of the manuscript.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Abstract Publication: The findings were presented at the American College of Cardiology 2012 Scientific Sessions and published in an abstract format in the Journal of the American College of Cardiology.
Financial Disclosure: This work was performed with the support of the General Clinical Research Centers at Yale University (National Institutes of Health M01-RR-00125), University of Rochester (NIH 5M01-RR-00847), and Tulane University (NIH 6M01-RR-05096).
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hage FG, Iskandrian AE. Cardiovascular imaging in diabetes mellitus. J Nucl Cardiol. 2011;18:959–65. doi: 10.1007/s12350-011-9431-7. [DOI] [PubMed] [Google Scholar]
- 2.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhalla AK, Wong MY, Wang WQ, Biaggioni I, Belardinelli L. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J Pharmacol Exp Ther. 2006;316:695–702. doi: 10.1124/jpet.105.095323. [DOI] [PubMed] [Google Scholar]
- 5.Hage FG, Heo J, Franks B, et al. Differences in heart rate response to adenosine and regadenoson in patients with and without diabetes mellitus. Am Heart J. 2009;157:771–6. doi: 10.1016/j.ahj.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Hage FG, Dean P, Bhatia V, Iqbal F, Heo J, Iskandrian AE. The prognostic value of the heart rate response to adenosine in relation to diabetes mellitus and chronic kidney disease. Am Heart J. 2011;162:356–62. doi: 10.1016/j.ahj.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Hage FG, Dean P, Iqbal F, Heo J, Iskandrian AE. A blunted heart rate response to regadenoson is an independent prognostic indicator in patients undergoing myocardial perfusion imaging. J Nucl Cardiol. 2011;18:1086–94. doi: 10.1007/s12350-011-9429-1. [DOI] [PubMed] [Google Scholar]
- 8.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–61. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 9.Wackers FJ, Chyun DA, Young LH, et al. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007;30:2892–8. doi: 10.2337/dc07-1250. [DOI] [PubMed] [Google Scholar]
- 10.Bansal S, Wackers FJ, Inzucchi SE, et al. Five-year outcomes in high-risk participants in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study: a post hoc analysis. Diabetes Care. 2011;34:204–9. doi: 10.2337/dc10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the international journal of cardiology. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 12.Hage FG, Perry G, Heo J, Iskandrian AE. Blunting of the heart rate response to adenosine and regadenoson in relation to hyperglycemia and the metabolic syndrome. The American journal of cardiology. 2010;105:839–43. doi: 10.1016/j.amjcard.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Chan SY, Brunken RC, Czernin J, et al. Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol. 1992;20:979–85. doi: 10.1016/0735-1097(92)90201-w. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 15.Faerman I, Faccio E, Milei J, et al. Autonomic neuropathy and painless myocardial infarction in diabetic patients. Histologic evidence of their relationship. Diabetes. 1977;26:1147–58. doi: 10.2337/diab.26.12.1147. [DOI] [PubMed] [Google Scholar]
- 16.Roos CJ, Djaberi R, Schuijf JD, et al. Relationship between vascular stiffness and stress myocardial perfusion imaging in asymptomatic patients with diabetes. Eur J Nucl Med Mol Imaging. 2011;38:2050–7. doi: 10.1007/s00259-011-1894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 18.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33:434–41. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pop-Busui R, Evans GW, Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–84. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–97. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson AF, Senior R, Cerqueira MD, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Yoon JK, Lee MG, Lee SH, Lee WR, Kim BT. Dipyridamole myocardial SPECT with low heart rate response indicates cardiac autonomic dysfunction in patients with diabetes. J Nucl Cardiol. 2001;8:129–35. doi: 10.1067/mnc.2001.111798. [DOI] [PubMed] [Google Scholar]


