Abstract
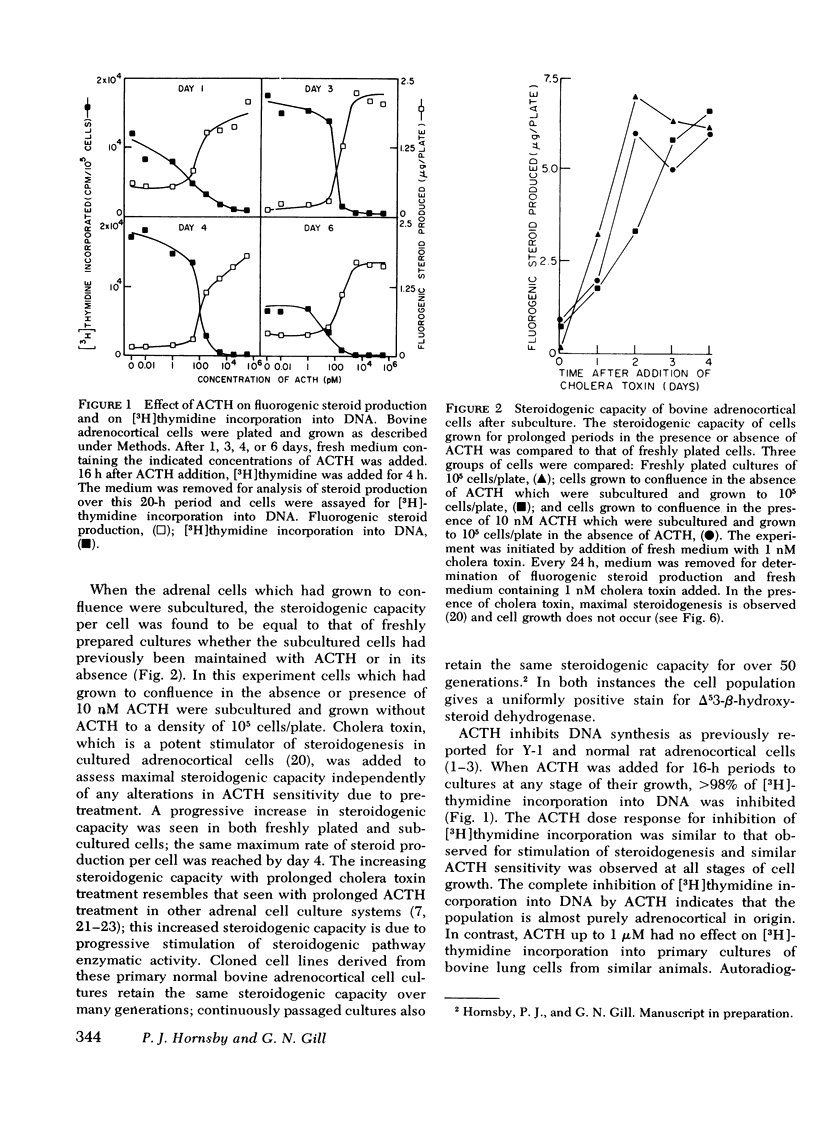
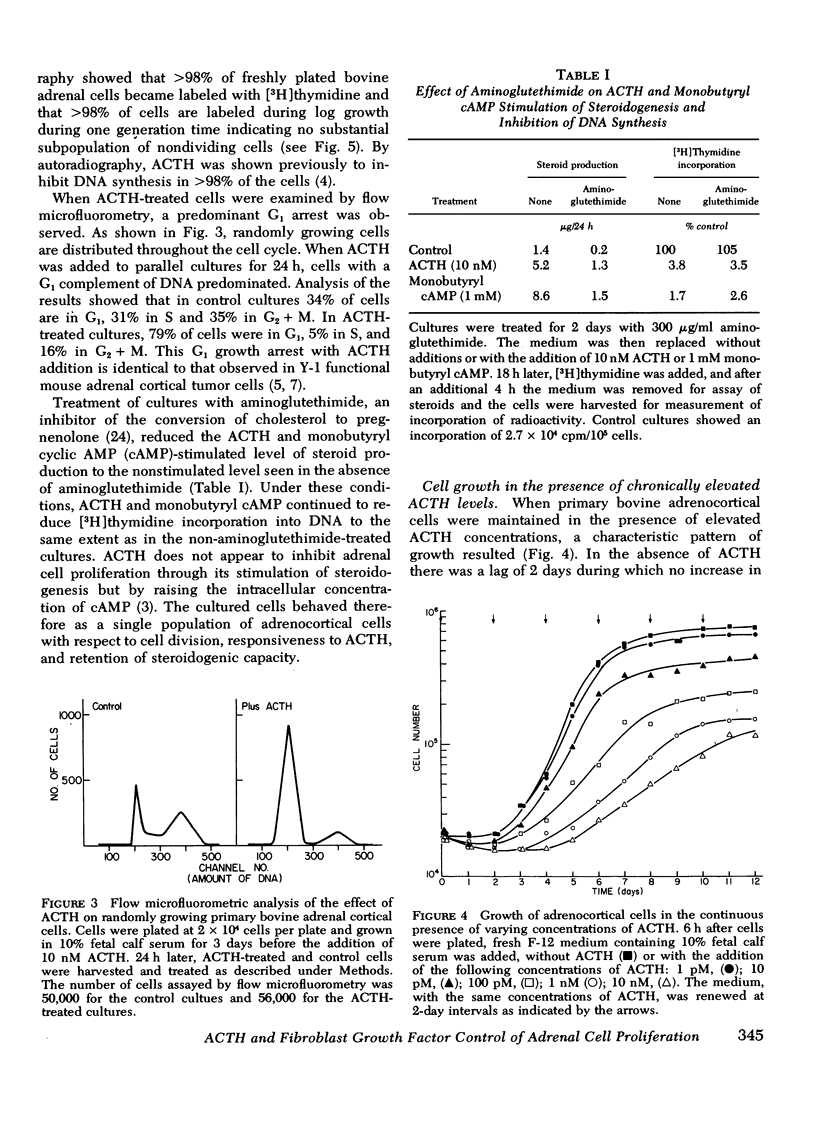
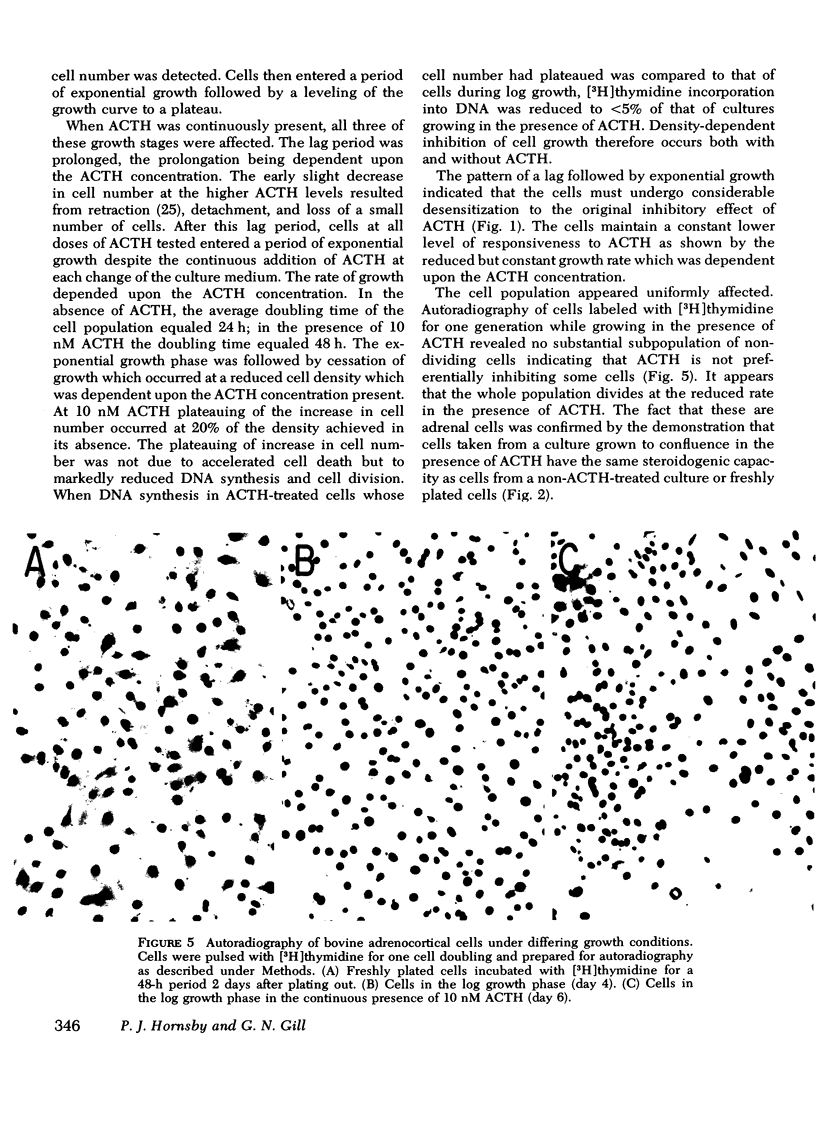
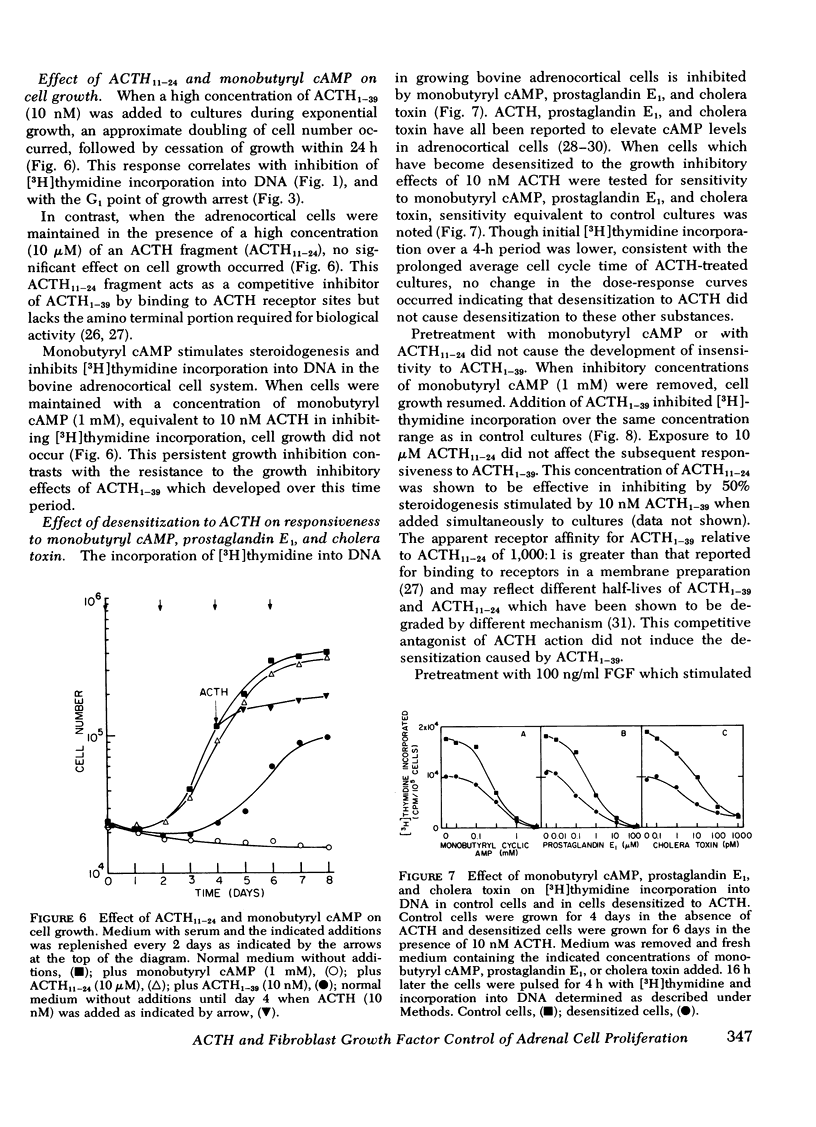
A primary bovine adrenocortical cell culture system responsive to physiological concentrations of ACTH has been established. When added to cultures, ACTH inhibited DNA synthesis and cell division over the same concentration range required for stimulation of fluorogenic steroid production (0.01-10 nM). With chronic exposure to ACTH, cells became desensitized to the growth inhibitory effects of ACTH. Though cell growth was initially completely inhibited by ACTH, cells ultimately began to grow in its continued presence. The lag time to initiation of cell growth, the rate of growth, and the final density achieved depended on the ACTH concentration. Desensitization to ACTH1-39 was not induced by monobutyryl cyclic AMP nor by ACTH11-24. Specificity of desensitization was apparent because cells which had become desensitized to ACTH1-39 remained fully responsive to monobutyryl cyclic AMP, prostaglandin E1, and cholera toxin. Though the effects of ACTH on cell growth were readily reversible upon hormone removal, the desensitized response to readdition of ACTH persisted for at least 8 h.
