Abstract
Objectives
To examine the effect of socioeconomic status (SES) on mortality in patients with bacteremia and the underlying factors that may mediate differences in mortality.
Methods
We conducted a population-based cohort study in two Danish regions. All patients 30 to 65 years of age with first time bacteremia from 2000 through 2008 were identified in a population-based microbiological bacteremia database (n = 8,653). Individual-level data on patients’ SES (educational level and personal income) and comorbid conditions were obtained from public and medical registries. We used Cox regression to examine mortality within 30 days after bacteremia with and without cumulative adjustment for potential mediators.
Results
Bacteremia patients of low SES were more likely to live alone and be unmarried than patients of high SES. They also had more pre-existing comorbidity, more substance abuse, more Staphylococcus aureus and nosocomial infections, and more admissions to small nonteaching hospitals. Overall, 1,374 patients (15.9%) died within 30 days of follow-up. Patients of low SES had consistently higher mortality after bacteremia than those of high SES crude hazard ratio for low vs. high education, 1.38 [95% confidence interval (CI), 1.18–1.61]; crude hazard ratio for low-income vs. high-income tertile, 1.58 [CI, 1.39–1.80]. Adjustment for differences in social support, pre-existing comorbidity, substance abuse, place of acquisition of the infection, and microbial agent substantially attenuated the effect of SES on mortality (adjusted hazard ratio for low vs. high education, 1.15 [95% CI, 0.98–1.36]; adjusted hazard ratio for low-income vs. high-income tertile, 1.29 [CI, 1.12–1.49]). Further adjustment for characteristics of the admitting hospital had minimal effect on observed mortality differences.
Conclusions
Low SES was strongly associated with increased 30-day mortality after bacteremia. Less social support, more pre-existing comorbidity, more substance abuse, and differences in place of acquisition and agent of infection appeared to mediate much of the observed disparities in mortality.
Introduction
Bacteremia is an increasingly prevalent and life-threatening condition with a reported 30-day mortality above 15% in studies from industrialized countries [1], [2]. In addition to increased risk of infections, low socioeconomic status (SES) may also worsen infection outcomes [3]–[5]. However, few studies have examined the association between SES and mortality after a severe infection, including after bacteremia.
A recent US study of patients hospitalized for sepsis adjusted for demographic factors and pre-existing comorbidity and found that, compared with married patients, widowed, single, and legally separated patients had greater odds of in-hospital death [6]. In another US study, Mendu et al. found an unadjusted relationship between neighborhood poverty rate and mortality within 1 year after bacteremia in patients admitted to intensive care units [7]. In contrast, in a population-based study from New Zealand, Huggan et al. found no relationship between an area-based measure of SES and mortality after Staphylococcus aureus bacteremia [8]. Thus, these previous studies have reached conflicting conclusions and used marital status as a proxy for SES or area-based measures of SES, with no data on detailed individual-level measures of SES. Moreover, none of them examined which prognostic factors may mediate socioeconomic disparities in mortality.
Compared with patients of higher SES, patients of lower SES tend to experience less social support, which may lead to more severe infection at admission and a more severe prognosis [6], [9], [10]. Several studies have documented the adverse impact of pre-existing comorbidity and conditions related to substance abuse on survival after bacteremia [11]–[13]. Furthermore, treatment in hospitals with high patient volume and teaching status may be associated with improved outcome and patients of high SES may have a better chance of being admitted to large university hospitals [14], [15].
To examine the effect of SES on mortality after bacteremia, we designed a population-based cohort study. We used two different individual-level indicators of SES, educational level and personal income, to capture different aspects of socioeconomic stratification. We further evaluated if differences in social support, pre-existing comorbidity, substance abuse, infection characteristics, and characteristics of the admitting hospital could explain socioeconomic differences in mortality after bacteremia.
Methods
Study Design
We conducted this study as a population-based cohort study. The geographic area included two Danish regions (North Denmark Region and Capital Region) with a total population of 1.7 million persons. All hospitalized patients aged 30 to 65 years with first time bacteremia from 2000 through 2008 were included in the cohort.
Setting
The Danish tax-funded welfare system provides free access to health care, education, and benefits such as pensions and unemployment coverage. All citizens are granted free services at general practitioners and public hospitals. Only 1% of hospital beds are in the private sector.
Since April 1, 1968, all citizens in Denmark have been registered in the Civil Registration System. A unique personal identification number (civil registration number) allows accurate linkage of information among national registries, including medical registries.
Identification of Patients with Bacteremia
We obtained data from the Danish Collaborative Bacteremia Network (DACOBAN). This network includes the Departments of Clinical Microbiology in the North Denmark Region (Aalborg Hospital) and the greater Copenhagen area (Hvidovre Hospital and Herlev Hospital). DACOBAN was established to enable coordinated surveillance of all cases of bacteremia in the two regions and to study risk factors and prognostic factors for bacteremia [16]. The three departments that serve these regions record data on all microbiological specimens, including blood cultures, in an electronic laboratory information system (ADBakt, Autonik, Ramsta, Sweden). A research database that consists of all patients with a first-time diagnosis of bacteremia between January 1, 2000 and December 31, 2008 has been established from data from these laboratory information systems. Bacteremia was defined as bacterial or fungal growth in blood cultures where contamination had been ruled out. Coagulase-negative staphylococci, Corynebacterium spp., Bacillus spp. and Propionibacterium acnes were regarded as contaminants unless they were isolated from two or more separate blood culture sets within a 5-day period [17].
We only included patients in the age group of 30 to 65 years because we assumed that most of them had completed their education and were of working age. The civil registration number, age, sex, the date on which the first positive blood culture was drawn (date of bacteremia diagnosis), hospital and specialty, microbial agent, and place of acquisition of infection (community-acquired or nosocomial) were included in the database for all patients. Bacteremia was defined as community-acquired if the first positive blood culture was obtained within 48 hours after hospital admission and as nosocomial if it was drawn more than 48 hours after hospital admission.
