Abstract
Background
The US prevalence of reduced estimated glomerular filtration rate (eGFR) based on serum creatinine increased over the decade ending in 2002. National Health and Nutrition Examination Survey (NHANES) cystatin C measurements were recently calibrated to the international standard, allowing for an independent test of the trend in prevalence of reduced eGFR using cystatin C.
Study Design
Cross-sectional surveys performed during two periods.
Setting & Participants
Nationally representative subsamples of adult participants from NHANES III (1988–1994) and the NHANES 1999–2002 surveys.
Predictor
Survey period.
Outcomes
Prevalence of reduced GFR, defined as eGFR<60ml/min/1.73m2 based on serum creatinine, cystatin C, or both (eGFRcr, eGFRcys, eGFRcr-cys), using estimating equations developed by the Chronic Kidney Disease Epidemiology Collaboration (CKDEPI).
Measurements
Serum cystatin C, measured from stored samples in 2006, calibrated to the international standard in 2012.
Results
Between 1988–1994 and 1999–2002, the prevalence of reduced eGFRcr, eGFRcys and eGFRcr-cys increased from 4.7% (95% CI, 4.1%–5.3%) to 6.5% (95% CI, 5.9%–7.1%; p<0.001), from 5.5% (95% CI, 4.6%–6.5%) to 8.7% (95% CI, 7.5%–10.0%; p<0.001), and from 4.4% (95% CI, 3.7%–5.2%) to 7.1% (95% CI, 6.2%–8.0%; p<0.001), respectively. The higher prevalence of reduced GFR in the later period was observed in all subgroups of age, race, sex, and GFR categories. After adjusting for changes in the US population by age, sex, race, diabetes, hypertension, and body mass index, the prevalence ratio of reduced GFR in the later versus earlier survey was 1.24 (95% CI, 1.09–1.45), 1.34 (95% CI, 1.15–1.67), and 1.33 (95% CI, 1.17–1.65) using eGFRcr, eGFRcys, and eGFRcr-cys, respectively.
Limitations
Likely under-ascertainment of persons with GFR<15 ml/min/1.73m2; GFR was estimated and not measured; comparability of laboratory assays based on a calibration subsample.
Conclusions
The prevalence of reduced eGFRcys in the US civilian, non-institutionalized population increased between 1988–1994 and 1999–2002, confirming the increase observed in the prevalence of reduced eGFRcr.
Index words: cystatin C, chronic kidney disease, estimating equations, prevalence
Chronic kidney disease (CKD) is common, costly, and a risk factor for excess morbidity and mortality.1–4 Over the decade ending in 2004, estimates of CKD prevalence in the US population rose by 30%, reflecting an increase in the prevalence of both albuminuria and reduced estimated glomerular filtration rate (eGFR), and only partially explained by concomitant increases in hypertension, diabetes, and body mass index.5 Similar analysis over longer periods has produced mixed results.1, 6 Some have questioned the validity of this “CKD epidemic,” noting that increases in reduced eGFR based on serum creatinine (eGFRcr) may be due to non-GFR determinants of serum creatinine, such as muscle mass and diet, and drift in laboratory assays over time.7, 8
Cystatin C is an alternative biomarker used to estimate GFR (eGFRcys). Cystatin C and creatinine are the products of very different metabolic pathways and are measured by independent assays, and cystatin C is less sensitive to muscle mass and diet.9, 10 As an estimator of GFR, neither biomarker has proved superior, perhaps due to distinct non-GFR determinants of cystatin C.11–14 GFR estimates based on both serum creatinine and cystatin C (eGFRcr-cys) tend to perform better than estimates based on either filtration marker alone, presumably because of the smaller contributions of non-GFR determinants of each marker when both are included in an estimating equation. Analysis of national trends in the prevalence of reduced eGFRcys and eGFRcr-cys would allow confirmation of trends based on eGFRcr.
The recent development of survey-specific equations that calibrate National Health and Nutrition Examination Survey (NHANES) cystatin C values to the international standard enables the analysis of US trends in eGFRcys and eGFRcr-cys.15 We examined changes in prevalence of reduced eGFR in the U.S. population between 1998–1994 and 1999–2002 using standardized serum creatinine and cystatin C values as well as GFR estimating equations expressed for these assays. We hypothesized that appropriately calibrated cystatin C data would show trends in the prevalence of reduced eGFR similar to those reported using serum creatinine, thus validating previous findings.5
METHODS
Study Population
The NHANES are nationally representative cross-sectional surveys of the noninstitutionalized civilian population in the US.16 Cystatin C concentrations were measured in subsamples of the NHANES III (1988–1994) and the NHANES 1999–2002 populations aged 12 years and older with non-missing serum creatinine. The sampling strategy included all participants aged 60 years and older, a 25% random sample of those aged 12 to 59 years, and all male (female) participants with a serum creatinine over 1.2 mg/dl (1.0 mg/dl).17 For our study, we used all non-pregnant participants aged 20 and older with available serum creatinine and urine albumin-creatinine ratio (ACR) for consistency with a previous study using eGFRcr.5 In analyses using eGFRcr, we used the entire population (N=15,133 in the 1988–1994 survey; N=8,238 in the 1999–2002 survey); in analyses using eGFRcys and eGFRcr-cys, we used the subsample with available cystatin C (n=6,660 in the 1988–1994 survey; n=4,343 in the 1999–2002 survey).