Fibroblast growth factor (FGF) stimulated both the growth rate and saturation density achieved. FGF did not alter the growth inhibitory effects of ACTH nor the reduced growth rate observed in desensitized cells maintained in ACTH. However, FGF greatly increased the saturation density achieved by cultures maintained with ACTH.
Through the process of desensitization, adrenocortical cells are able to grow in the presence of high concentrations of ACTH and to respond to the effects of a growth factor by increasing the cell density achieved. This pattern of response may be a general one for growth control under the combined effects of antimitotic and mitotic factors.
Full text
PDF

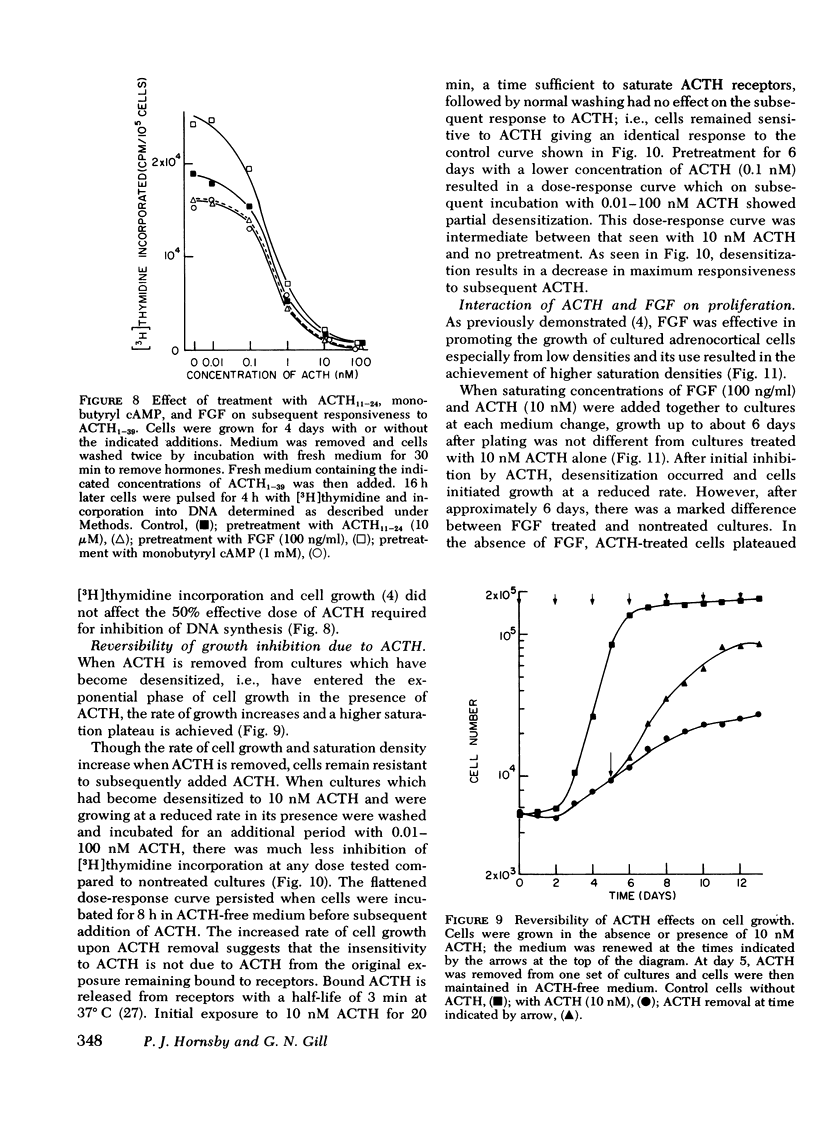
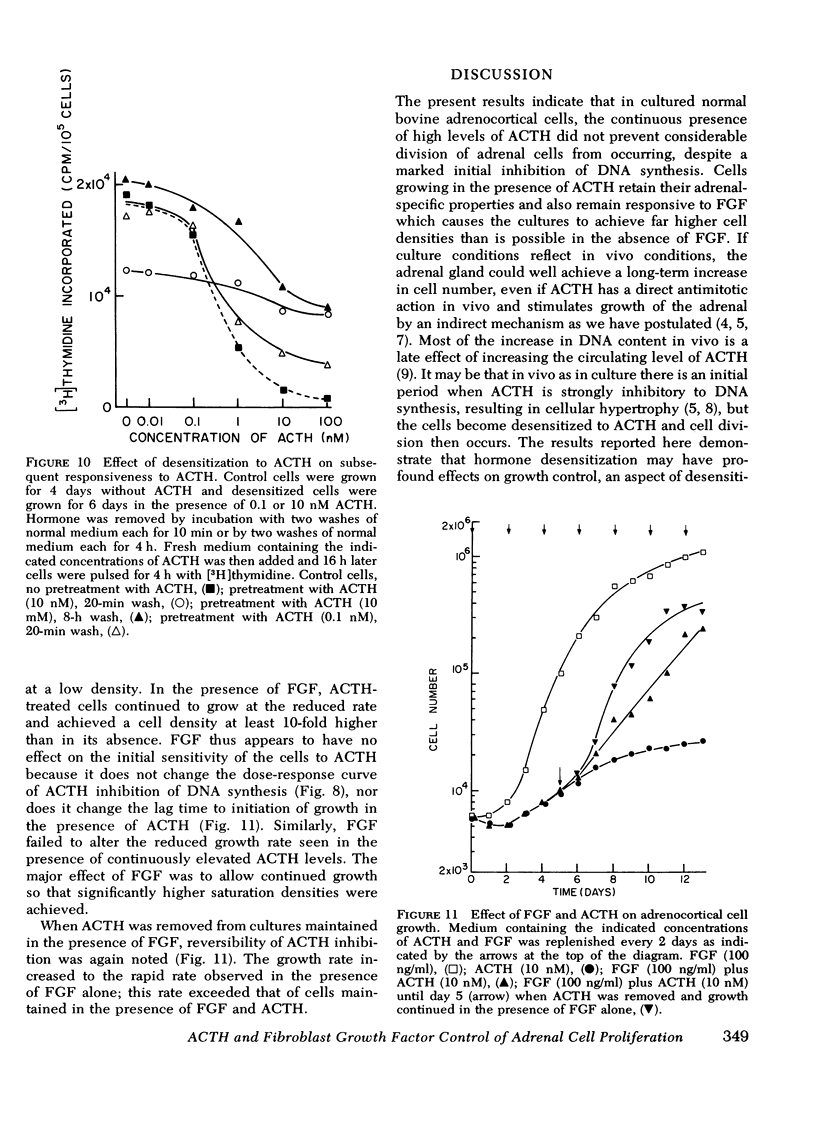



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURY H. P., CRANE W. A. EFFECT OF AGE AND HORMONAL STATE ON THE NUMBERS OF DEOXYRIBONUCLEIC ACID SYNTHESIZING NUCLEI IN RAT ADRENAL CORTEX. Nature. 1965 Jan 16;205:301–302. doi: 10.1038/205301a0. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- CATER D. B., STACK-DUNNE M. P. The effects of growth hormone and corticotrophin upon the adrenal weight and adrenocortical mitotic activity in the hypophysectomized rat. J Endocrinol. 1955 May;12(3):174–184. doi: 10.1677/joe.0.0120174. [DOI] [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L., Liddle G. W. Inhibition of adrenal corticosteroid synthesis by aminoglutethimide: studies of the mechanism of action. J Clin Endocrinol Metab. 1967 Apr;27(4):473–480. doi: 10.1210/jcem-27-4-473. [DOI] [PubMed] [Google Scholar]
- Engeland W. C., Dallman M. F. Compensatory adrenal growth is neurally mediated. Neuroendocrinology. 1975;19(4):352–362. doi: 10.1159/000122456. [DOI] [PubMed] [Google Scholar]
- Engeland W. C., Shinsako J., Dallman M. F. Corticosteroids and ACTH are not required for compensatory adrenal growth. Am J Physiol. 1975 Nov;229(5):1461–1464. doi: 10.1152/ajplegacy.1975.229.5.1461. [DOI] [PubMed] [Google Scholar]