Socioeconomic Status
SES was based on patients’ educational level and personal income. Although the two indicators are related they measure different aspects of socioeconomic stratification. Formal education is normally completed in young adulthood and will therefore to some extent measure early life SES. In contrast, income can change over a life course, but may better capture aspects of SES later in life [18], [19]. To assess the effect of both early life SES and later life SES on mortality after bacteremia, we examined both SES indicators.
SES data were obtained for all patients through registries maintained by the government agency Statistics Denmark [20]–[22]. These registries contain detailed individual-level socioeconomic data, updated yearly, for all Danish citizens. Information on patients’ highest completed education was obtained from the Population’s Education Register, which consists of data generated from surveys and from the administrative records of educational institutions. In 2008 the register contained valid information on education for 97% of the Danish population born from 1945 to 1990 [20]. We categorized educational level into primary/lower secondary education (low), upper secondary education (medium), and tertiary education (high) according to the International Standard Classification of Education (1997) [23].
Patients’ personal annual income was all income subject to income taxation (wages and salaries, and all types of benefits and pensions). Income data was obtained from the Income Statistics Register. Data in the register are primarily supplied by tax authorities and the income data are assumed to equal the real income [21]. Personal annual income was adjusted for inflation according to the year 2000 value of the Danish crown (DKK) and was grouped into tertiles: low-income (1st tertile), middle-income (2nd tertile) and high-income (3rd tertile).
We also obtained data on patients’ employment status, nationality, cohabitation status, and marital status. Employment status was grouped into employed/self-employed, unemployed/employment subsidized by labor market arrangement and early retirement pensioners. We used cohabitation status and marital status as markers for social support. For all variables, we used data from the year preceding the index date of the bacteremia diagnosis.
Pre-existing Comorbidity
We obtained data from the Danish National Patient Registry for all diagnoses recorded from the start of the registry until the date of bacteremia diagnosis. The registry contains information for almost 100% of all inpatient admissions to public and private non-psychiatric hospitals in Denmark since 1977 and from outpatient and emergency room visits since 1995. Each record includes one primary diagnosis and up to 20 secondary diagnoses, which have been classified according to the International Classification of Diseases [24].
Pre-existing comorbidity was summarized according to the Charlson Comorbidity Index, which was originally developed to predict 1-year mortality in hospitalized medical patients [25]. Since then, the index has been adapted and validated for use with hospital diagnoses and has been used in previous studies of the association between comorbidity and survival after bacteremia [13], [26], [27]. The index consists of 19 disease groups and each disease group is assigned a specific weight depending on the severity of the pre-existing condition. Based on the Charlson index scores three levels of comorbidity were defined: 0 (low), corresponding to patients with no recorded pre-existing comorbidity; 1–2 (medium), and >2 (high).
Diagnoses related to substance abuse (alcohol and drug abuse) are not included in the Charlson index and may influence prognosis after bacteremia. Therefore, we also collected data on previous alcohol- and drug-abuse-related diagnoses from the National Patient Registry.
Hospital Characteristics
Patients were treated in 1 of 16 public hospitals. We characterized these hospitals according to number of hospital beds, hospital volume, and medical school affiliation. We categorized hospital beds, setup and staffed for use, as less than 300 beds or 300 beds or more. Hospital volume was defined as the annual number of bacteremia patients treated at the institution and categorized as low-volume (≤99 bacteremia patients treated per year), medium-volume (100–299 per year), and high-volume (≥300 per year) hospitals. Teaching hospitals were defined as hospitals directly affiliated with a medical school.
Follow-up and Mortality
Our outcome was all-cause mortality within 30 days after the bacteremia diagnosis. We obtained data on each patient’s vital status from the Danish Civil Registration System [28]. This registry contains daily updated information on all changes in vital status and migration for all Danish citizens. Patients were followed from the date of their diagnosis to the time of death, date of emigration, or completion of 30 days of follow-up, whichever occurred first.
Statistical Analysis
We first constructed contingency tables to provide information on baseline characteristics and crude outcomes according to SES, which were inferred on the basis of patients’ educational level and personal income. Using Kaplan-Meier plots we examined mortality within 30 days after bacteremia according to SES.
A Cox proportional hazards model was constructed to determine the association between SES and 30-day mortality. We performed a sequential cumulative adjustment analysis to assess whether differences in social support (cohabitation and marital status), pre-existing comorbidity (comorbidity included in the Charlson Comorbidity Index and conditions related to substance abuse), infection characteristics (place of acquisition, microbial agent, and admitting specialty) or hospital characteristics (number of hospital beds, hospital volume, and medical school affiliation) accounted for socioeconomic differences in mortality. We included the potential mediators in our analyses in a sequence that reflected the temporal relation of the potential mediators (e.g., we assumed that SES would normally precede comorbidity existing at the time of the bacteremia diagnosis).
Log-minus-log plots were used to confirm that the proportional hazards assumption was not violated [29]. All analyses were performed with Stata statistical software, version 11.2 (StataCorp, College Station, TX). The study was approved by the Danish Data Protection Agency (Record no. 2010-41-5650). Informed consent was not required by Danish law.