Measurements
Serum cystatin C was measured at the Cleveland Clinic Reference Laboratory in 2006 using stored samples. Measurements were conducted in two batches corresponding to survey period (NHANES 1988–1994 or 1999–2002), using a particle-enhanced immunonephelometric assay with a nephelometer (BNII; Dade Behring).18, 19 Due to concern of assay drift between batches, survey-specific equations were developed to calibrate cystatin C levels to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) standard: for NHANES 1988–1994, IFCC standard cystatin C (mg/l) = 1.12×[0.022+0.80×(cystatin C)]; for the 1999–2002 survey, IFCC standard cystatin C (mg/l) = 1.12×[(cystatin C)−0.12].15 The calibration procedure involved repeating cystatin C measurements on a randomly selected 200-aliquot subsample (University of Minnesota, 2009), using Deming regressions to relate the original Cleveland Clinic Reference Laboratory measurements to the re-analyzed sample values, and converting University of Minnesota values to standardized ERM (European Reference Material)471/IFCC-traceable values using a multiplier of 1.12, as established recently.20 Serum creatinine was measured by the Jaffe modified kinetic method, using a Roche/Hitachi 737 analyzer in NHANES 1988–1994 and a Roche Hitachi 917 analyzer in NHANES 1999–2002. Creatinine values for each survey were standardized as previously described.21
NHANES participants completed a standardized interview and physical examination.16 Race/ethnicity was self-reported and categorized as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Systolic and diastolic blood pressures were measured according to standardized protocols. Hemoglobin A1c levels were measured in whole blood samples. Body mass index (BMI) was calculated from measured height and weight conducted during the physical examination.
Estimating Equations
GFR was estimated using equations developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). eGFRcr was computed using the CKD-EPI creatinine 2009 equation22; eGFRcys and eGFRcr-cys were computed using the CKD-EPI cystatin C 2012 equation and the CKD-EPI creatinine−cystatin C 2012 equations, respectively.11 eGFRcys was used to assess changes in prevalence of reduced eGFR independent of possible creatinine-based effects. eGFRcr-cys was used to assess changes in prevalence with the most accurate estimate of GFR. Non-physiologic (> 200 ml/min/1.73m2) values of eGFR were truncated at 200 ml/min/1.73 m2 (4 values for eGFRcr, 3 values for eGFRcys, and 3 values for eGFRcr-cys).
Definitions
Reduced GFR was defined as eGFR <60 ml/min/1.73m2. GFR categories were classified as G1 (>90 ml/min/1.73m2), G2 (60–89 ml/min/1.73m2), G3a (45–59 ml/min/1.73m2), G3b (30–44 ml/min/1.73m2), G4 (15–29 ml/min/1.73m2), and G5 (<15 ml/min/1.73m2).23 Age was categorized as 20–39, 40–59, 60–69, 70–79, and ≥ 80 years. Hypertension and diabetes were defined by patient self-report; in sensitivity analyses, mean systolic blood pressure > 140 mmHg or mean diastolic blood pressure > 90 mmHg and hemoglobin A1c > 6.5% were included in the definitions. Obesity was defined as BMI ≥ 30 kg/m2.
Statistical analysis
All statistical analyses incorporated sampling weights, primary sampling units, and strata specific to each survey to generate nationally representative estimates of the U.S. civilian, non-institutionalized population.24 Standard errors were estimated using the Taylor series (linearization) method. For analyses of the subsample with available cystatin C, modified sampling weights were used as previously described.17 To check the sensitivity of our results to these weights, analyses of baseline characteristics and eGFRcr were performed both in the full sample and in the subsample with available cystatin C.
We formally compared weighted prevalence estimates in the 1988–1994 and 1999–2002 survey periods using adjusted Wald tests. Kernel density plots (incorporating sampling weights) were used to demonstrate the distribution of kidney function per 1 ml/min/ 1.73 m2 increment in eGFR in the two survey populations. To evaluate whether differences in prevalence were due to differences in mean serum cystatin C across survey periods (and thus potentially a laboratory calibration issue), we compared cystatin C levels among a subsample of young, healthy individuals (age < 40 years, without diabetes or hypertension). While no statistically significant difference was found, we performed a conservative trends analysis as done in a previous study, adjusting filtration marker values so that the mean level with the subsample of young, healthy individuals was identical between surveys. Modified Poisson regression (with standard errors estimated using the Taylor series method) was used to evaluate possible mediation by age (years), sex (male; female), race (non-Hispanic white; non-Hispanic black; Mexican-American; or other), diagnosed diabetes (yes; no), diagnosed hypertension (yes; no), and category of BMI (<25, 25 to <30, ≥ 30 kg/m2) on the association of survey period with CKD prevalence. Finally, reclassification tables were constructed, comparing eGFR categorization by eGFRcr, eGFRcys, and eGFRcr-cys. All analyses were carried out using Stata SE, Version 11.2 (StataCorp LP, College Station, TX).
