Abstract
Age-related hearing loss (AHL), also known as presbycusis, is a universal feature of mammalian aging and is characterized by a decline of auditory function, such as increased hearing thresholds and poor frequency resolution. The primary pathology of AHL includes the hair cells, stria vascularis, and afferent spiral ganglion neurons as well as the central auditory pathways. A growing body of evidence in animal studies has suggested that cumulative effect of oxidative stress could induce damage to macromolecules such as mitochondrial DNA (mtDNA) and that the resulting accumulation of mtDNA mutations/deletions and decline of mitochondrial function play an important role in inducing apoptosis of the cochlear cells, thereby the development of AHL. Epidemiological studies have demonstrated four categories of risk factors of AHL in humans: cochlear aging, environment such as noise exposure, genetic predisposition, and health co-morbidities such as cigarette smoking and atherosclerosis. Genetic investigation has identified several putative associating genes, including those related to antioxidant defense and atherosclerosis. Exposure to noise is known to induce excess generation of reactive oxygen species (ROS) in the cochlea, and cumulative oxidative stress can be enhanced by relatively hypoxic situations resulting from the impaired homeostasis of cochlear blood supply due to atherosclerosis, which could be accelerated by genetic and co-morbidity factors. Antioxidant defense system may also be influenced by genetic backgrounds. These may explain the large variations of the onset and extent of AHL among elderly subjects.
1. Introduction
Age-related hearing loss (AHL), or presbycusis, is a complex degenerative disease and is one of the most prevalent chronic conditions of the aged, affecting tens of millions of people world-wide. AHL is a multifactorial condition, representing the end stage sequela of multiple intrinsic (e.g. genetic predisposition) and extrinsic (e.g. noise exposure) factors acting on the inner ear over a lifetime that cumulatively lead to impairments in cochlear transduction of acoustic signals (Ohlemiller, 2009; Schuknecht, 1955).
Potential sites of pathology include the inner and outer hair cells, the stria vascularis, and afferent spiral ganglion neurons (Schuknecht et al., 1993). The stria vascularis and hair cells are particularly susceptible to injury. The stria vascularis is highly metabolically active and depends on an elaborate cellular machinery to maintain the steady-state endocochlear resting potential. Consequently, injury from multiple different pathways (e.g. age-related cell losses within the stria, oxidative stress from noise exposure, genetic polymorphisms leading to inefficient oxidative pathways or dysfunctional supporting cells, or microvascular disease in the strial vessels) could all affect strial function (Ohlemiller, 2009). The resulting loss of the endocochlear potential would impair the function of the cochlear amplifier and lead to an increase in hearing thresholds (Schmiedt et al., 2002; Schuknecht et al., 1974).
A similar multimodal pathway of injury and dysfunction is also observed in the cochlear hair cells and cochlear nerve. Post-mitotic hair cells are susceptible to accumulated injury over time from a combination of poor cellular repair mechanisms associated with aging, direct mechanical or mitochondrial oxidative injury from noise, and toxicity from aminoglycosides or other ototoxic medications (Liu et al., 2007; Ohlemiller, 2004; Pickles, 2008). Neuronal degeneration of spiral ganglion afferents can also be triggered by cumulative exposures to loud noise leading to glutamate excito-toxicity and loss of the afferent dendrites (Kujawa et al., 2006). Interestingly, such a mechanism of injury may allow for relative preservation of pure tone threshold sensitivity but disproportionate effects on speech perception in noise and speech understanding given the complexity of speech sounds and the need for precise temporal and frequency coding by the spiral ganglion afferents.
The complexity of factors (aging, genetic, epigenetic, environmental, health co-morbidity) and importantly the interaction of the different mechanistic pathways that can cause AHL have greatly complicate our interpretation of basic and clinical research into AHL (Van et al., 2007) and have led to some latent cynicism about the precise value of key factors contributing to AHL (Ohlemiller, 2009). In particular, the same functional consequences of increased hearing thresholds and poor frequency resolution generally occur regardless of etiology of AHL or the cochlear mechanistic pathway (Pickles, 2008). Consequently, for elderly with AHL, the main issue is often the inability to understand words rather than the inability to hear, leading to the refrains of “I can hear you but I can’t understand you” or perhaps more commonly, “My hearing is fine. You’re just mumbling”. Most importantly, AHL gradually impairs an individual’s ability to understand the meaning of everyday language (e.g. “I’ll see you Sunday” versus “I’ll see you someday”), in which fine auditory cues encoding semantic meaning are critical for understanding communicative meaning.