- FARESE R. V., REDDY W. J. OBSERVATIONS ON THE INTERRELATIONS BETWEEN ADRENAL PROTEIN, RNA AND DNA DURING PROLONGED ACTH ADMINISTRATION. Biochim Biophys Acta. 1963 Sep 17;76:145–148. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Handley H. H. Stimulation of division of Y1 adrenal cells by a growth factor isolated from bovine pituitary glands. Endocrinology. 1975 Jul;97(1):102–107. doi: 10.1210/endo-97-1-102. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Hornsby P. J., Gill G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology. 1977 Apr;100(4):1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Haeuber H. D. Zur Regeneration des Nebennierenrindenparenchyms beim Meerschweinchen nach sog. 3/4-Resektion der Nebennierenrinde. Endokrinologie. 1965;48(5):255–265. [PubMed] [Google Scholar]
- Haksar A., Maudsley D. V., Péron F. G. Stimulation of cyclic adenosine 3':5'-monophosphate and corticosterone formation in isolated rat adrenal cells by cholera enterotoxin. Comparison with the effects of ACTH. Biochim Biophys Acta. 1975 Feb 13;381(2):308–323. doi: 10.1016/0304-4165(75)90237-8. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry. 1975 Aug 26;14(17):3845–3851. doi: 10.1021/bi00688a017. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. L., Gospodarowicz D. Biological activity of a growth factor for ovarian cells. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3372–3376. doi: 10.1073/pnas.71.9.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J. Adrenal cells in tissue culture. 3. Effect of adrenocorticotropin and 3',5'-cyclic adenosine monophosphate on 11 beta-hydroxylase and other steroidogenic enzymes. Biochemistry. 1969 May;8(5):1821–1831. doi: 10.1021/bi00833a007. [DOI] [PubMed] [Google Scholar]
- MACHEMER R., OEHLERT W. AUTORADIOGRAPHISCHE UNTERSUCHUNGEN UEBER DEN PHYSIOLOGISCHEN ZELLUMSATZ UND DIE GESTEIGERTE ZELLNEUBILDUNG DER NEBENNIERE DER AUSGEWACHSENEN RATTE NACH BEHANDLUNG MIT ACTH. Endokrinologie. 1964 Feb;46:77–91. [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui H., Garren L. D. On the mechanism of action of adrenocorticotropic hormone. Stimulation of deoxyribonucleic acid polymerase and thymidine kinase activities in adrenal glands. J Biol Chem. 1970 May 25;245(10):2627–2632. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Regulation of adenylate cyclase coupled beta-adrenergic receptors by beta-adrenergic catecholamines. Endocrinology. 1976 Aug;99(2):347–357. doi: 10.1210/endo-99-2-347. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson P. A. Quantitative study on the effect of an ACTH-producing pituitary tumor on the ultrastructure of the mouse adrenal gland. Am J Pathol. 1975 Aug;80(2):295–308. [PMC free article] [PubMed] [Google Scholar]