RESULTS
Population Characteristics by Survey Period
The population in 1999–2002 was older (mean age, 46.2 vs. 44.6 years; p=0.007) and more likely to have diabetes, hypertension, and BMI ≥ 30 kg/m2 compared with the population in 1988–1994 (Table 1). A higher proportion self-identified as Mexican American or other race/ethnicity (17.3% vs. 12.9%, p=0.04). Mean albuminuria was slightly higher in the later period (mean ACR, 33.7 vs. 25.7 mg/g; p=0.05), as were serum creatinine (0.90 vs. 0.84 mg/dL; p<0.001) and cystatin C (0.86 vs. 0.83 mg/L; p=0.005). Estimates using the subsample of participants with available cystatin C measurements were very similar to those using the full sample (data not shown).
Table 1.
Population characteristics of US adults aged ≥20 years based on NHANES 1988–1994 and 1999–2002
| Sample with Available SCr | ||
|---|---|---|
| NHANES 1988–1994 (n=15,133) |
NHANES 1999–2002 (n=8,238) |
|
| Age (y)* | 44.6 (0.5) | 46.2 (0.4) |
| Male sex | 47.9 (0.5) | 48.9 (0.4) |
| Race/Ethnicity | ||
| Non-Hispanic white | 76.8 (1.3) | 72.6 (1.8) |
| Non-Hispanic black | 10.4 (0.6) | 10.1 (1.2) |
| Mexican American | 5.1 (0.4) | 7.0 (0.9) |
| Other | 7.8 (0.8) | 10.3 (1.8) |
| Self-reported diabetes* | 3.6 (0.2) | 6.5 (0.3) |
| Hemoglobin A1c ≥ 6.5% | 5.2 (0.3) | 6.1 (0.4) |
| Self-reported hypertension | 23.7 (0.7) | 25.7 (0.9) |
| Systolic blood pressure (mmHg)* | 122.4 (0.4) | 123.7 (0.4) |
| Diastolic blood pressure (mmHg)* | 74.2 (0.2) | 72.5 (0.3) |
| Body Mass Index categories* | 26.6 (0.1) | 28.0 (0.1) |
| <25 kg/m2 | 44.5 (0.9) | 35.1 (0.9) |
| 25-<30 kg/m2 | 33.0 (0.6) | 34.9 (0.8) |
| ≥30 kg/m2 | 22.5 (0.7) | 29.9 (1.0) |
| ACR (mg/g)* | 25.7 (1.6) | 33.7 (3.6) |
| Non-standardized SCr (mg/dl)* | 1.07(0.003) | 0.83 (0.004) |
| Standardized SCr (mg/dl)* | 0.84 (0.003) | 0.90 (0.005) |
| eGFRcr (ml/min/1.73m2)* | 99.0 (0.5) | 93.5 (0.5) |
| Sample with Available SCysC‡ | ||
| NHANES 1988–1994 (n=6,660) |
NHANES 1999–2002 (n=4,343) |
|
| Non-standardized SCr (mg/dl)* | 1.07 (0.005) | 0.82 (0.007) |
| Standardized SCr (mg/dl)* | 0.84 (0.004) | 0.90 (0.007) |
| Non-standardized SCysC (mg/l) | 0.90 (0.01) | 0.89 (0.01) |
| Standardized SCysC (mg/l)* | 0.83 (0.01) | 0.86 (0.01) |
| eGFRcr (ml/min/1.73m2)* | 99.4 (0.8) | 93.6 (0.6) |
| eGFRcys (ml/min/1.73m2)* | 102.9 (0.9) | 99.7 (0.9) |
| eGFRcr-cys (ml/min/1.73m2)* | 102.4 (0.8) | 97.7 (0.7) |
Note: Values for categorical variables are given as percentage (standard error); values for continuous variables are given as weighted mean (standard error). Conversion factor for creatinine in mg/dL to µmol/L, ×88.4.
P<0.05 in the comparison between survey periods.
SCysC-based estimates use analytic weights customized for the sampling strategy.
ACR, albumin-creatinine ratio; SCysC, serum cystatin C; NHANES, National Health and Nutrition Examination Survey; eGFR, estimated glomerular filtration rate; eGFRcr, eGFR based on SCr; eGFRcys, eGFR based on SCysC; eGFRcr-cys, eGFR based on SCr and SCysC; SCr, serum creatinine.
In a subsample of young healthy individuals, there was no difference in weighted mean cystatin levels between surveys (0.76 mg/l in 1999–2002 vs. 0.75 mg/l in 1988– 1994; p=0.9), whereas there was a small increase in mean serum creatinine (0.86 mg/dl in 1999–2002 vs. 0.80 mg/dl in 1988–1994; p<0.001). Similarly, mean eGFRcys among young healthy individuals was stable between surveys (116 vs. 117 ml/min/1.73m2 in 1999–2002 vs. 1988–1994; p=0.2), yet mean eGFRcr was significantly lower in the later period (107 vs. 114 ml/min/1.73 m2; p<0.001).