In this review, we have chosen to focus on recent works that have improved our understanding of the cellular and molecular mechanisms that could cause age-related degeneration of the cochlea. Particularly, we have emphasized the role of oxidative stress and mitochondrial dysfunction due to accumulation of mitochondrial DNA (mtDNA) mutations/deletions in the development of AHL.
2. Human studies
2.1. Prevalence of ARHL
Estimating hearing loss prevalence and identifying epidemiologic risk factors can be ascertained from large cohorts where audiometric testing was performed. A sampling of such studies include Beaver Dam (Cruickshanks et al., 2003), Framingham (Gates et al., 1990), Blue Mountains (Gopinath et al., 2009), Baltimore Longitudinal Study of Aging (BLSA) (Brant et al., 1990), and National Health and Nutrition Examination Survey (NHANES) (Agrawal et al., 2008). Reports of hearing loss prevalence across these studies vary because of different tonal frequencies utilized to obtain a pure tone average (PTA), monaural or binaural definition of hearing loss, and audiometric cutoffs used to define hearing loss. Differences in cohort characteristics (volunteer cohort or recruitment of population sample) and the age of the cohort also limit comparisons across studies.
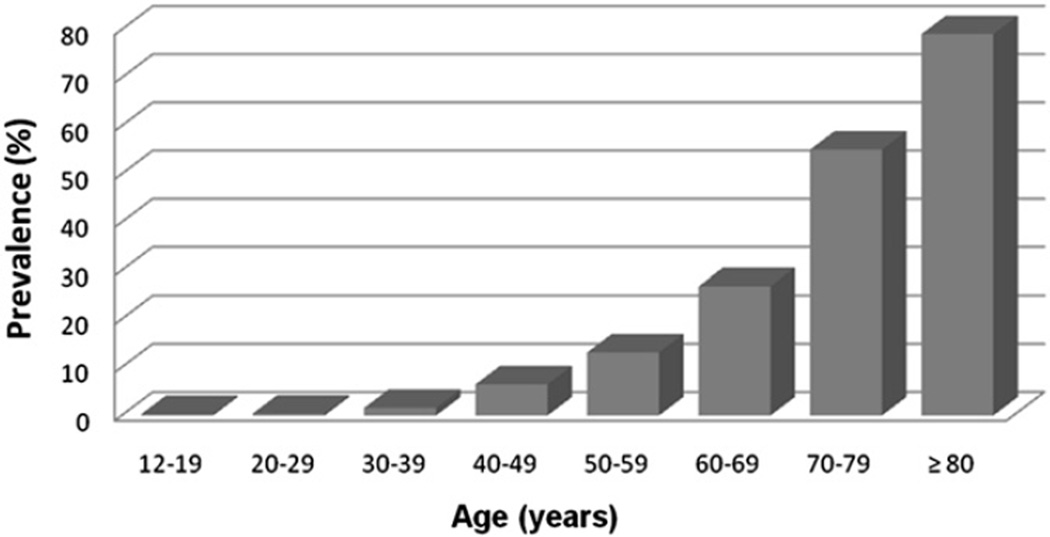
A useful audiometric definition of hearing loss has been adopted by the World Health Organization as a speech-frequency pure tone average of thresholds at 0.5, 1, 2 and 4 kHz tones in the better-hearing ear of >25 dB (World Health Organization). The selected tonal frequency range and the use of the better-hearing ear are useful from a pragmatic perspective that emphasizes communication since 0.5–4 kHz represents the critical frequency range of speech, and the better-hearing ear would be the principal determinant of a person’s communicative abilities. Using this definition of hearing loss and NHANES data (representing a cross-section of the non-institutionalized U.S. population), hearing loss prevalence approximately doubles every decade of life from the second through seventh decades (Fig. 1) (Lin et al., 2011a). Using the same definition of hearing loss, national Institute for longevity sciences-longitudinal study of aging (NILS-LSA) in Japan has reported that the prevalence rates of AHL are 29% in late sixties, 39% in early seventies, and 65% in late seventies in male, and 23%, 37%, and 59% in female, respectively (http://www.ncgg.go.jp/department/ep/monograph5th/sensory.htm).
Fig. 1.
Prevalence of hearing loss in the United States by age, 2001–2008. Hearing loss is defined by a PTA of 0.5–4 kHz thresholds in the better-hearing ear >25 dB.
