Abstract
Background
Burkitt lymphoma (BL) occurs at all ages, but the patterns of Epstein-Barr virus (EBV) positivity in relation to human immunodeficiency virus (HIV), immunoprofiles and age have not been fully explored.
Design and methods
BL tissues from residual tissue repositories, and 2 academic centers in the United States were examined by expert hematopathologists for morphology, immunohistochemistry, MYC rearrangement, EBV early RNA (EBER), and diagnosed according to the 2008 WHO lymphoma classification. Analysis was done using frequency tables, Chi-squared statistics, and Student’s t-test.
Results
Of 117 cases examined, 91 were confirmed as BL. The age distribution was 26%, 15%, 19%, and 29% for 0-19, 20-34, 35-59, 60+ years, and missing in 11%. MYC rearrangement was found in 89% and EBER positivity in 29% of 82 cases with results. EBER positivity varied with age (from 13% in age-group 0-19 to 55% in age-group 20-34, and fell to 25% in age-group 60+ years, P=0.08); with race (56% in Blacks/Hispanics versus 21% in Whites/Asians/Pacific Islanders, P=0.006); and by HIV status (64% in HIV positive versus 22% in HIV negative cases, P=0.03).
Conclusions
EBER positivity was demonstrated in about one-third of tumors and it was strongly associated with race and HIV status, and marginally with age-group.
Introduction
Burkitt lymphoma (BL) is a rapidly growing B cell non-Hodgkin lymphoma (NHL) (1). First described in 1958 in African children (2), three subtypes have been described worldwide as endemic, sporadic, and immunodeficiency-associated BL (1). The subtypes are indistinguishable by routine pathology, so they are considered a single biological entity, albeit with distinct epidemiological patterns. Endemic BL occurs as a rapidly growing tumor with male preponderance involving the jaw or abdomen in children in Africa. The peak age is 5-9 years, and childhood exposure to Epstein-Barr virus (EBV) and Plasmodium falciparum have been identified as important risk factors (3). Sporadic BL occurs worldwide, shows male preponderance, but more commonly involves the ileocecal region (mucosa, appendix, ascending colon), lymph nodes and, occasionally, the bone marrow, kidney, ovary and other miscellaneous regions (4). The peak age is early adolescence and young adulthood, but cases can occur at any age. The risk factors for sporadic BL have not been identified. Immunodeficiency-associated BL, which have been described mostly in countries in the West, occurs in the context of human immunodeficiency virus (HIV) and solid organ transplantation and, similar to endemic and sporadic BL, shows male predominance (1). Except for immunosuppression, the risk factors for immunodeficiency-associated BL have not been identified. EBV is considered less important in both sporadic and immunodeficiency-associated BL because it is detected in at most 20% of cases in the former (5-8), and in about 30-40% of cases in the later.
We recently identified separate incidence peaks near 10, 40, and 70 years in BL cases in the United States in the general population (9) and in persons with AIDS (10). Similar, separate, incidence peaks were observed in BL cases from four continents, excluding the U.S.(11). Data from Africa were sparse or incomplete, the hypothesis that BL in Africa is multimodal could not be evaluated. Multiple incidence peaks of BL suggest BL with distinct etiology and/or biology, which may vary according to age. This reasoning is in keeping with conclusions drawn about Hodgkin lymphoma (12-14), whose multiple incidence peaks are generally accepted as epidemiologic evidence for Hodgkin lymphoma variants with distinct biology or etiology (12, 15). We investigated the hypothesis that BL in the U.S. may have distinct immunophenotype, molecular and viral profile, which varies with age, using tumors retrieved from residual tissue repositories (RTRs) and diagnostic referral centers located in three SEER population-based cancer registry areas that were reviewed centrally by experienced hematopathologists (BNN, LMW, and STP).
Design and Methods
Institutional Review Board Approvals
Institutional Review Boards at the University of Southern California, University of California at Los Angeles, University of Hawaii, University of Iowa, and Cedars Sinai Medical Center and at the National Institutes of Health gave ethical approval to conduct the study.
Selection of cases
Formalin-fixed paraffin embedded tissue (FFPET) for 81 cases diagnosed as BL between 1979 and 2009 were retrieved from three SEER RTRs in Los Angeles County, Hawaii, and Iowa (16). A diagnosis of BL was based on morphology code 9687 in the International Classification of Diseases in Oncology, third edition, (ICD-O-3)(1). These cases were augmented with an additional 36 FFPET specimens diagnosed for BL cases at the University of California at Los Angeles (UCLA) or the City of Hope Cancer Center. Each case’s age, year of diagnosis, sex, and race/ethnicity (categorized as non-Hispanic white, Hispanic white, Black, and Asian/Native American) were compiled and computerized. HIV status was available for some cases. When not available, HIV status was coded as missing. Patient or hospital identifiers were not used in this study.
Pathology review of cases
Hematoxylin and eosin (H&E) and immunohistochemistry using monoclonal antibodies for CD20, CD3, Bcl-6, Bcl-2, Ki-67, (DAKO, Carpinteria, California) and CD10 (Novocastra, Bannockburn, Illinois) were performed on thin sections prepared from FFPET following standard procedures.