Change in Prevalence of Reduced GFR Over Time
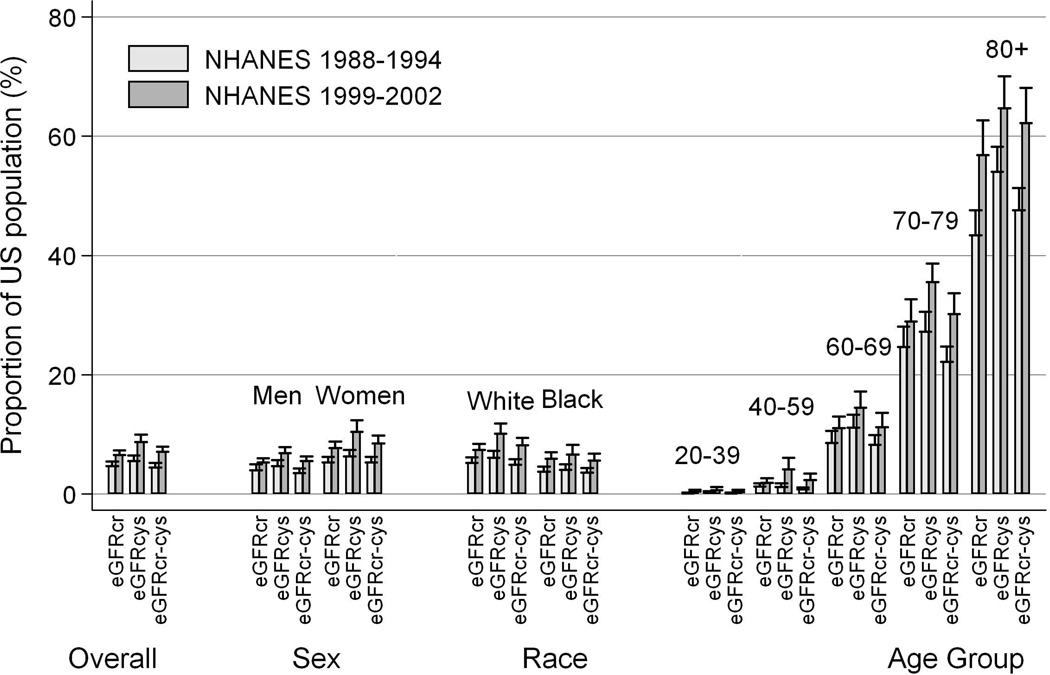
The prevalence of reduced GFR increased between the 1988–1994 and 1999–2002 survey periods by all methods of GFR estimation (Table 2). The prevalence of reduced eGFRcr, eGFRcys, and eGFRcr-cys increased from 4.7% (95% CI, 4.1%–5.3%) to 6.5% (95% CI, 5.9%–7.1%;p<0.001), from 5.5% (95% CI, 4.6%–6.5%) to 8.7% (95% CI, 7.5%–10.0%; p<0.001), and from 4.4% (95% CI, 3.7%–5.2%) to 7.1% (95% CI, 6.2%- 8.0%; p<0.001), respectively. Increases in prevalence were consistent across subsamples with eGFRcr (Table 2, columns 5 and 6), across categories of reduced GFR (G3a-G5), and across subgroups of sex, race, and age (Figure 1). On the raw scale, both the prevalence of reduced eGFR and the increase in prevalence were highest among the oldest segment of the population (62.2% of those aged 80 and older had eGFRcr-cys <60 ml/min/1.73 m2 in 1999–2002, compared with 47.6% in 1988–1994). On a relative scale, the reverse was true: the greatest increase was seen among those younger than 60 years.
Table 2.
Prevalence of GFR Categories, by sample, estimating equation and survey
| GFR | eGFRcr | eGFRcr** | eGFRcys | eGFRcr-cys | |||
|---|---|---|---|---|---|---|---|
| Category | Terms | Range* | |||||
| NHANES 988–1994 | |||||||
| n=15,131 | n=6657 | n=6657 | n=6657 | ||||
| G1 | High and optimum |
>105 | 42.1% | 43.7% | 56.7% | 51.5% | |
| 90–104 | 27.1% | 26.4% | 20.1% | 24.1% | |||
| G2 | Mild | 75–89 | 18.1% | 17.1% | 11.0% | 13.1% | |
| 60–74 | 8.0% | 8.0% | 6.7% | 7.0% | |||
| G3a | Mild- moderate |
45–59 | 3.3% | 3.3% | 3.5% | 2.7% | |
| G3b | Moderate- severe |
30–44 | 1.1% | 1.1% | 1.6% | 1.4% | |
| G4 | Severe | 15–29 | 0.2% | 0.2% | 0.4% | 0.2% | |
| G5 | Kidney failure | <15 | 0.0% | 0.0% | 0.0% | 0.0% | |
| NHANES 999–2002 | |||||||
| n=8236 | n=4343 | n=4343 | n=4343 | ||||
| G1 | High and optimum |
>105 | 30.7% | 31.0% | 51.5% | 42.0% | |
| 90–104 | 27.6% | 27.4% | 17.8% | 25.1% | |||
| G2 | Mild | 75–89 | 23.5% | 23.3% | 13.5% | 17.6% | |
| 60–74 | 11.7% | 11.8% | 8.5% | 8.3% | |||
| G3a | Mild- moderate |
45–59 | 4.5% | 4.5% | 5.2% | 4.5% | |
| G3b | Moderate- severe |
30–44 | 1.6% | 1.6% | 2.4% | 1.7% | |
| G4 | Severe | 15–29 | 0.3% | 0.4% | 0.9% | 0.7% | |
| G5 | Kidney failure |
<15 | 0.1% | 0.1% | 0.2% | 0.1% | |
Note: Values are given as percentages.