Other reports of hearing loss prevalence have generally focused on older adults using differing definitions of hearing loss. Prevalence rates have been 29% (>26 dB in the standard PTA [0.5–2 kHz] in the better ear, subjects >60 years), 73% (>25 dB in the speech frequency [0.5–4 kHz] PTA in the worse ear, subjects >70 years), and 60% (>25 dB in the standard PTA in the worse ear, subjects 73–84 years) in the Framingham (Gates et al., 1990), Beaver Dam (Cruickshanks et al., 1998b), and Health ABC (Helzner et al., 2005) studies, respectively. Using identical definitions of hearing loss and age ranges from the latter two studies, prevalence figures calculated using the 2005–2006 NHANES dataset would be 76% and 64%, respectively (Lin et al., 2011a). However, comparing results across different studies is difficult even when applying the same definition of hearing loss given the different demographic characteristics across cohorts particularly with regard to age and race. For example, both the Framingham cohort and Beaver Dam cohorts included few African American individuals, but the Health ABC cohort included 36.3% African American. Age distributions and ranges also varied across these study cohorts. Strength of using NHANES estimates of hearing loss prevalence is that these results are generalizable to the entire civilian, non-institutionalized U.S. population.
2.2. Risk factors for AHL
Epidemiologic studies also provide insight into the modifiable and non-modifiable risk factors associated with hearing loss and provide further insight into the mechanistic pathways underlying AHL. Studied risk factors can generally be divided into four categories as discussed previously (Cooper, 1994; Cruickshanks et al., 1998a, 2003): cochlear aging (individual age), environment (occupational and leisure noise exposure, ototoxic medications, socioeconomic status), genetic predisposition (sex, race, specific genetic loci/genes), and health co-morbidities (hypertension, diabetes, stroke, cigarette smoking). Strong and consistent associations of hearing loss have generally been found with the non-modifiable risk factors of increasing age (increased risk), male sex (increased risk), and African American (decreased risk) (Agrawal et al., 2008; Brant et al., 1990; Gates et al., 1990; Helzner et al., 2005; Ishii et al., 1998; Jerger et al., 1986).
Genetic predisposition as shown by heritability studies among twins and longitudinal studies of family cohorts have also shown heritability indices of 0.35–0.55 (Christensen et al., 2001; Gates et al., 1999; Karlsson et al., 1997), indicating that genetic phenotype accounts for a substantial portion of hearing loss risk. Using general estimation equation analysis, Shimokata (2008) found that 28 out of 177 single nucleotide polymorphisms (SNPs) were associated with impaired hearing in the elderly subjects. Of these, 5 SNPs were significantly related to hearing impairment at low frequencies (125–500 Hz) and other 5 SNPs at high frequencies (2–8 kHz), respectively. The SNPs associated hearing loss at low frequencies were distinct from those at high frequencies, but all these SNPs are known to be associated with atherosclerosis or obesity. The odds ratio of hearing impairment between subjects with all 5 SNPs and those with none of them was 18.6 (95% confidence interval, 4.9–70.8) at low frequencies and 6.5 (95% confidence interval, 3.3–12.7) at high frequencies.
Other factors that have associations with the risk of hearing loss include hypertension and cardiovascular disease, cerebrovascular disease, smoking, diabetes, noise exposure, and alcohol consumption, with all factors being associated with increased risk of hearing loss except for alcohol consumption (Cruickshanks et al., 1998a, 1998b; Dalton et al., 1998; Gates et al., 1993; Helzner et al., 2005; Van et al., 2007; Shimokata, 2008).Cruickshanks et al. (1998a) evaluated the association between smoking and hearing loss in 3753 adults aged 48–92 years, and found that after adjusting for other factors, current smokers were 1.69 times as likely to have a hearing loss as nonsmokers (95% confidence interval, 1.31–2.17), with weak evidence of a dose–response effect. Similarly,Fransen et al. (2008) conducted a multicenter study to elucidate the environmental and medical risk factors contributing to AHL and found that in 4083 subjects between 53 and 67 years, smoking significantly increased high-frequency hearing loss with dose-dependent effect. There have been some inconsistent findings with the latter group of risk factors, which may be a consequence of how hearing loss was defined and the characteristics of the study cohort. For example, noise exposure may primarily lead to high-frequency hearing loss, whereas cardiovascular risk-factors affect both low and high-frequencies. Averaging across frequencies when defining a pure tone average could, therefore, obscure certain associations depending on which tonal frequencies are selected for the PTA. Characteristics of the study cohort may also obscure potential associations depending on the risk factors present in the risk group. For example, in a study focused on only older adults, the factors associated with older age and cochlear aging may overshadow associations with these weaker risk factors. Genetic heterogeneity within cohorts with consequent variability in gene-risk factor interactions (Liu et al., 2007; Van et al., 2007) would also likely bias any possible association toward the null hypothesis.