Results
Patient Characteristics
The cohort consisted of 8,653 hospitalized patients aged 30 to 65 years with a first time diagnosis of bacteremia, which corresponded to an incidence of 114 episodes per 100,000 person-years in our study population. The median age of the cohort was 55 years (interquartile range, 47 to 61), and 3,853 (44.5%) were women. Table 1 shows baseline characteristics according to educational level. Only small differences with respect to age and gender were seen. Patients with higher education were slightly younger. The medium-educated patients were more likely to be male. On average, patients with lower education were much less affluent than those with higher education (i.e., 44.7% vs. 17.4% in the lowest income tertile). They were more likely to live alone and be unmarried, and were substantially more likely to be out of the workforce (70.6%) than the patients with higher education (34.6%). Virtually all pre-existing comorbidities were more prevalent among patients with lower education. Only solid cancer, leukaemia, and lymphoma were more prevalent among those with a higher education. Similarly, the prevalence of conditions related to alcohol and drug abuse were substantially increased among those with lower education. Patients with lower education were also more likely to have Staphylococcus aureus bacteremia, to have a nosocomial infection, and to receive intensive care. In addition, patients with lower education were more likely to be admitted to small, low-volume, and nonteaching hospitals.
Table 1. Baseline characteristics of 8,382 patients with bacteremia, aged 30 to 65 years, categorized according to educational level.
| Educational level | ||||||
| Low | Medium | High | ||||
| Variable | (n = 3,457; 41.2%) | (n = 3,312; 39.5%) | (n = 1,613; 19.2%) | |||
| Demographic charateristic | ||||||
| Median age, y | 56 | 56 | 54 | |||
| Men, n (%) | 1,770 | (51.2) | 2,041 | (61.6) | 841 | (52.1) |
| Nationality, n (%) | ||||||
| Danish | 3,187 | (92.2) | 3,057 | (92.3) | 1,448 | (89.8) |
| Immigrants from Western countries | 72 | (2.1) | 102 | (3.1) | 67 | (4.2) |
| Immigrants from non-Western countries | 198 | (5.7) | 153 | (4.6) | 98 | (6.1) |
| Socioeconomic indicators, n (%) | ||||||
| Income category | ||||||
| Low (1st tertile) | 1,544 | (44.7) | 965 | (29.1) | 281 | (17.4) |
| Middle (2nd tertile) | 1,309 | (37.9) | 1,145 | (34.6) | 335 | (20.8) |
| High (3rd tertile) | 600 | (17.4) | 1,194 | (36.1) | 995 | (61.7) |
| Data missing | 4 | (0.1) | 8 | (0.2) | 2 | (0.1) |
| Employment | ||||||
| Employed/self-employed | 1,007 | (29.1) | 1,693 | (51.1) | 1,049 | (65.0) |
| Unemployed/labor market arrangement | 910 | (26.3) | 793 | (23.9) | 309 | (19.2) |
| Early retirement pension | 1,530 | (44.3) | 812 | (24.5) | 249 | (15.4) |
| Data missing | 10 | (0.3) | 14 | (0.4) | 6 | (0.4) |
| Social support, n (%) | ||||||
| Living alone | ||||||
| Yes | 1,788 | (51.7) | 1,325 | (40.0) | 581 | (36.0) |
| No | 1,669 | (48.3) | 1,987 | (60.0) | 1,032 | (64.0) |
| Marital status | ||||||
| Married | 1,458 | (42.2) | 1,826 | (55.1) | 971 | (60.2) |
| Divorced or widowed | 1,006 | (29.1) | 833 | (25.2) | 317 | (19.7) |
| Never married | 983 | (28.4) | 639 | (19.3) | 319 | (19.8) |
| Data missing | 10 | (0.3) | 14 | (0.4) | 6 | (0.4) |
| Pre-existing comorbidity, n (%) | ||||||
| Clinical conditions included in the CCI | ||||||
| Previous myocardial infarction | 207 | (6.0) | 185 | (5.6) | 64 | (4.0) |
| Congestive cardiac insufficiency | 213 | (6.2) | 157 | (4.7) | 53 | (3.3) |
| Peripheral vascular disease | 236 | (6.8) | 192 | (5.8) | 62 | (3.8) |
| Cerebrovascular disease | 332 | (9.6) | 330 | (10.0) | 113 | (7.0) |
| Dementia | 36 | (1.0) | 44 | (1.3) | 13 | (0.8) |
| Hemiplegia | 49 | (1.4) | 28 | (0.9) | 11 | (0.7) |
| Chronic pulmonary disease | 475 | (13.7) | 297 | (9.0) | 98 | (6.1) |
| Connective tissue disease | 146 | (4.2) | 142 | (4.3) | 58 | (3.6) |
| Peptic ulcer disease | 450 | (13.0) | 333 | (10.1) | 81 | (5.0) |
| Mild liver disease | 454 | (13.1) | 364 | (11.0) | 99 | (6.1) |
| Moderate or severe liver disease | 212 | (6.1) | 166 | (5.0) | 53 | (3.3) |
| Diabetes, without complications | 488 | (14.1) | 398 | (12.0) | 140 | (8.7) |