In-situ hybridization for EBV-encoded small ribonucleic acid (EBER) was performed (LMW) as previously described (17). This technique has 100% sensitivity for detecting EBER in Hodgkin lymphoma, thus a known EBER positive Hodgkin lymphoma case was included as a positive control (18). A poly-d(T) was included in tests as a control for total RNA preservation. Cases with adequate staining were classified as EBER positive if the nucleus of a tumor cell was stained dark blue or black, otherwise as negative.
Fluorescence in-situ hybridization (FISH) to detect rearrangement of MYC was performed using dual color fluorescent MYC break-apart probes (Abbott #32-191096: Abbot/Vysis, Downers Grove, IL, USA) following standard procedures (LMW). When hybridization was deemed adequate, the results were read and coded as negative or positive based on the fluorescent signal patterns (LMW). A minimum of 20 cell nuclei (five fields of 4 cells per field) were scored per slide. For negative cases, the entire tumor slide was evaluated again with a manual scope.
The stained sections were reviewed by three experienced lymphoma hematopathologists (BNN, LMW, and STP). A single hematopathologist (BNN) reviewed all the H&E stains for adequacy of diagnostic material, compatible histological features of BL and classified the cases as typical BL morphology or as compatible but variable or atypical BL. A qualitative evaluation (positive or negative) was applied for immunohistochemistry results for CD10, CD20, Bcl-2 and Bcl-6 (19). A semi-quantitative evaluation was applied on the number of cells staining positive as a percent (%) of all malignant cells considered to have positive intensity pattern, results as follows: <10% (+), 10-50% (++), and >50% (+++) staining in tumor cells for the marker. For Ki-67, a similar semi-quantitative evaluation was as follows: <50% (+), 50-90% (++), 91-95% (+++), 96-99% or 100% (++++) of tumor cells.
Results of MYC and EBER FISH were reviewed by one hematopathologist (LMW) who recorded the result as positive or negative. These results were also reviewed by another hematopathologist (BNN) who combined them with the H&E and immunohistochemistry results and made a diagnosis according to the criteria established in the 2008 World Health Organization (WHO) classification of lymphoma(1). A case was diagnosed as BL when the lymphoma showed a diffuse and starry sky pattern under the microscope, and was composed of a monotonous hypercellular population of medium-sized lymphoid cells with round nuclei, open chromatin, multiple distinct nucleoli, moderate amounts of basophilic cytoplasm and frequent mitotic figures. The malignant cells were positive for CD10, CD20, Bcl-6, negative for Bcl-2, and had Ki-67 (proliferation activity). Some cases with variability in tumor cell morphology or absence of the typical immunoprofile were identified. These cases were jointly reviewed by two hematopathologists (BNN and STP), to make a consensus on the diagnosis.
Statistical analysis
Demographic and pathologic characteristics were analyzed using frequency tables, the Chi-squared statistic for categorical variables and Student’s t-test for continuous variables. Although we have previously studied variation of BL across three age groups (0-19, 20-59, 60+ years) (9, 10, 20), we split the middle age group into 2 groups of 20-34 years (young adults) and 35-59 year olds (older adults) to create four categories (0-19, 20-34, 35-59 and 60+ years). This was done to ascertain differences in patterns in young adults, who may have had recent EBV or HIV infection, and in older adults, who may have had chronic EBV or HIV infection. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association of HIV with EBER positivity in BL were estimated using unconditional logistic regression. Two-sided P-values of < 0.05 were considered statistically significant.
Results
Out of the 117 cases whose H&E slides were initially reviewed, 91 cases were confirmed as BL (Figure 1). The remaining 26 cases were excluded because of insufficient diagnostic tissue (n=10) or reclassification as other lymphoma subtypes after detailed review (n=16). The demographic characteristics of the 91 cases are described in Table 1.
Figure 1.