GFR, glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; eGFR, estimated glomerular filtration rate; eGFRcr, eGFR based on serum creatinine; eGFRcys, eGFR based on serum cystatin C; eGFRcr-cys, eGFR based on serum creatinine and cystatin C;
GFR in mL/min/1.73 m2.
Based on SCr, but restricted to those individuals with SCr and SCysC.
Figure 1.
Prevalence of reduced eGFR by survey period, estimating equation, and subgroups of sex, race, and age
Effect of Demographic Variables and Comorbid Conditions on Change in Reduced GFR Prevalence
The increase in reduced GFR in NHANES 1999–2002 compared to NHANES 1988– 1994 was partially explained by underlying changes in the US population (Table 3). For each estimating equation, the magnitude of the prevalence ratio of eGFR < 60 ml/min/1.73m2 decreased with sequential adjustment for age, sex and race, diabetes and hypertension, and BMI. The addition of these covariates explained 38%, 40%, and 44% of the increased prevalence of reduced eGFRcr, eGFRcys, and eGFRcr-cys, respectively. Results were similar using alternate definitions of hypertension and diabetes (data not shown). In conservative trends analysis, the fully adjusted prevalence ratios of eGFR < 60 ml/min/1.73m2 associated with survey period were 1.02 (95% CI, 0.91–1.15), 1.25 (95% CI, 1.07–1.46), and 1.20 (95% CI, 1.06–1.37) for eGFRcr, eGFRcys, and eGFRcr-cys, respectively.
Table 3.
Prevalence ratios of reduced GFR comparing NHANES 1999–2002 with NHANES 1988–1994
| PR (95% CI) | P-value | |
|---|---|---|
| eGFRcr < 60 mL/min/1.73 m2 | ||
| Unadjusted | 1.39 (1.19, 1.62) | <0.001 |
| Adjusted for age | 1.31 (1.16, 1.53) | <0.001 |
| + sex & race | 1.32 (1.16, 1.54) | <0.001 |
| + diagnosed DM & HTN | 1.24 (1.09, 1.45) | 0.002 |
| + above + BMI† | 1.24 (1.09, 1.45) | 0.002 |
| eGFRcys < 60 mL/min/1.73 m2 | ||
| Unadjusted | 1.57 (1.26, 1.96) | <0.001 |
| Adjusted for age | 1.46 (1.25, 1.83) | <0.001 |
| + sex & race | 1.47 (1.25, 1.83) | <0.001 |
| + diagnosed DM & HTN | 1.38 (1.17, 1.72) | <0.001 |
| + above + BMI† | 1.34 (1.15, 1.67) | <0.001 |
| eGFRcr-cys < 60mL/min/1.73 m2 | ||
| Unadjusted | 1.59 (1.28, 1.96) | <0.001 |
| Adjusted for age | 1.48 (1.30, 1.83) | <0.001 |
| + sex & race | 1.49 (1.30, 1.84) | <0.001 |
| + diagnosed DM & HTN | 1.37 (1.20, 1.70) | <0.001 |
| + above + BMI† | 1.33 (1.17, 1.65) | <0.001 |
Note: NHANES 1988–1994 is the reference group. All models (unadjusted and adjusted) are composed of the same subsample with available cystatin C. Potential mediators are added sequentially; in other words, “+ above + BMI” indicates adjustment for age, sex and race, diagnosed DM and HTN, and BMI.