| Diabetes with complications | 311 | (9.0) | 248 | (7.5) | 86 | (5.3) |
| Moderate or severe kidney disease | 264 | (7.6) | 282 | (8.5) | 91 | (5.6) |
| Solid cancer | 590 | (17.1) | 628 | (19.0) | 312 | (19.3) |
| Metastatic solid cancer | 135 | (3.9) | 171 | (5.2) | 97 | (6.0) |
| Leukemia | 47 | (1.4) | 74 | (2.2) | 37 | (2.3) |
| Lymphoma | 102 | (3.0) | 160 | (4.8) | 98 | (6.1) |
| HIV/AIDS | 67 | (1.9) | 24 | (0.7) | 6 | (0.4) |
| Charlson comorbidity index score | ||||||
| Low (0) | 1,103 | (31.9) | 1,182 | (35.7) | 733 | (45.4) |
| Medium (1–2) | 1,291 | (37.3) | 1,182 | (35.7) | 507 | (31.4) |
| High (>2) | 1,063 | (30.8) | 948 | (28.6) | 373 | (23.1) |
| Substance abuse, n (%) | ||||||
| Alcohol abuse | 719 | (20.8) | 624 | (18.8) | 192 | (11.9) |
| Drug abuse | 344 | (10.0) | 115 | (3.5) | 38 | (2.4) |
| Characteristics of infection, n (%) | ||||||
| Microbial agent | ||||||
| Staphylococcus aureus | 565 | (16.3) | 546 | (16.5) | 226 | (14.0) |
| Streptococcus pneumoniae | 394 | (11.4) | 375 | (11.3) | 190 | (11.8) |
| Other gram-positive organisms | 645 | (18.7) | 641 | (19.4) | 356 | (22.1) |
| Escherichia coli | 866 | (25.1) | 803 | (24.3) | 411 | (25.5) |
| Other enterobacteria | 1,302 | (37.7) | 1,206 | (36.4) | 609 | (37.8) |
| Other gram-negative organisms | 268 | (7.8) | 295 | (8.9) | 126 | (7.8) |
| Polymicrobial or fungal | 396 | (11.5) | 384 | (11.6) | 172 | (10.7) |
| Acquisition | ||||||
| Community-acquired | 2,175 | (62.9) | 2,089 | (63.1) | 1,054 | (65.3) |
| Nosocomial | 1,266 | (36.6) | 1,207 | (36.4) | 550 | (34.1) |
| Data missing | 16 | (0.5) | 16 | (0.5) | 9 | (0.6) |
| Specialty | ||||||
| Internal medicine | 2,269 | (65.6) | 2,110 | (63.7) | 996 | (61.8) |
| Surgery | 847 | (24.5) | 899 | (27.1) | 495 | (30.7) |
| Intensive care | 327 | (9.5) | 286 | (8.6) | 108 | (6.7) |
| Data missing | 14 | (0.4) | 17 | (0.5) | 14 | (0.9) |
| Hospital characteristics, n (%) | ||||||
| Bed size | ||||||
| Low (<300 beds) | 725 | (21.0) | 601 | (18.2) | 242 | (15.0) |
| High (>300 beds) | 2,732 | (79.0) | 2,711 | (81.9) | 1,371 | (85.0) |
| Hospital volume a | ||||||
| Low (≤99/year) | 678 | (19.6) | 561 | (16.9) | 271 | (16.8) |
| Medium (100–299/year) | 517 | (15.0) | 492 | (14.9) | 190 | (11.8) |
| High (≥300/year) | 2,262 | (65.4) | 2,259 | (68.2) | 1,152 | (71.4) |
| Teaching hospital b | ||||||
| No | 497 | (14.4) | 404 | (12.2) | 177 | (11.0) |
| Yes | 2,960 | (85.6) | 2,908 | (87.8) | 1,436 | (89.0) |
| Mortality, n (%) | ||||||
| 30-day | 602 | (17.4) | 529 | (16.0) | 209 | (13.0) |
Abbreviation: CCI, Charlson comorbidity index.
Hospital volume was defined as the annual number of bacteremia patients treated at the institution.
Teaching hospitals were defined as hospitals directly affiliated with a medical school.
A similar but more extreme pattern of differences was seen for income categories: bacteremia patients with low versus high income were 1.5 times more likely to live alone and be unmarried, had a 1.5 to 4 times higher prevalence of many comorbidities, and had a more than 4-fold higher risk of substance abuse (Table 2).
Table 2. Baseline characteristics of 8,633 patients with bacteremia, aged 30 to 65 years, categorized according to income.
| Income category | ||||||
| Low | Middle | High | ||||
| Variable | (1st tertile; n = 2,878) | (2nd tertile; n = 2,878) | (3rd tertile; n = 2,877) | |||
| Demographic charateristic | ||||||
| Median age, y | 55 | 55 | 55 | |||
| Men, n (%) | 1,450 | (50.4) | 1,481 | (51.5) | 1,858 | (64.6) |
| Nationality, n (%) | ||||||
| Danish | 2,524 | (87.7) | 2,633 | (91.5) | 2,713 | (94.3) |
| Immigrants from Western countries | 109 | (3.8) | 78 | (2.7) | 84 | (2.9) |
| Immigrants from non-Western countries | 245 | (8.5) | 167 | (5.8) | 80 | (2.8) |
| Socioeconomic indicators, n (%) | ||||||
| Educational level | ||||||
| Low | 1,515 | (52.6) | 1,320 | (45.9) | 618 | (21.5) |
| Medium | 943 | (32.8) | 1,138 | (39.5) | 1,223 | (42.5) |
| High | 274 | (9.5) | 337 | (11.7) | 1,000 | (34.8) |
| Data missing | 146 | (5.1) | 83 | (2.9) | 36 | (1.3) |
| Employment | ||||||
| Employed/self-employed | 412 | (14.3) | 1,023 | (35.6) | 2,367 | (82.3) |
| Unemployed/labor market arrangement | 843 | (29.3) | 995 | (34.6) | 260 | (9.0) |
| Early retirement pension | 1,605 | (55.8) | 858 | (29.8) | 248 | (8.6) |