- O'Hare M. J. Monolayer cultures of normal adult rat adrenocortical cells: steroidogenic responses to nucleotides, bacterial toxins and antimicrotubular agents. Experientia. 1976 Feb 15;32(2):251–253. doi: 10.1007/BF01937794. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. Morphological responses to corticotrophin and cyclic AMP by adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Mar;56(3):529–536. doi: 10.1677/joe.0.0560529. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. Steroid metabolism by adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Sep;58(3):447–462. doi: 10.1677/joe.0.0580447. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. The steroidogenic response of adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Mar;56(3):537–549. doi: 10.1677/joe.0.0560537. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. M., Dazord A., Morera A. M., Bataille P. Interactions of adrenocorticotropic hormone with its adrenal receptors. Degradation of ACTH-1-24 and ACTH-11-24. J Biol Chem. 1975 Mar 10;250(5):1683–1689. [PubMed] [Google Scholar]
- Saruta T., Kaplan N. M. Adrenocortical steroidogenesis: the effects of prostaglandins. J Clin Invest. 1972 Sep;51(9):2246–2251. doi: 10.1172/JCI107033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig S., Sayers G. Isolated adrenal cortex cells: ACTH agonists, partial agonists, antagonists; cyclic AMP and corticosterone production. Arch Biochem Biophys. 1973 Jan;154(1):230–239. doi: 10.1016/0003-9861(73)90053-2. [DOI] [PubMed] [Google Scholar]
- Segal B. M., Christy N. P. Potentiation of the biologic activity of ACTH by human plasma. A preliminary study. J Clin Endocrinol Metab. 1968 Oct;28(10):1465–1472. doi: 10.1210/jcem-28-10-1465. [DOI] [PubMed] [Google Scholar]
- Silber R. H. Fluorimetric analysis of corticoids. Methods Biochem Anal. 1966;14:63–78. doi: 10.1002/9780470110324.ch3. [DOI] [PubMed] [Google Scholar]
- Weidman R. E., Gill G. N. Differential effects of ACTH or 8-Dr-cAMP on growth and replicationin a functional adrenal tumor cell line. J Cell Physiol. 1977 Jan;90(1):91–103. doi: 10.1002/jcp.1040900112. [DOI] [PubMed] [Google Scholar]