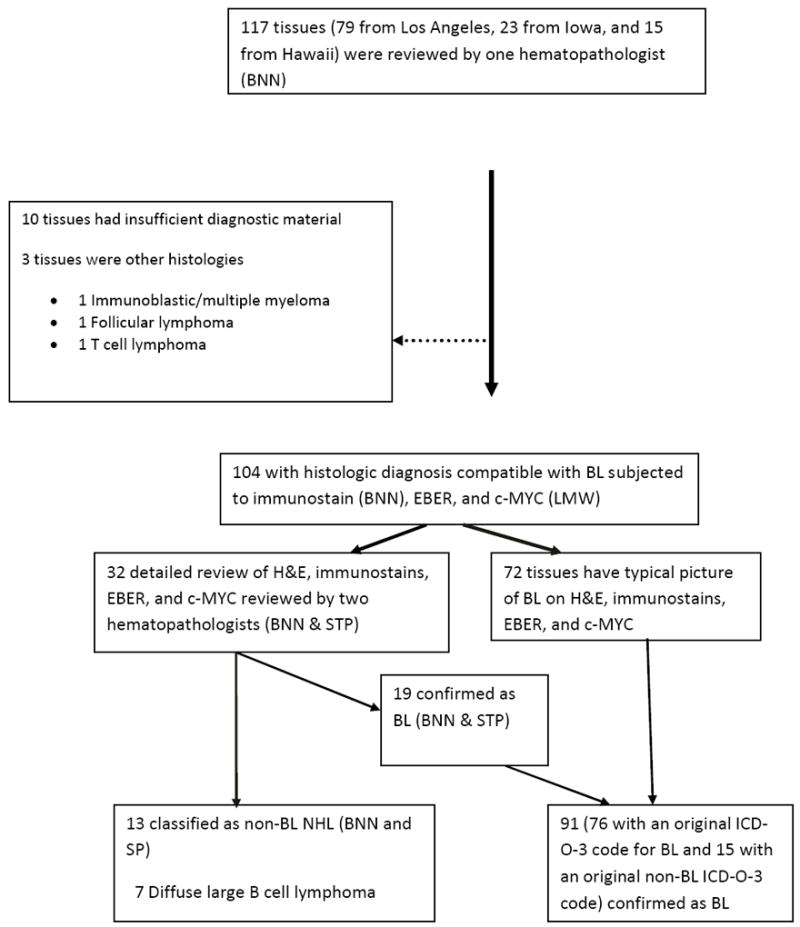
Flow diagram showing number of cases evaluated and the results of pathology evaluation at each stage.
Table 1.
Demographic characteristics of confirmed Burkitt lymphoma cases
| Characteristics | Number of cases (%) |
|---|---|
| All subjects | 91 (100) |
| Repository | |
| Los Angeles | 54 (59) |
| Hawaii | 15 (16) |
| Iowa | 22 (24) |
| Diagnosis year | |
| 1979 | 2 (2) |
| 1980-1989 | 14 (15) |
| 1990-1999 | 27 (30) |
| 2000-2009 | 42 (46) |
| Missing | 6 (7) |
| Sex | |
| Male | 73 (80) |
| Female | 12 (13) |
| Missing | 6 (7) |
| Race | |
| Non-Hispanic White | 51 (56) |
| Black/African American | 3 (3) |
| Hispanic White | 15 (16) |
| Asian/Pacific Islander* | 16 (18) |
| Unknown/missing | 6 (7) |
| Age at diagnosis, years | |
| 0-19 | 24 (26) |
| 20-34 | 14 (15) |
| 35-59 | 26 (29) |
| 60+ | 17 (19) |
| Missing± | 10 (11) |
| HIV status | |
| Negative | 18 (20) |
| Positive | 14 (15) |
| Unknown/missing | 59 (65) |
or American Indian or Alaska Native
includes 4 cases whose age was provided as in range 20-59 years
Characteristics of BL cases
All 91 confirmed BL cases were positive for CD20, 98% were CD10 positive, 91% were Bcl-6 positive, and 92% were Bcl-2 negative. MYC gene rearrangement was positive in 89% (Table 2). Positivity for Bcl-6 and MYC rearrangement did not correlate with age (Table 3).
Table 2.
Immunoprofile of 91 Burkitt lymphoma cases, including findings of 19 cases that were considered non-typical
| Case profiles | Immunohistochemistry results | In-situ hybridization | |||||
|---|---|---|---|---|---|---|---|
| CD20 | CD10 | Bcl-6 | Bcl-2 | Ki-67* | c-MYC± | EBER§ | |
| Typical cases (n=72) | 100% | 100% | 100% | 0% | 100% | 90% | 34% |
| Non-typical cases (n=19) | |||||||
| CD10 Negative & Bcl-2 positive ¶ | |||||||
| Case 1 | + | - | + | + (>50%) | + (96-99%) | + | - |
| Case 2 | + | - | + | + (>50%) | + (90-95%) | + | - |
| Bcl-2 positive¶ | |||||||
| Case 3 | + | + | + | + (10-50%) | + (>99%) | ND | - |
| Case 4 | + | + | + | + (10-50%) | + (96-99%) | + | - |
| Case 5 | + | + | + | + (>50%) | + (90-95%) | + | - |
| Case 6 | + | + | + | + (>50%) | + (96-99%) | ND | ND |
| Case 7 | + | + | + | + (>50%) | + (>99%) | ND | - |
| Bcl-6 un-evaluable | |||||||
| Case 8 | + | + | ± | - | + (>99%) | - | + |
| Case 9 | + | + | ± | - | + (>99%) | + | - |
| Case 10 | + | + | ± | - | + (<50%) | ND | + |
| Case 11 | + | + | ± | - | + (>99%) | + | - |
| Case 12 | + | + | ± | - | + (>99%) | - | - |
| Ki-67 un-evaluableΔ | |||||||
| Case 13 | + | + | + | - | ± | + | - |
| Case 14 | + | + | + | - | ± | + | - |
| Case 15 | + | + | + | - | ± | ND | - |
| Case 16 | + | + | + | - | ± | + | - |
| Ki-67 & Bcl-6 un-evaluable Δ | |||||||
| Case 17 | + | + | ± | - | ± | ND | ND |
| Case 18 | + | + | ± | - | ± | + | - |
| Case 19 | + | + | ± | - | ± | + | - |
| All BL cases (n=91) | 100% | 98% | 91% | 8% | 92% | 89% | 29% |
+ Positive; - Negative; ± un-evaluable; ND not done
Ki-67 based on 83 cases with evaluable results.