| Data missing | 18 | (0.6) | 2 | (0.1) | 2 | (0.1) |
| Social support, n (%) | ||||||
| Living alone | ||||||
| Yes | 1,513 | (52.6) | 1,428 | (49.6) | 923 | (32.1) |
| No | 1,365 | (47.4) | 1,450 | (50.4) | 1,954 | (67.9) |
| Marital status | ||||||
| Married | 1,239 | (43.1) | 1,315 | (45.7) | 1,789 | (62.2) |
| Divorced or widowed | 883 | (30.7) | 775 | (26.9) | 578 | (20.1) |
| Never married | 738 | (25.6) | 786 | (27.3) | 508 | (17.7) |
| Data missing | 18 | (0.6) | 2 | (0.1) | 2 | (0.1) |
| Pre-existing comorbidity, n (%) | ||||||
| Clinical conditions included in the CCI | ||||||
| Previous myocardial infarction | 170 | (5.9) | 161 | (5.6) | 135 | (4.7) |
| Congestive cardiac insufficiency | 167 | (5.8) | 157 | (5.5) | 115 | (4.0) |
| Peripheral vascular disease | 207 | (7.2) | 178 | (6.2) | 117 | (4.1) |
| Cerebrovascular disease | 310 | (10.8) | 284 | (9.9) | 207 | (7.2) |
| Dementia | 40 | (1.4) | 39 | (1.4) | 14 | (0.5) |
| Hemiplegia | 21 | (0.7) | 48 | (1.7) | 24 | (0.8) |
| Chronic pulmonary disease | 397 | (13.8) | 328 | (11.4) | 175 | (6.1) |
| Connective tissue disease | 138 | (4.8) | 128 | (4.5) | 93 | (3.2) |
| Peptic ulcer disease | 406 | (14.1) | 323 | (11.2) | 167 | (5.8) |
| Mild liver disease | 498 | (17.3) | 321 | (11.2) | 135 | (4.7) |
| Moderate or severe liver disease | 227 | (7.9) | 160 | (5.6) | 61 | (2.1) |
| Diabetes, without complications | 436 | (15.2) | 378 | (13.1) | 238 | (8.3) |
| Diabetes with complications | 285 | (9.9) | 238 | (8.3) | 136 | (4.7) |
| Moderate or severe kidney disease | 235 | (8.2) | 238 | (8.3) | 180 | (6.3) |
| Solid cancer | 430 | (14.9) | 500 | (17.4) | 624 | (21.7) |
| Metastatic solid cancer | 100 | (3.5) | 138 | (4.8) | 169 | (5.9) |
| Leukemia | 40 | (1.4) | 55 | (1.9) | 66 | (2.3) |
| Lymphoma | 70 | (2.4) | 114 | (4.0) | 177 | (6.2) |
| HIV/AIDS | 62 | (2.2) | 32 | (1.1) | 13 | (0.5) |
| Charlson comorbidity index score | ||||||
| Low (0) | 872 | (30.3) | 981 | (34.1) | 1,259 | (43.8) |
| Medium (1–2) | 1,064 | (37.0) | 1,035 | (36.0) | 976 | (33.9) |
| High (>2) | 942 | (32.7) | 862 | (30.0) | 642 | (22.3) |
| Substance abuse, n (%) | ||||||
| Alcohol abuse | 829 | (28.8) | 526 | (18.3) | 235 | (8.2) |
| Drug abuse | 338 | (11.7) | 148 | (5.1) | 45 | (1.6) |
| Characteristics of infection, n (%) | ||||||
| Microbial agent | ||||||
| Staphylococcus aureus | 513 | (17.8) | 463 | (16.1) | 409 | (14.2) |
| Streptococcus pneumoniae | 308 | (10.7) | 295 | (10.3) | 384 | (13.4) |
| Other gram-positive organisms | 556 | (19.3) | 547 | (19.0) | 582 | (20.2) |
| Escherichia coli | 694 | (24.1) | 749 | (26.0) | 693 | (24.1) |
| Other enterobacteria | 1,031 | (35.8) | 1,108 | (38.5) | 1,066 | (37.1) |
| Other gram-negative organisms | 223 | (7.8) | 253 | (8.8) | 230 | (8.0) |
| Polymicrobial or fungal | 345 | (12.0) | 318 | (11.1) | 322 | (11.2) |
| Acquisition | ||||||
| Community-acquired | 1,766 | (61.4) | 1,831 | (63.6) | 1,880 | (65.4) |
| Nosocomial | 1,096 | (38.1) | 1,036 | (36.0) | 983 | (34.2) |
| Data missing | 16 | (0.6) | 11 | (0.4) | 14 | (0.5) |
| Specialty | ||||||
| Internal medicine | 1,857 | (64.5) | 1,862 | (64.7) | 1,830 | (63.6) |
| Surgery | 719 | (25.0) | 745 | (25.9) | 835 | (29.0) |
| Intensive care | 289 | (10.0) | 254 | (8.8) | 196 | (6.8) |
| Data missing | 13 | (0.5) | 17 | (0.6) | 16 | (0.6) |
| Hospital characteristics, n (%) | ||||||
| Bed size | ||||||
| Low (<300 beds) | 575 | (20.0) | 607 | (21.1) | 443 | (15.4) |
| High (>300 beds) | 2,303 | (80.0) | 2,271 | (78.9) | 2,434 | (84.6) |
| Hospital volume a | ||||||
| Low (≤99/year) | 552 | (19.2) | 559 | (19.4) | 457 | (15.9) |
| Medium (100–299/year) | 417 | (14.5) | 434 | (15.1) | 383 | (13.3) |
| High (≥300/year) | 1,909 | (66.3) | 1,885 | (65.5) | 2,037 | (70.8) |
| Teaching hospital b | ||||||
| No | 377 | (13.1) | 437 | (15.2) | 291 | (10.1) |
| Yes | 2,501 | (86.9) | 2,441 | (84.8) | 2,586 | (89.9) |
| Mortality, n (%) | ||||||
| 30-day | 566 | (19.7) | 433 | (15.1) | 371 | (12.9) |
Abbreviation: CCI, Charlson comorbidity index.
Hospital volume was defined as the annual number of bacteremia patients treated at the institution.
Teaching hospitals were defined as hospitals directly affiliated with a medical school.