Previous research into hearing loss epidemiology has emphasized the study of modifiable risk factors in order to form the basis for possible hearing loss prevention strategies. However, the contribution of these modifiable risk factors (e.g. hypertension, etc.) is relatively weak in comparison to the non-modifiable risk factors of genetic predisposition and race as demonstrated by the consistency and strength of associations seen in epidemiologic studies. Further study of these non-modifiable risk factors, particularly the physiologic basis of black race being a protective factor for hearing loss and the identification of the genetic loci and genes contributing to AHL, could possibly offer the most substantial and profound insights into actual hearing loss prevention.
2.3. Impact of race on AHL
Previous observational studies investigating the role of race and hearing loss have consistently demonstrated that black race is associated with a 60–70% lower odd of noise-induced hearing loss and AHL compared to white subjects (Agrawal et al., 2008; Cooper, 1994; Helzner et al., 2005; Lin et al., 2011b). Other epidemiologic studies using a case–control approach recruiting individuals with similar occupational exposures have also demonstrated a reduced risk of hearing loss in black subjects (Ishii et al., 1998; Jerger et al., 1986). A recent epidemiologic study suggests that skin color and hence melanocytic functioning in the cochlea is the mechanism underlying the protective association of race with hearing (Lin et al., 2011b).
Melanin produced by strial melanocytes (intermediate cells) in the cochlea has been hypothesized to serve a protective role as a free radical scavenger, metal chelator, or regulator of calcium homeostasis in the stria vascularis, which is involved with generating and maintaining the endolymphatic potential necessary for normal hearing (Murillo-Cuesta et al., 2010; Riley, 1997). A recent study has also demonstrated that deficiency in strial melanin is associated with marginal cell loss and decline in the endocochlear potential (Ohlemiller et al., 2009). There have not been any further epidemiologic studies exploring the issue of race and hearing loss and little basic science research into mechanistic pathways leading to hearing preservation in individuals with darker skin. The lack of research exploring these topics is surprising, given the strength of the epidemiologic association between race and hearing loss and the fact that melanin pathways in the inner ear could potentially be pharmacologically targeted for hearing loss prevention.
2.4. Candidate genes associated with AHL
The number of genetic investigations on AHL has increased at a surprising rate recently. Association studies analyze genetic variations in unrelated individuals and try to identify those variations that are more frequent in affected individuals compared to unaffected individuals. The ultimate in association studies is a genome-wide association study (GWAS), in which hundreds of thousands of SNPs across the entire genome are analyzed in unrelated individuals. Although the use of GWAS to understand human disease is maturing, GWAS remain prohibitively expensive, and sometimes association studies are limited to a carefully selected set of candidate genes. To date, only several GWAS studies have been performed (Huyghe et al., 2008; Konings et al., 2009; Van et al., 2007, 2008, 2010; Friedman et al., 2009; Girotto et al., 2011); however, these studies have been limited in only studying a certain subset of potential genes or markers (i.e. those associated with monogenic forms of deafness) rather than examining a broad array (>106) of various polymorphisms.
Candidate-gene-based association studies also have been extensively carried out recently. This approach is based on the selection of candidate genes, which are usually implicated in a biological pathway that is plausibly related to a specific disease. A whole range of candidate genes can be proposed because perception of sound involves many complex pathways and age-related changes in any component of one such pathway could contribute to AHL. Genes causing monogenic forms of hearing loss are candidate susceptibility genes for AHL and other genes can be candidates because of a known or presumed function in the inner ear. With these considerations in mind, a number of researchers have speculated that oxidative stress, and consequently, mitochondrial DNA mutations, have important causative roles in the development of AHL. Several genes and loci have been proposed using candidate gene approaches (see review by Uchida et al., 2011), which included DFNA18 and DFNA5 loci, chromosome 8q24, 13-kb region of KCNQ4 (Potassium channel, voltage gated, subfamilyQ, member 4), N-acetyltransferase 2 grainyhead like 2, glutamate receptor metabotropic 7, glutathione S-transferase (GST), apolipoprotein E allele 34, endothelin-1 (EDN1), mitochondrial uncoupling protein 2 (UCP2), and mitochondrial DNA mutations.