MYC results based on 75 cases with results;
EBER results based on 82 cases with results.
None of the Bcl-2 positive cases had BCL2/IGH translocations. Gene duplication (polysomy) was detected in five of the cases.
Staining results for Ki-67 for 4 cases and for Ki-67 & Bcl-6 for 3 cases were deemed un-evaluable for technical reasons due to loss of antigenicity due to tissue fixative or old archived specimen.
Table 3.
Immunophenotype and in-situ hybridization results of 81* confirmed Burkitt lymphoma cases, overall and according to age groups
| Age groups (years)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 0-19 | 20-34 | 35-59 | 60+ | P-value± | ||||
| n | % | n | % | n | % | n | % | ||
| All subjects | 24 | 29% | 14 | 17% | 26 | 32% | 17 | 21% | |
| Immunohistochemistry | |||||||||
| Bcl-6 | NE | ||||||||
| Positive | 20 | 83% | 12 | 86% | 24 | 92% | 17 | 100% | |
| Un-evaluable§Δ | 4 | 17% | 2 | 14% | 2 | 8% | 0 | 0% | |
| Bcl-2 | 0.96 | ||||||||
| Positive§ | 2 | 8% | 1 | 7% | 2 | 8% | 2 | 12% | |
| Negative | 22 | 92% | 13 | 93% | 24 | 92% | 15 | 88% | |
| Ki-67 | NE | ||||||||
| Positive | 19 | 79% | 13 | 93% | 25 | 96% | 17 | 100% | |
| Un-evaluable §Δ | 5 | 21% | 1 | 7% | 1 | 4% | 0 | 0% | |
| Fluorescent in-situ hybridization | |||||||||
| MYC | 0.39 | ||||||||
| Positive | 20 | 91% | 8 | 80% | 20 | 95% | 11 | 79% | |
| Negative | 2 | 9% | 2 | 20% | 1 | 5% | 3 | 21% | |
| Missing ¶ | 0 | 1 | 1 | 3 | |||||
| EBER | 0.08 | ||||||||
| Positive | 3 | 13% | 6 | 55% | 8 | 33% | 4 | 25% | |
| Negative | 20 | 87% | 5 | 45% | 16 | 67% | 12 | 75% | |
| Missing ¶ | 0 | 3 | 2 | 1 | |||||
10 of 91 BL cases did not have specific age indicated.
P value for heterogeneity based on Chi-square statistic or Fisher’s exact test when there were fewer than 5 cases in a cell. NE Statistical pattern not evaluated because there was no relevant group for comparison;
Immunoprofile patterns of 19 cases of Burkitt lymphoma are shown in Table 2.
Staining results for 7 Ki-67 cases were considered un-evaluable because of technical reasons due to loss of antigenicity due to tissue fixative or old archived specimen.
Missing values for result in the age category not included when calculating percent or P values.
Of the 91 BL cases, 19 did not have the immunoprofile of typical BL and hence were classified as not typical BL (Table 2). These 19 cases did not differ from the typical BL cases with respect to age (Table 3). Two of the 19 cases were CD10 negative and Bcl-2 positive, but they had classic BL morphology and were positive for CD20, Bcl-6, Ki-67>90% and MYC. Five non-typical BL cases had Bcl-2 positivity (representative micrographs in Figure 2A-G), which may occur at low levels in BL (21). These five cases exhibited classic morphology and were positive for CD20, CD10, and Bcl-6, with a Ki-67 of 90% to>99%. MYC rearrangement results were available for 2 of these cases and both were positive, but not in the remaining three cases. In 5 more cases Bcl-6 was un-evaluable (negative due to technical reasons), but they had the classic BL morphology, CD20 and CD10 positivity, Ki-67 >90%, and Bcl-2 negativity. In the remaining 7 non-typical BL cases, staining for Ki-67 and/or Bcl-6 were un-evaluable because these cases showed no internal positive staining, implying that the negative results were likely artifactual due to loss of antigenicity, the tissue fixative used, decalcification process or long duration of sample storage. However these 7 cases displayed classic morphology, were positive for CD20, CD10 and were BCL-2 negative, and hence classified as BL. In addition, in 5 of these 7 cases, MYC rearrangement by FISH was positive in all 5 cases.
Figure 2.