Mortality
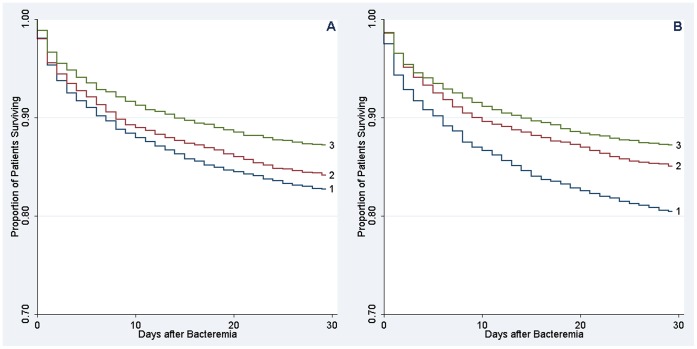
Overall, 1,374 patients (15.9%) died within 30 days of follow-up. There was a substantial gradient in mortality according to both educational level and income categories. Survival curves for the different levels of education and income diverged early after the bacteremia diagnosis and the differences in mortality persisted throughout the 30 days of follow-up (Figure 1). The 30-day mortality among the lower educated patients was 17.4% compared with 13.0% for those with higher education, which was an absolute difference in 30-day mortality of 4.5% [95% confidence interval (CI): 2.4–6.5%] and corresponded to a crude hazard ratio of 1.38 [95% CI: 1.18–1.61]. The difference in 30-day mortality was 6.7% [95% CI: 4.8–8.6%] when patients in the low-income tertile (30-day mortality, 19.7%) were compared with patients in the high-income tertile (30-day mortality, 12.9%). This corresponded to a crude hazard ratio of 1.58 [95% CI: 1.39–1.80].
Figure 1. Crude Kaplan-Meier survival curves according to socioeconomic status.
A) Educational level (low 1, medium 2, high 3), B) Income (low 1, middle 2, high 3).
Sequential adjustment for social support, pre-existing comorbidity, substance abuse, and infection characteristics attenuated the effect of SES on mortality (Table 3 and 4). Further adjustment for differences in characteristics of the admitting hospital had only a marginal impact on the adjusted 30-day mortality hazard ratios. The fully adjusted risk estimates showed that there was still a residual difference in mortality according to both educational level (low vs. high education, 1.14 [95% CI: 0.97–1.35] and income categories (low vs. high income, 1.30 [95% CI: 1.13–1.49] after adjustment for a range of known prognostic factors.
Table 3. 30-day mortality risk after first time diagnosis of bacteremia according to educational level and effect of adjustment for social support, pre-existing comorbidity, substance abuse, characteristics of infection, and hospital characteristics.
| Educational level | |||
| Low | Medium | High | |
| Unadjusted | 1.38 (1.18–1.61) | 1.25 (1.07–1.47) | 1.00 (reference) |
| Adjusted | |||
| Demographiccharacteristicsa | 1.33 (1.14–1.56) | 1.17 (0.99–1.37) | 1.00 (reference) |
| + social supportb | 1.27 (1.08–1.49) | 1.15 (0.98–1.35) | 1.00 (reference) |
| + pre-existingcomorbidityc | 1.20 (1.02–1.41) | 1.08 (0.92–1.28) | 1.00 (reference) |
| + characteristics ofinfectiond | 1.15 (0.98–1.36) | 1.04 (0.88–1.22) | 1.00 (reference) |
| + hospitalcharacteristicse | 1.14 (0.97–1.35) | 1.03 (0.88–1.22) | 1.00 (reference) |
Age, sex, and nationality.
Cohabitation and marital status.
Comorbidities included in the Charlson comorbidity index and conditions related to substance abuse.
Microbial agent, place of acquisition, and admitting specialty.
Number of hospital beds, hospital volume, and medical school affiliation.
Table 4. 30-day mortality risk after first time diagnosis of bacteremia according to income and effect of adjustment for social support, pre-existing comorbidity, substance abuse, characteristics of infection, and hospital characteristics.
| Income Category | |||
| Low (1st tertile) | Middle (2nd tertile) | High (3rd tertile) | |
| Unadjusted | 1.58 (1.39–1.80) | 1.18 (1.02–1.35) | 1.00 (reference) |
| Adjusted | |||
| Demographic characteristicsa | 1.69 (1.48–1.93) | 1.22 (1.07–1.41) | 1.00 (reference) |
| + social supportb | 1.58 (1.38–1.81) | 1.16 (1.01–1.33) | 1.00 (reference) |
| + pre-existing comorbidityc | 1.37 (1.19–1.57) | 1.08 (0.92–1.22) | 1.00 (reference) |
| + characteristics of infectiond | 1.29 (1.12–1.49) | 1.03 (0.89–1.18) | 1.00 (reference) |
| + hospital characteristicse | 1.30 (1.13–1.49) | 1.03 (0.89–1.19) | 1.00 (reference) |
Age, sex, and nationality.
Cohabitation and marital status.
Comorbidities included in the Charlson comorbidity index and conditions related to substance abuse.
Microbial agent, place of acquisition, and admitting specialty.
Number of hospital beds, hospital volume, and medical school affiliation.
To examine whether income mediated the effect of education, we also included income as a covariate in our statistical model examining the effect of education on mortality. The effect of education on mortality after bacteremia was further attenuated after inclusion of income as a covariate (low vs. high education, 1.08 [95% CI: 0.91–1.28]), showing that income in part mediated the effect of education.
Discussion
In this population-based cohort study we found that patients of lower SES, inferred on the basis of educational level and income, had higher 30-day mortality after bacteremia than those of higher SES. The association between SES and mortality was most pronounced when we used income as SES indicator. More than half of the socioeconomic differences in mortality could be explained by differences in social support, pre-existing comorbidity, alcohol and drug abuse, and characteristics of the infection. In contrast, characteristics of the admitting hospital had only a marginal explanatory effect on the socioeconomic mortality differences. Our findings thus suggest that socioeconomic disparities in mortality after bacteremia are to a large extent explained by a range of adverse prognostic factors that are present before hospital admission and include severe pre-existing comorbidity, unhealthy lifestyle, and lack of social support.