Interestingly, some of the candidate genes are well known to be associated with oxidative stress and atherosclerosis. For example, GSTs, one of glutathione-related antioxidant enzymes, catalyze conjugation of glutathione with xenobiotics and other compounds and play an important role in the antioxidant protection of the cochlea (el Barbary et al., 1993). Decreased glutathione and GST activity levels cause increased susceptibility of cells to insults and cell damage. When glutathione level is lower, cochlea becomes more vulnerable to intense noise (Yamasoba et al., 1998) and aminoglycoside-induced hearing loss (Lautermann et al., 1995). Van Eyken et al. (2007) investigated an association between AHL and genes related to oxidative stress using a large set of 2111 independent samples from two population groups, the general European and the Finnish population. Although they did not detect an association between GSTM1 (mu, chromosome 1p13.3), or GSTT1 (theta, chromosome 22q11.2) and AHL in the former population, there were significant associations between both genes and AHL in the latter population.
UCPs are members of the larger family of mitochondrial anion carrier proteins They facilitate the transfer of anions from the inner to the outer mitochondrial membrane and the return transfer of protons from the outer to the inner mitochondrial membrane and also reduce the mitochondrial membrane potential in mammalian cells. UCPs play a role in non-shivering thermogenesis, obesity, diabetes and atherosclerosis, but the main function of UCP2 is the control of mitochondria-derived ROS (Arsenijevic et al., 2000). Recently,Sugiura et al. (2010) reported that UCP2 Ala55Val polymorphisms, but not UCP1 A-3826G polymorphism, exhibited significant association with AHL in the Japanese population.
Endothelin is a potent vasoactive peptide that is synthesized and released by the vascular endothelium and the best-characterized endothelin, EDN1, is involved in the development of atherosclerosis. Several SNPs in EDN1 gene have been shown to be associated with atherosclerosis, coronary disease and hypertension (for example, Yasuda et al., 2007). Further, EDN1 can induce a strong, long-lasting constriction of the spiral modiolar artery, causing an ischemic stroke of the inner ear (Scherer et al., 2005).Uchida et al. (2009) has observed significant association between the Lys198Asn (G/T) polymorphism (rs5370) in the EDN1 gene and hearing loss in middle-aged and elderly Japanese.
2.5. Mitochondrial DNA mutations and AHL
Increases of deletions, mutations, or both in mtDNA have been reported in human archival temporal bone samples from people with AHL compared to normal hearing control tissues.Bai et al. (1997) examined mtDNA from celloidin-embedded temporal bone sections of 34 human temporal bones, 17 with normal hearing and 17 with AHL, and found that a 4977-base pair (bp) deletion, called a ‘common ageing deletion,’ was significantly more frequent in the cochlear tissues from patients with AHL compared to those with normal hearing.Markaryan et al. (2009) evaluated the association between the common ageing deletion level in cochlear tissue and the severity of hearing loss in elderly subjects and found that a mean level of the deletionwas 32 ± 14% in subjects with AHL and 12 ± 2% in the normal-hearing age-matched controls, with statistical significance. They also observed the reduction of cytochrome c oxidase subunit 3 (COX3) expression in spiral ganglion cells from individuals with AHL, and in addition to the mtDNA common ageing deletion, other deletions involving the mtDNA major arc contributed to the observed deficit in COX 3 expression (Markaryan et al., 2010). Sporadic mtDNA mutations are also likely to contribute to the manifestation of AHL. Fischel-Ghodsian et al. (1997) examined the archival temporal bones from five patients with AHL for mutations within the mitochondrially-encoded cytochrome oxidase II gene and when compared to controls, the mutations occurred more commonly with AHL despite great individual variability in both quantity and location of mutation accumulation.