BMI was modeled as an ordinal variable: 1 = BMI 0–24.9 kg/m2, 2 = BMI 25–29.9 kg/m2, 3 = BMI ≥30 kg/m2
PR, prevalence ratio; DM, diabetes mellitus; HTN, hypertension; CI, confidence interval; NHANES, National Health and Nutrition Examination Survey; GFR, glomerular filtration rate ; eGFR, estimated GFR; eGFRcr, eGFR based on serum creatinine; eGFRcys, eGFR based on serumcystatin C; eGFRcr-cys, eGFR based on serum creatinine and cystatin C;
Comparisons of eGFR Distributions by Estimating Equations in Both Survey Periods
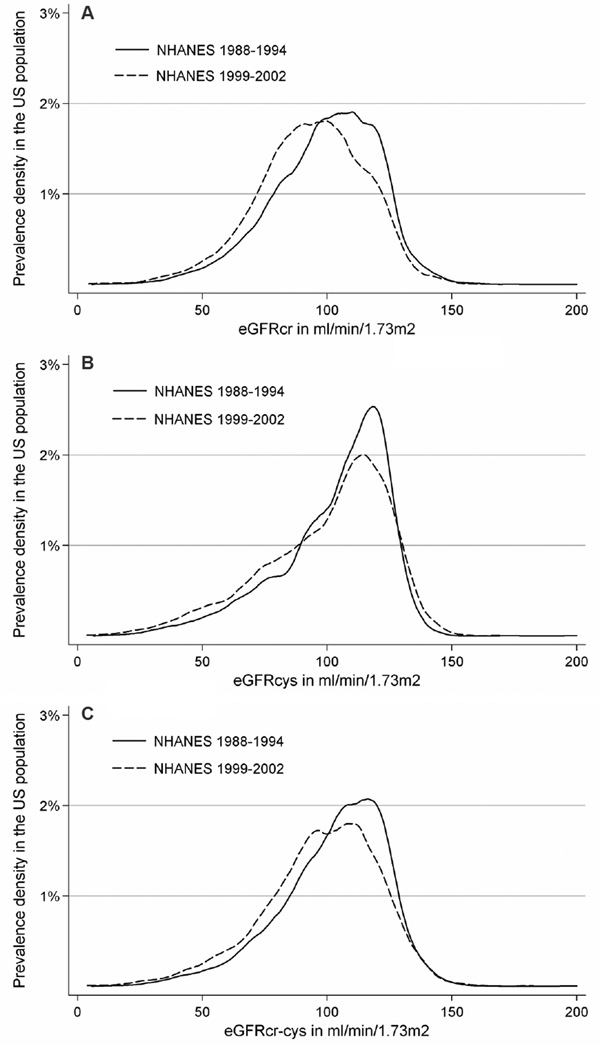
The distributions of eGFRcr, eGFRcys, and eGFRcr-cys prevalence densities were all shifted toward lower eGFR in the later survey period (Figure 2A-C). Median eGFRcr, eGFRcys and eGFRcr-cys decreased from 102 (interquartile range [IQR], 86–115) ml/min/1.73m2 to 94 (IQR, 80–109) ml/min/1.73m2, 109 (IQR, 92–119) ml/min/1.73m2 to 106 (IQR, 84–118) ml/min/1.73m2, and 106 (IQR, 90–118) ml/min/1.73m2 to 100 (IQR, 84–114) ml/min/1.73m2 in 1988–1994 and 1999–2002, respectively. This was demonstrated in all subgroups except for Mexican Americans, who had a slight increase in median eGFRcys and eGFRcr-cys, and other races, who had a slight increase in median eGFRcr (Table S1, available as online supplementary material).
Figure 2.
Distribution in eGFR in the United States by survey period: (A) eGFRcr, (B) eGFRcys, (C) eGFRcr-cys
Reclassification by Estimating Equations in Both Survey Periods Combined
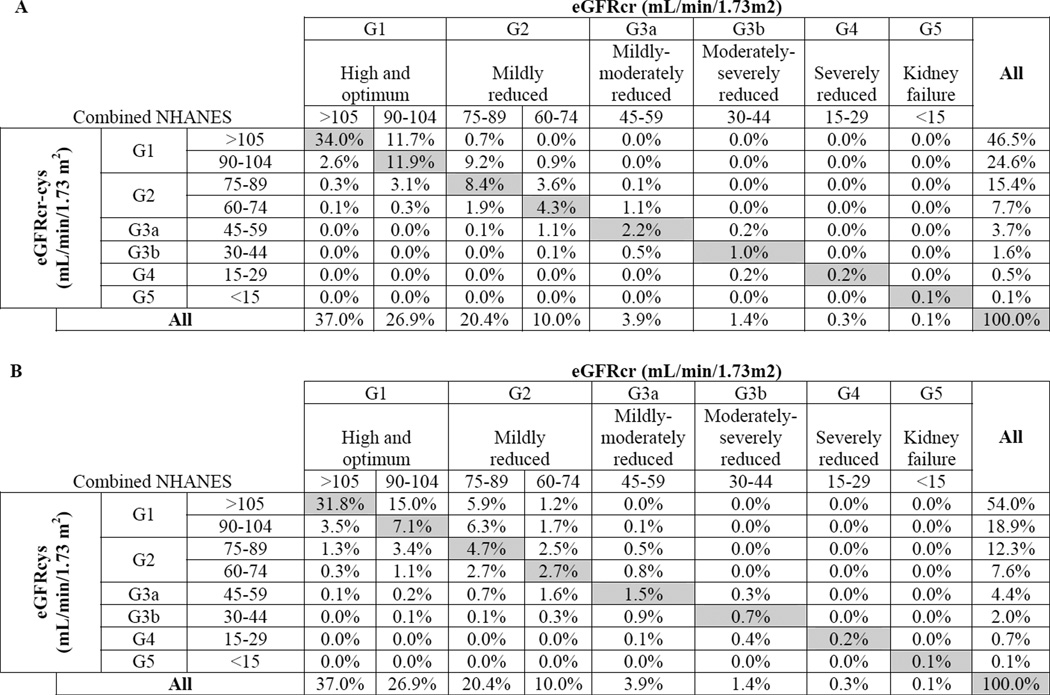
In the combined survey populations, GFR category was reclassified in 37.7% of the population using eGFRcr-cys vs. eGFRcr: 27.5% were reclassifed upwards (higher eGFR) vs. 10.2% downwards (lower eGFR) (Figure 3A). For eGFRcr <60 ml/min/1.73 m2 (5.7% of the total population), 37.8% were reclassified using eGFRcr-cys (24.6% upwards vs. 13.2% downwards). Of the 3.9% of the population classified as eGFRcr 45–59 ml/min/1.73 m2 (G3a), 29.0% were classified upward by eGFRcr-cys, and 13.3% were classified downward. Conversely, of the 10% of the population classified as eGFRcr 60–89 ml/min/1.73 m2 (G2), 11.9% were classified downward by eGFRcr-cys and 44.7% were classified upward. Overall, when classifying reduced eGFR using eGFRcr-cys as the “gold standard”, the false positive and false negative rates among eGFRcr 45–89 ml/min/1.73 m2 were 3.3% and 3.8%, respectively. The false positive and false negative rates compared with eGFRcys were 4.2% and 7.7%, respectively (Figure 3B).
Figure 3.
Comparison of GFR classification in combined surveys: (A) eGFRcr-cys vs. eGFRcr; (B) eGFRcys vs.eGFRcr
DISCUSSION
In this nationally representative study, the prevalence of reduced GFR (defined as CKD stage 3+) increased between the periods 1988–1994 and 1999–2002. This increase was manifest using either a cystatin C-based or creatinine-based GFR estimating equation to classify reduced eGFR. Overall, the prevalence of reduced eGFRcr, eGFRcys and eGFRcr-cys rose by 39%, 57% and 59%, respectively, based on the most accurate estimating equations currently available.11, 25 Much of the increase in reduced eGFR during the later survey period could be explained by differences in demographic characteristics of the two populations and concomitant increases in the prevalence of diabetes, hypertension, and obesity.