For the clinician it is important to know if certain groups of patients with severe infection have a poorer prognosis than others and our study therefore has clinical implications. Worse prognosis among bacteremia patients of lower SES imply that these patients would benefit from increased clinical attention. Our findings of a much higher prevalence of comorbidities among bacteremia patients of lower SES suggest that special attention should be paid to improved management of these patients’ comorbidities, which may reduce their excess mortality risk.
We are aware of only three studies that have examined socioeconomic disparities in mortality in cohorts of patients with sepsis or bacteremia. Seymour et al. conducted a population-based cohort study of 37,524 hospitalizations for sepsis in New Jersey, US [6]. After adjusting for demographic factors and comorbid conditions, they found that single and divorced men and single women had greater odds of in-hospital mortality than married persons. In contrast to our study, this study used marital status as a proxy for social factors and did not include data on more precise measures of SES, such as educational level and income. Furthermore, identification of patients with infections was based on administrative ICD-9-CM codes and the infections were not microbiologically verified. Mendu et al. analyzed data from 2,435 patients with bacteremia who were admitted to intensive care units at two hospitals in Boston, Massachusetts, US [7]. This study reported an unadjusted ‘dose-response’ relationship between neighborhood poverty rate and mortality within one year after bacteremia among patients receiving critical care. Adjustment for demographic factors, patient type, comorbidity, laboratory data, and severity of illness attenuated this association substantially and the authors concluded that neighborhood poverty was not associated with mortality after bacteremia. However, their use of an aggregate area-based measure of SES may have led to some inaccuracy due to misclassification of individual SES. A third study by Huggan et al. included 779 patients with Staphylococcus aureus bacteremia admitted to hospitals in Canterbury, New Zealand [8]. The authors reported that there was no relationship between an address-based measure of deprivation and mortality but did not present any estimates. This study also used an area-based measure of SES, which may have resulted in misclassification of individual SES [30].
In contrast with previous studies we used a sequential cumulative adjustment analysis to evaluate a range of recognized prognostic factors as potential mediators of the socioeconomic mortality differences. Even after full adjustment for all the potential mediators included in our study, we still found a residual difference in mortality. It is likely that the residual difference may be explained partly by greater levels of undiagnosed comorbidity among patients of lower SES, which again may be due to poor self-care and late presentation of clinical disease. However, we speculate that unmeasured variation in severity of infection at admission may also explain some of the residual mortality differences. Furthermore, since we did not have precise data on bacteremia patients’ care and treatment, we cannot rule out that difference in the quality of care contributed to the residual mortality differences.
Our study has several important limitations. First, we used information collected during routine clinical work and data from administrative registries, which limited clinical detail in our study. More information on clinical parameters would have enabled us to better characterize severity of the infection and to assess any differences in severity according to SES. On the other hand, the use of routinely collected microbiological data and accurate linkage of high-quality registries enabled us to avoid some major methodological problems, such as selection and surveillance bias. Use of prospectively recorded individual data on SES indicators, which were collected independently, also reduced misclassification of patients SES. Second, our outcome measure was all-cause mortality, and we did not have any data on other important outcomes, such as recovery and functional status. When interpreting all-cause mortality, it must be considered that patients may have died from causes unrelated to their infection. Nevertheless, by including only deaths that occurred within 30 days after the date of bacteremia diagnosis we assume that most deaths would be at least to some extent related to the infection. Third, even though we studied a large cohort of bacteremia patients statistical precision was still limited. Fourth, we included bacteremia patients with a microbiologically confirmed infection, which allowed us to assess the mediating effect of the microbial agent on the association between SES and mortality. We can assume that the vast majority of the bacteremia patients in our cohort fulfilled the criteria for sepsis [31], [32]. However, since less than 50% of patients with sepsis have documented bacteremia, our findings cannot necessarily be generalised to sepsis patients [33], [34].
In conclusion, we found that patients of low SES had higher mortality within 30 days after bacteremia than those of high SES. Differences in social support, pre-existing comorbidity, substance abuse, and characteristics of the infection were important explanatory factors for these SES-mortality gradients. In contrast, characteristics of the admitting hospital seemed to have a negligible role in explaining disparities in mortality. The residual impact of SES on mortality might be explained by differences in bacteremia severity and treatment of the infection. Future studies should assess the importance of potential differences in severity of illness and quality of care in explaining socioeconomic mortality differences after bacteremia.
Acknowledgments
Contributing members of DACOBAN
Christian Østergaard Andersen (Department of Clinical Microbiology, Copenhagen University Hospital, Hvidovre Hospital, Copenhagen, Denmark), Magnus Arpi (Department of Clinical Microbiology, Copenhagen University Hospital, Herlev Hospital, Copenhagen, Denmark), Kim Oren Gradel (Center for National Clinical Databases - South, Odense University Hospital, Odense, Denmark), Ulrich Stab Jensen (Department of Clinical Microbiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark), Jenny Dahl Knudsen (Department of Clinical Microbiology, Copenhagen University Hospital, Hvidovre Hospital, Copenhagen, Denmark), Kristoffer Koch (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark), Mette Pinholt (Department of Clinical Microbiology, Copenhagen University Hospital, Herlev Hospital, Copenhagen, Denmark), Henrik Carl Schønheyder (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark), Mette Søgaard (Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark).