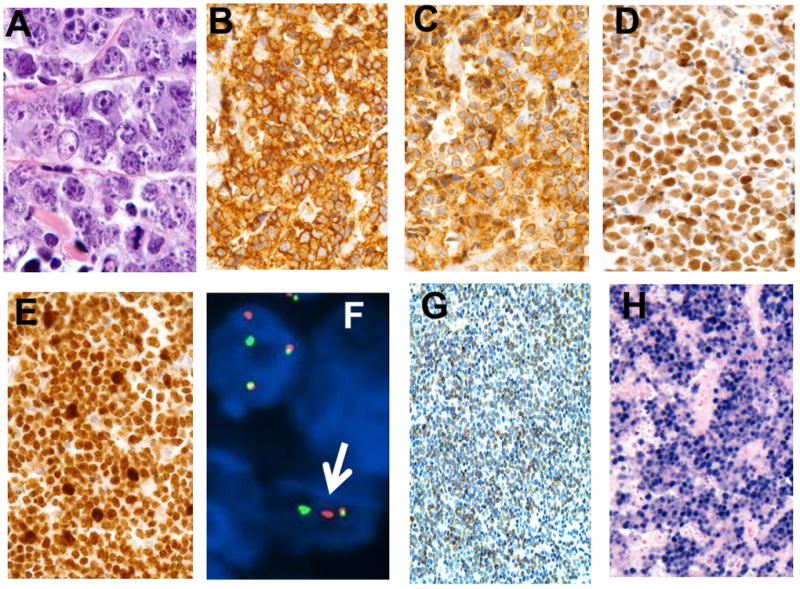
(A) Burkitt lymphoma cells showing hypermitotic activity (hematoxylineosin stain, magnification ×400). (B) CD20 immunostain showing strong membrane staining (magnification ×400). (C) CD10 immunostain showing strong membrane staining (magnification ×400). (D) Bcl-6 showing strong nuclear staining in the lymphoma cells (magnification ×400). (E) Ki-67 showing proliferation index > 99% (magnification ×400). (F) Positive dual color c-MYC gene break apart probe fluorescent in-situ hybridization (FISHba): A separate red and green signal indicates rearrangement of the MYC gene (see white arrow). A fused one orange/green (yellow) fused signal pattern indicates lack of rearrangement (magnification ×400). (G) Section showing cases with Bcl-2 positivity in the lymphoma cells. Note: all Bcl-2 positive cases were EBER negative (magnification ×20). (H) EBER in-situ hybridization showing strong nuclear staining in tumor cells (magnification ×400).
EBER and HIV status in the BL cases
Overall, EBER positivity (Figure 2H) was found in 24 (29%) of 82 cases with complete EBER results, and the proportion varied according to age (Figure 3A, based on 74 cases whose age was known), race/ethnicity (Figure 3B), and HIV status (Figure 3C). Among 74 cases with known age, the percentage of EBER positive cases increased from 13% (3/23) among cases aged 0-19 years to 55% (6/11) among those aged 20-34 years, and then decreased to 33% (8/24) among those aged 35-59 years and to 25% (4/16) in 60+ years and older (Pheterogeneity=0.08). Among 77 cases with both ethnicity and EBER results, EBER positivity was 56% (9/16) in Blacks/Hispanics and 21% (13/61) in the non-Hispanic/White/Asian/Pacific Islanders (Pheterogeneity=0.006). Among 32 BL cases with HIV results, 18 were HIV negative and 14 were HIV positive. The 14 HIV positive cases were between ages 20-59 years, representing 67% of the 21 BL cases in this age group with HIV results. EBER positivity was 64% (7/11) in the HIV positive cases and 22% (4/18) in the HIV negative cases (P=0.03, Figure 3C). The EBER positivity remained 64% among HIV positive cases in age group 20-59 years, but it was lower at 14% in the HIV negative cases. HIV positivity was associated with EBER positivity (OR 6.8, 95% CI 1.6-28.4) in analyses adjusting for age group. Adjusted for HIV status, age group was not independently associated with EBER positivity (P=0.16).
Figure 3.
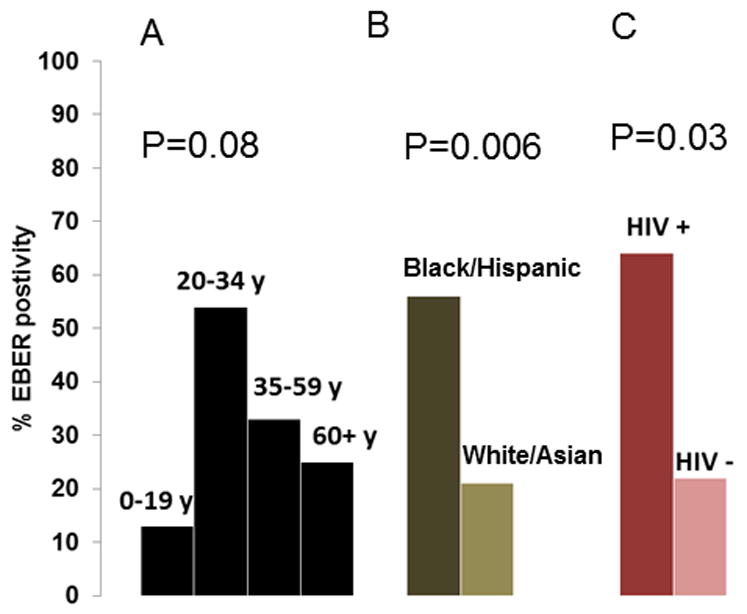
Bar graph showing the percent of confirmed BL cases with EBV Early RNA protein (EBER) detected according to age group (A), race/ethnicity (B), and HIV status (C).