These results are fully consistent with previously published trends in prevalence of reduced eGFRcr,5, 26 but they differ from a prior study, which reported no change in the prevalence of reduced eGFRcys in NHANES over time.7 The difference in findings by eGFRcys is primarily due to the use of calibrated cystatin C values in our analysis compared with non-calibrated values in the previous study. Laboratory drift in the cystatin C assay has proved substantial, even when measured using the same assay from the same manufacturer, and our results underscore the importance of careful calibration of cystatin C measurements in NHANES.27, 28 In addition, we used cystatin C-based GFR estimating equations developed in a diverse population and which include age and sex, rather than equations developed in a CKD population that did not use these terms.11, 13
The reason for the rise in prevalence of reduced GFR is not entirely clear. Similar to previous results, we found a marked association of reduced GFR with age.5, 29 Not only was the prevalence of reduced GFR over 50% in those aged 80 and older, but also the increase in prevalence between survey periods was greatest among this age group. This mirrors trends seen in end stage renal disease, where the highest incidence rates are observed in persons aged 75 and older.1 The increased use among older adults of health care interventions that cause reduced GFR (e.g., the administration of intravenous contrast/medications that cause kidney toxicity, and ACE inhibitors that reversibly alter renal hemodynamics) may decrease the likelihood of death but increase the prevalence of reduced GFR. However, using more recent data, there is evidence that the prevalence of CKD stage 3 has plateaued, although this may not be true for stages 4 and 5.6
While the overall trend in reduced eGFR prevalence over time was similar irrespective of the filtration marker, there were important differences in the distribution of eGFR between the markers. In general, the distribution of eGFRcys and eGFRcr-cys were shifted to higher values than that of eGFRcr, leading to higher mean eGFR. However, the distributions of eGFRcys and eGFRcr-cys were also more disperse, leading to higher prevalence estimates for reduced GFR. These differences most likely represent differences in non-GFR determinants of serum cystatin C vs. creatinine; for example, some believe that BMI differentially affects creatinine and cystatin C generation.30 They may also reflect differences in accuracy of the estimating equations in certain subgroups of the population (because of differences in the study populations in which the estimating equations were developed) or undetected differences in assay calibration used in NHANES or the development populations.