Funding Statement
This study received financial support from the Institute of Clinical Medicine, Aarhus University Hospital, Denmark. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Søgaard M, Nørgaard M, Dethlefsen C, Schønheyder HC (2011) Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clin Infect Dis 52: 61–69. [DOI] [PubMed] [Google Scholar]
- 2. Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, St Sauver JL, et al. (2007) Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 167: 834–839. [DOI] [PubMed] [Google Scholar]
- 3. Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, et al. (2012) Chronic medical conditions and risk of sepsis. PLoS One 7: e48307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stelianides S, Golmard JL, Carbon C, Fantin B (1999) Influence of socioeconomic status on features and outcome of community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 18: 704–708. [DOI] [PubMed] [Google Scholar]
- 5. Burton DC, Flannery B, Bennett NM, Farley MM, Gershman K, et al. (2010) Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health 100: 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seymour CW, Iwashyna TJ, Cooke CR, Hough CL, Martin GS (2010) Marital status and the epidemiology and outcomes of sepsis. Chest 137: 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mendu ML, Zager S, Gibbons FK, Christopher KB (2012) Relationship between neighborhood poverty rate and bloodstream infections in the critically ill. Crit Care Med 40: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 8. Huggan PJ, Wells JE, Browne M, Richardson A, Murdoch DR, et al. (2010) Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern Med J 40: 117–125. [DOI] [PubMed] [Google Scholar]
- 9. Iwashyna TJ, Christakis NA (2003) Marriage, widowhood, and health-care use. Soc Sci Med 57: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 10. Gordon HS, Rosenthal GE (1995) Impact of marital status on outcomes in hospitalized patients. Evidence from an academic medical center. Arch Intern Med 155: 2465–2471. [PubMed] [Google Scholar]
- 11. Pittet D, Thievent B, Wenzel RP, Li N, Gurman G, et al. (1993) Importance of pre-existing co-morbidities for prognosis of septicemia in critically ill patients. Intensive Care Med 19: 265–272. [DOI] [PubMed] [Google Scholar]
- 12. Laupland KB, Gregson DB, Zygun DA, Doig CJ, Mortis G, et al. (2004) Severe bloodstream infections: a population-based assessment. Crit Care Med 32: 992–997. [DOI] [PubMed] [Google Scholar]
- 13. Søgaard M, Schønheyder HC, Riis A, Sørensen HT, Nørgaard M (2008) Short-term mortality in relation to age and comorbidity in older adults with community-acquired bacteremia: a population-based cohort study. J Am Geriatr Soc 56: 1593–1600. [DOI] [PubMed] [Google Scholar]
- 14. Peelen L, de Keizer NF, Peek N, Scheffer GJ, van der Voort PH, et al. (2007) The influence of volume and intensive care unit organization on hospital mortality in patients admitted with severe sepsis: a retrospective multicentre cohort study. Crit Care 11: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahin J, Harrison DA, Rowan KM (2012) Relation between volume and outcome for patients with severe sepsis in United Kingdom: retrospective cohort study. BMJ 344: e3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gradel KO, Knudsen JD, Arpi M, Østergaard C, Schønheyder HC, et al. (2012) Classification of positive blood cultures: computer algorithms versus physicians’ assessment - development of tools for surveillance of bloodstream infection prognosis using population-based laboratory databases. BMC Med Res Methodol 12: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trick WE, Zagorski BM, Tokars JI, Vernon MO, Welbel SF, et al. (2004) Computer algorithms to detect bloodstream infections. Emerg Infect Dis 10: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey SG (2006) Indicators of socioeconomic position (part 1). J Epidemiol Community Health 60: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geyer S, Hemstrom O, Peter R, Vagero D (2006) Education, income, and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against a common practice. J Epidemiol Community Health 60: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen VM, Rasmussen AW (2011) Danish Education Registers. Scand J Public Health 39: 91–94. [DOI] [PubMed] [Google Scholar]
- 21. Baadsgaard M, Quitzau J (2011) Danish registers on personal income and transfer payments. Scand J Public Health 39: 103–105. [DOI] [PubMed] [Google Scholar]
- 22. Petersson F, Baadsgaard M, Thygesen LC (2011) Danish registers on personal labour market affiliation. Scand J Public Health 39: 95–98. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Educational, Scientific and Cultural Organization (UNESCO) (1997) International Standard Classification of Education. Available: http://www.uis.unesco.org/Library/Pages/DocumentMorePage.aspx?docIdValue=144&docIdFld=ID. Accessed: 2012 Dec 22.
- 24. Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH (1999) The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 46: 263–268. [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 26. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT (2011) The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM (2003) How to measure comorbidity. a critical review of available methods. J Clin Epidemiol 56: 221–229. [DOI] [PubMed] [Google Scholar]
- 28. Pedersen CB (2011) The Danish Civil Registration System. Scand J Public Health 39: 22–25. [DOI] [PubMed] [Google Scholar]
- 29. Bewick V, Cheek L, Ball J (2004) Statistics review 12: survival analysis. Crit Care 8: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLoone P, Ellaway A (1999) Postcodes don’t indicate individuals’ social class. BMJ 319: 1003–1004. [PMC free article] [PubMed] [Google Scholar]
- 31. Madsen KM, Schønheyder HC, Kristensen B, Nielsen GL, Sørensen HT (1998) Can hospital discharge diagnosis be used for surveillance of bacteremia? A data quality study of a Danish hospital discharge registry. Infect Control Hosp Epidemiol 19: 175–180. [PubMed] [Google Scholar]
- 32. Christensen JS, Jensen TG, Kolmos HJ, Pedersen C, Lassen A (2012) Bacteremia with Streptococcus pneumoniae: sepsis and other risk factors for 30-day mortality–a hospital-based cohort study. Eur J Clin Microbiol Infect Dis 31: 2719–2725. [DOI] [PubMed] [Google Scholar]
- 33. Bates DW, Sands K, Miller E, Lanken PN, Hibberd PL, et al. (1997) Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis 176: 1538–1551. [DOI] [PubMed] [Google Scholar]
- 34. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, et al. (1995) The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273: 117–123. [PubMed] [Google Scholar]