Bcl-2, EBER, HIV status and age in BL cases
Overall, Bcl-2 positivity by immunohistochemistry was low (8%, n=7), as expected, and did not differ by age group (Table 3, P=0.96). None of the Bcl-2 positive cases was positive for IgH@-BCL2 fusion signals by FISH, but 5 of 7 cases with interpretable results showed evidence of multiple copies of (polysomy) the IgH@ and/or BCL2 gene loci (data not shown). Bcl-2 and EBER positivity results were mutually exclusive; 10% (6/58) of EBER negative BL cases were Bcl-2 positive compared to 0% (0/24) among EBER positive cases (P=0.10). Similarly, Bcl-2 and HIV positivity results were also mutually exclusive; 22% (4/18) of HIV negative cases were Bcl-2 positive compared to 0% (0/14) among HIV positive cases (P=0.09). All seven BL cases that were Bcl-2 positive tested negative for both viruses (P=0.03).
Discussion
We investigated the hypothesis that the morphology, immunophenotype, molecular, and viral (EBV and HIV) patterns of BL in the United States may vary with demographic characteristics. Our data suggest that one-third of BL tumors were EBV positive. EBV positivity in BL in the United States was strongly associated with ethnicity, HIV status, and, marginally associated with age group. These results confirm previous impressions that EBV is not implicated in the majority of BL in the general population in the United States. The distinct associations between EBV positive and EBV negative BL with demographic characteristics suggest EBV may be a marker of distinct BL entities, which may arise via distinct molecular pathways (22-25).
Our finding that some BL tumors were Bcl-2 protein positive is intriguing, but not new. The current criteria for diagnosing BL exclude tumors with strong Bcl-2 positivity (26, 27), but previous reports have noted Bcl-2 positivity in BL diagnosed after careful evaluation of all evidence (21, 28). Our results, based on careful evaluation using the WHO classification, expand on those results with the observation that the Bcl-2 positive tumors in our study were negative for both EBV and HIV. This finding might be due to chance or it may be a clue to differences in BL entities that arise and/or progress via distinct pathways that are influenced by abnormalities induced by viral co-factors or in host genes (29). The molecular mechanism for Bcl-2 expression in BL cases is uncertain, however, IgH@-BCL2 translocations were not found in the cases. We note Bcl-2 protein expression can occur in the absence of BCL2 gene rearrangement(19) and is observed in many types of B and T cell lymphomas as well as in benign mantle cells, benign marginal zone B-cells, and benign small-T-cells (19). In keeping with these results, polysomy of the IgH@ and/or BCL2 gene loci might be related to Bcl-2 positivity as a secondary event.
The cases in our tissue study are similar in age to the 3058 cases in the SEER study where we reported trimodal incidence peaks in the United States (30). In both studies, cases aged 0-19 years, 20-59 years, and 60+ years, contributed approximately 30%, 50% and 20%, respectively. The substantial proportion of BL among adults (20-59 years) is in keeping with the impact of HIV on BL. In our study, 67% of BL cases in the 20-59 year age group were HIV positive and is consistent with a substantial impact of HIV in this age group. In keeping with the large number of our cases from Los Angeles, which has a relatively high prevalence of HIV and contributed the majority of BL cases in the current series, our study may overestimate the proportion of BL due to HIV. An analysis of the United States HIV/AIDS Cancer Match Study, albeit based on cases not verified by hematopathologist review, estimated that about 20% of BL since 1980 could be attributed to HIV/AIDS (31).
Our finding that 29% of BL cases were EBER positive confirms the comparatively low prevalence of EBV in BL reported in the United States(5). EBV positivity was slightly higher in the present analysis than that reported in earlier case series (10-20%)(6-8), but those studies were conducted before the HIV epidemic. Of interest, EBV positivity in BL bears some similarity to EBV positivity in Hodgkin lymphoma (32). In both conditions, EBV positivity is generally higher among African-Americans and Hispanic Whites than in non-Hispanic Whites or Asian/Pacific Islanders (32). In both, EBV positivity is also higher in HIV positive cases than in HIV negative cases (14). These similarities suggest that EBV infection is a co-factor for both tumors in these racial groups (33), and that EBV reactivation due to HIV-related immunosuppression is a risk factor for both tumors.