Cystatin C is a promising filtration marker and may be particularly useful for GFR estimation in certain subgroups of the population. Consistent with prior reports, our results suggest it may be useful to measure cystatin C to confirm reduced GFR in people with eGFRcr 45–59 ml/min/1.73 m2, or to detect reduced eGFR in people with eGFRcr 60– 89 ml/min/1.73 m2.11, 31, 32 It may also be useful to measure cystatin C in patients with a wider range of eGFRcr in whom alterations in muscle mass or diet are suspected, which might affect creatinine independent of GFR.10, 14 This may be especially important in the elderly in whom reduced GFR is common.12 In addition, eGFRcys appears to be superior to eGFRcr for risk estimation, probably due in part to the wider distribution of eGFRcys vs. eGFRcr.11, 33–35
This study has certain strengths and limitations. It uses a nationally representative sample, confirming trends in reduced GFR prevalence using two separate biomarkers. Both biomarkers have undergone extensive evaluation for accurate standardization and calibration.15, 21 Results were robust to conservative trends analysis such as those performed previously.5 However, NHANES is a cross-sectional sample, and GFR was estimated but not measured – a limitation that reflects standard clinical practice. The accuracy of the 2012 CKD-EPI creatinine- and cystatin C-based equations in the general population elderly would benefit from additional data. Because we focus solely on eGFR, this study was restricted to CKD stage 3+; whether the prevalence of CKD stages 1 and 2 has changed over time was not conclusively evaluated. Finally, the time span is limited, as cystatin C data are only available for the 1988–2002 survey periods.
In conclusion, the prevalence of reduced GFR in the United States increased over the decade ending in 2002. This observation is similar irrespective of filtration marker used to estimate GFR, and the increase in prevalence was seen across subgroups of race, age, sex, and GFR category. These results emphasize the need for preventative measures to reduce and forestall the development of reduced GFR.
Supplementary Material
Acknowledgements
Support: MEG is supported by National Institutes of Hleath (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K08DK092287. LAI is supported by NIH/NIDDK grant K23DK081017-05. SPJ and MCF were supported by NIH/National Heart, Lung and Blood Institute grant T32 HL007024. Siemens Healthcare Diagnostics provided a grant to the University of Minnesota for labor and reagents to conduct some cystatin C assays. This project was partially funded by NIH/NIDDK grant U01 DK067651.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: JC has consulted for Amgen and Merck and has an investigator-initiated grant from Amgen. The other authors declare that they have no other relevant financial relationships to disclose.
Supplementary Material
Table S1: Median eGFR by subgroup and survey period, using subsample with available cystatin C.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
REFERENCES
- 1.U.S. Renal Data System: USRDS 2012 annual data report: Atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 2.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative metaanalysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. United States. Chronic Kidney Disease Surveillance System 2011. Atlanta: U.S. Department of Health and Human Services; 2013. Jan 8, Available at http://www.cdc.gov/ckd. [Google Scholar]
- 7.Foley RN, Wang C, Snyder JJ, Collins AJ. Cystatin C levels in U.S. adults, 1988–1994 versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009;4(5):965–972. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Amin AN. Toward more accurate detection and risk stratification of chronic kidney disease. JAMA. 2012;307(18):1976–1977. doi: 10.1001/jama.2012.4623. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58(4):680–689. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangri N, Stevens LA, Schmid CH, et al. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79(4):471–477. doi: 10.1038/ki.2010.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Calibration of cystatin C in the National Health and Nutrition Examination Surveys (NHANES) Am J Kidney Dis. 2012 doi: 10.1053/j.ajkd.2012.09.013. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics: Plan and operations of the third national health and nutrition examination survey, 1988–94. series 1: Programs and collection procedures. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 17.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the united states: The third national health and nutrition examination survey (NHANES III) Am J Kidney Dis. 2008;51(3):385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the dade behring N latex cystatin C assay on the dade behring nephelometer II system. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 19.Uhlmann EJ, Hock KG, Issitt C, et al. Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the dade behring BN II system, and correlation with creatinine. Clin Chem. 2001;47(11):2031–2033. [PubMed] [Google Scholar]
- 20.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (chronic kidney disease epidemiology collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58(4):682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, de Jong PE, Coresh J, et al. The definition, classification and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 2010 doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics, Center for Disease Control and Prevention. Analytic and reporting guidelines: The national health and nutrition examination survey (NHANES) 2012. Sep 25, [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO): Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;(Suppl 3):1–150. [Google Scholar]
- 26.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the united states. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. Calibration of the siemens cystatin C immunoassay has changed over time. Clin Chem. 2011;57(5):777–778. doi: 10.1373/clinchem.2010.159848. [DOI] [PubMed] [Google Scholar]
- 28.White CA, Rule AD, Collier CP, et al. The impact of interlaboratory differences in cystatin C assay measurement on glomerular filtration rate estimation. Clin J Am Soc Nephrol. 2011;6(9):2150–2156. doi: 10.2215/CJN.00130111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of newonset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 30.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine-versus cystatin C-based estimating equations by category of body mass index. Am J Kidney Dis. 2009;53(6):993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. Journal of the American Society of Nephrology. 2010;22(1):147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA: The Journal of the American Medical Association. 2011;305(15):1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: The health, aging, and body composition study. J Am Soc Nephrol. 2006;17(1):254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. Journal of the American Society of Nephrology. 2009;20(10):2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.