However, there are certain differences between BL and Hodgkin lymphoma. In BL, EBV positivity peaked among young adults, whereas in Hodgkin lymphoma, EBV positivity peaks among young children and elderly adults (32). These contrasting patterns in BL and Hodgkin lymphoma suggest that both primary EBV infection and EBV reactivation may influence the risk for Hodgkin lymphoma, while primary EBV may be more relevant for BL. As such, the peak of EBV positive Hodgkin lymphoma in young children is in keeping with the contribution of primary EBV at an early age, when the immune system is immature, whereas a peak in the elderly is in keeping with the contribution of reactivated latent EBV in the elderly (14), as immunity declines. In BL, EBV positivity peaks in young adults in keeping with a contribution from primary EBV infection among adolescents and young adulthoods. Thus, although EBV reactivation occurs in the elderly, it may not play an important role in BL in the elderly (14).
Taken together, our and other studies (9, 10, 20) suggest that BL is comprised of EBV positive and EBV negative entities. EBV positive and EBV negative BL is observed in HIV positive and negative individuals and at all ages, and in all races. The common abnormality in BL entities is hyper-proliferation of B-cells due to MYC deregulation, which is triggered by chromosomal translocations of the MYC oncogene on chromosome 8 (8q24) into the vicinity of the regulatory elements of one of the immunoglobulin heavy or light chains at chromosome positions 14q32, 2p12, or 22q11.2(34, 35). The strong proliferative signals induced by these translocations deregulate MYC (1) triggering strong apoptosis signals to protect the cell from progressing to malignancy (36, 37). We suggest that EBV positivity is a clue to different pathways initiated B cells take to progress to malignancy. In one instance, the cells may rely on viral factors, whereas in other instances, they may rely on abnormalities developed in host genes.
Strengths of this study include using population-based BL cases that were defined according to the current WHO classification system for lymphoma (1) after expert pathology review, which heretofore has not been done in most studies. To increase sample size, we included cases diagnosed up to 30 years ago. However, one of the drawbacks of using long-term archived specimens is loss of antigenicity, and the other is lack of HIV and demographic data, in some cases. For example, incomplete results in some of the non-typical BL cases (cases 6 and 7 in Table 2 lack results on MYC FISH) preclude firm conclusions as to whether these cases truly represent BL or the intermediate BL/DLBCL.
In conclusion, our findings confirm that EBV is not implicated in the majority of cases in the United States. EBV positivity is strongly associated with race and HIV status and marginally associated with age group. The distinct associations of EBV positive and EBV negative BL with demographic and viral factors support the notion that EBV may be a marker of distinct BL entities, which may differ in biology and/or etiology.
Acknowledgments
The authors would like to thank Freda Selk and Dan Olson, University of Iowa for selecting and retrieving tissue blocks from the University of Iowa; Chris Hochstedler, University of Iowa, for supervising the cutting and staining of tissue blocks from the University of Iowa. We would also like to thank Catherine Grafel-Anderson, Hugh Luk at Hawaii Tumor Registry and Pathology Shared Resource of the University of Hawaii Cancer Center for cutting and staining of tissue blocks. The authors would like to thank James J. Goedert, Infections and Immunoepidemiology Branch, and Susan S. Devesa, Biostatistics Branch, at NCI for their editorial comments on the manuscript draft.
Funding
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services (HHSN261200900444P and HHSN261200900586P) by NCI grants to the participating SEER Registries (NO1-PC-35143 for Iowa, NO1-PC-35137 for Hawaii, and NO1-PC-35139 N01-PC-2010-00035 for Los Angeles), and by a grant from the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 and from the Centers for Disease Control and Prevention (1U58DP000807-3).
Footnotes
Author contribution
SMM was the principal investigator and takes primary responsibility for the paper. STP, BNN, LMW, NR performed laboratory work for this study; KB, DH, MGC, MW, VN and BE participated statistical analyses; CFL, BH, SA, MTG and WC obtained tissues used in this study and participated in statistical analyses. WC was co-principal investigator of the study.
The authors reported no potential conflicts of interests.
References
- 1.Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM, editors. Burkitt lymphoma. Lyon: International Agency for Research on Cancer (IARC); 2008. [Google Scholar]
- 2.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 3.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 4.Magrath IT, Sariban E. Clinical features of Burkitt’s lymphoma in the USA. IARC Sci Publ. 1985:119–127. [PubMed] [Google Scholar]
- 5.Levine PH, Kamaraju LS, Connelly RR, Berard CW, Dorfman RF, Magrath I, et al. The American Burkitt’s Lymphoma Registry: eight years’ experience. Cancer. 1982;49:1016–1022. doi: 10.1002/1097-0142(19820301)49:5<1016::aid-cncr2820490527>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler JL, Andersson M, Klein G, Henle W. Detection of Epstein-Barr virus DNA in American Burkitt’s lymphoma. Int J Cancer. 1976;17:701–706. doi: 10.1002/ijc.2910170603. [DOI] [PubMed] [Google Scholar]
- 7.Pagano JS, Huang CH, Levine P. Absence of Epstein-Barr viral DNA in American Burkitt’s lymphoma. N Engl J Med. 1973;289:1395–1399. doi: 10.1056/NEJM197312272892604. [DOI] [PubMed] [Google Scholar]
- 8.Gravell M, Levine PH, McIntyre RF, Land VJ, Pagano JS. Epstein-Barr virus in an American patient with Burkitt’s lymphoma: detection of viral genome in tumor tissue and establishment of a tumor-derived cell line (NAB) J Natl Cancer Inst. 1976;56:701–704. doi: 10.1093/jnci/56.4.701. [DOI] [PubMed] [Google Scholar]
- 9.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973-2005. Int J Cancer. 126:1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbulaiteye SM, Anderson WF, Ferlay J, Bhatia K, Chang C, Rosenberg PS, et al. Pediatric, elderly, and emerging adult-onset peaks in Burkitt’s lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87:573–578. doi: 10.1002/ajh.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macmahon B. Epidemiological evidence of the nature of Hodgkin’s disease. Cancer. 1957;10:1045–1054. doi: 10.1002/1097-0142(195709/10)10:5<1045::aid-cncr2820100527>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett RF. Viruses and Hodgkin’s lymphoma. Ann Oncol. 2002;13(Suppl 1):23–29. doi: 10.1093/annonc/13.s1.23. [DOI] [PubMed] [Google Scholar]
- 14.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Intern Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 15.Ries LAG, Devesa SS. Cancer incidence, mortality, and patient survival in the United States. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. [Google Scholar]
- 16.Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss LM, Chen YY, Liu XF, Shibata D. Epstein-Barr virus and Hodgkin’s disease. A correlative in situ hybridization and polymerase chain reaction study. Am J Pathol. 1991;139:1259–1265. [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong AA, Weiss LM, Gallagher A, Jones DB, Krajewski AS, Angus B, et al. Criteria for the definition of Epstein-Barr virus association in Hodgkin’s disease. Leukemia. 1992;6:869–874. [PubMed] [Google Scholar]
- 19.Nathwani BN, Sasu SJ, Ahsanuddin AN, Hernandez AM, Drachenberg MR. The critical role of histology in an era of genomics and proteomics: a commentary and reflection. Adv Anat Pathol. 2007;14:375–400. doi: 10.1097/PAP.0b013e318159479d. [DOI] [PubMed] [Google Scholar]
- 20.Mbulaiteye SM, Anderson WF, Ferlay J, Bhatia K, Chang C, Rosenberg PS, et al. Pediatric, elderly, and emerging adult-onset peaks in Burkitt lymphoma incidence diagnosed in four continents, excluding Africa. American J Hematology. 2012 doi: 10.1002/ajh.23187. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave SS, Fu K, Wright GW, Lam LT, Kluin P, Boerma EJ, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 22.Bellan C, Lazzi S, Hummel M, Palummo N, de Santi M, Amato T, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–1036. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander S, Calado DP, Srinivasan L, Kochert K, Zhang B, Rosolowski M, et al. Synergy between PI3K Signaling and MYC in Burkitt Lymphomagenesis. Cancer Cell. 2012;22:167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulino-Roth L, Wang K, Macdonald TY, Mathew S, Tam Y, Cronin MT, et al. Targeted genomic sequencing of pediatric Burkitt lymphoma identifies recurrent alterations in anti-apoptotic and chromatin-remodeling genes. Blood. 2012 doi: 10.1182/blood-2012-06-437624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naresh KN, Ibrahim HA, Lazzi S, Rince P, Onorati M, Ambrosio MR, et al. Diagnosis of Burkitt lymphoma using an algorithmic approach - applicable in both resource-poor and resource-rich countries. Br J Haematol. doi: 10.1111/j.1365-2141.2011.08771.x. [DOI] [PubMed] [Google Scholar]
- 27.Lukande R, Wabinga HR, Tumwine LK. Burkitt’s lymphoma in Uganda: the role of immunohistochemistry in diagnosis. East Afr Med J. 2008;85:207–212. doi: 10.4314/eamj.v85i5.9493. [DOI] [PubMed] [Google Scholar]
- 28.Klapper W, Szczepanowski M, Burkhardt B, Berger H, Rosolowski M, Bentink S, et al. Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood. 2008;112:1374–1381. doi: 10.1182/blood-2008-01-136465. [DOI] [PubMed] [Google Scholar]
- 29.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 30.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973-2005. Int J Cancer. 2010;126:1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiels MS, Pfeiffer RM, Hall HI, Li J, Goedert JJ, Morton LM, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 305:1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glaser SL, Gulley ML, Clarke CA, Keegan TH, Chang ET, Shema SJ, et al. Racial/ethnic variation in EBV-positive classical Hodgkin lymphoma in California populations. Int J Cancer. 2008;123:1499–1507. doi: 10.1002/ijc.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodsky AL, Heath CW., Jr Infectious mononucleosis: epidemiologic patterns at United States colleges and universities. Am J Epidemiol. 1972;96:87–93. doi: 10.1093/oxfordjournals.aje.a121444. [DOI] [PubMed] [Google Scholar]
- 34.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983;32:311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- 36.Klein G. Burkitt lymphoma-A stalking horse for cancer research? Semin Cancer Biol. 2009 doi: 10.1016/j.semcancer.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]