3. AHL studies in animals
3.1. General pathological and physiological findings
As discussed earlier, AHL is generally classified into three major types based on the relationship between cochlear pathology and hearing levels: sensory (loss of sensory hair cells), neuronal (loss of spiral ganglion neurons), and metabolic (strial atrophy) hearing loss (Schuknecht, 1955). Age-related stria atrophy or degeneration is one of the common features of AHL in both animals and humans (Gates and Mills, 2005; Ohlemiller, 2009; Fetoni et al., 2011). Aged gerbils display loss of stria capillaries (Gratton and Schulte, 1995), degeneration of marginal and intermediate cells of the stria vascularis (Gates and Mills, 2005; Spicer and Schulte, 2005), and loss of Na+K+ ATPase (Schulte and Schmiedt, 1992), which regulates stria function and endcochlear potential (EP) through transporting Na+ out, while transporting K+ into the cell (Spicer and Schulte, 2005). The loss of function of the cells in the stria vascularis and/or spiral ligament is thought to result in disruption of inner ear ion homeostasis, thereby causing a decline in EP. Consistent with this view, aged gerbils display an age-related decline in EP as well as disruption of ion homeostasis in the cochlea (Schmiedt, 1996).
There are several mouse models of aging and age-related diseases that display a variety of premature aging phenotypes, including a reduced lifespan and early onset of AHL. C57BL/6J mouse strain, one of the most widely used models for the study of aging and age-associated diseases, display loss of the hair cells and spiral ganglion neurons and increased hearing thresholds by 12 months of age (Zheng et al., 1999). Aged C57BL/6 mice display an age-related decline in the density of spiral ligament and stria vascularis (Ichimiya et al., 2000) and also an age-related decrease in the cross-sectional area of the stria vascularis as well as the survival of the Type IV fibrocytes in the spiral ligament (Hequembourg and Liberman, 2001). Interestingly, an age-related decline in EP was observed in CBA/CaJ mice and BALB/cJ mice, but not in C57BL/6 or CBA/J (Lang et al., 2002; Sha et al., 2008), which suggests that decreased EP may not be a key common feature of AHL. Since inbred mouse strains have a wide range of noise sensitivities and rates of hearing loss with age, they may not be good model for the heterogeneity of the human population. An animal population featuring a genetically heterogenous background, late onset of hearing loss and a well defined range of sensitivity to environmental factors might provide a more informative model for human AHL.Schacht et al. (2012) tested four-way cross mice from 4 parental strains, MOLF/Ei, C3H/HeJ, FVB/NJ, and 129/SvImJ, and identified several polymorphisms affecting hearing in later life (loci on chromosomes 2, 3, 7, 10, and 15 at 18 months, on chromosomes 4, 10, 12, and 14 at 22 months in noise-exposed mice, and on chromosomes 10 and 11 in those not exposed to noise). Such four-way cross mice, in which each in the progeny shares a random 50% of its genetic heritage with each other, are considered to have the advantages of providing robustness, reproducibility, and genetic tractability (Miller et al., 1999) and thus are worth for future AHL studies.
3.2. Role of ROS in AHL
It has been postulated that reactive oxygen species (ROS) play a major role in the degeneration of these cochlear cells during aging (Cheng et al., 2005; Someya et al., 2009). It is now well established that mitochondria are a major source of ROS (Balaban et al., 2005; Lin and Beal, 2006; Wallace, 2005) and that the majority of intra-cellular ROS are continuously generated as a by-product of mitochondrial respiration metabolism during the generation of ATP (Balaban et al., 2005; Beckman and Ames, 1998; Halliwell and Gutteridge, 2007). These ROS include superoxide (•O2−) and hydroxyl radical (•OH) which are extremely unstable, and hydrogen peroxide (H2O2) which is freely diffusible and relatively long-lived (Balaban et al., 2005; Beckman and Ames, 1998; Halliwell and Gutteridge, 2007). ROS generated inside mitochondria are hypothesized to damage key cell components such as nuclear DNA, mitochondrial DNA (mtDNA), membranes, and proteins. Such oxidative damage accumulates over time and leads to tissue dysfunction during aging. This by no means is in any way special to the inner ear, but has been ubiquitously found in all systems. An elaborate antioxidant system has evolved to control the damaging effects of those ROS. The system includes the antioxidant enzymatic scavengers, such as superoxide dismutase (SOD), catalase, GST, and glutathione peroxidase (Gpx) (see Halliwell and Gutteridge, 2007). SOD decomposes superoxide (O2−) into hydrogen peroxide (H2O2) and oxygen (O2), while catalase and Gpx decomposes hydrogen peroxide into water (H2O) and oxygen (Halliwell and Gutteridge, 2007).
It has been shown that increased Gpx activity was observed in the stria vascularis and spiral ligament in the cochlea of aged Fisher 344 rats (Coling et al., 2009). In the organ of Corti of CBA mice, glutathione-conjugated proteins, markers of H2O2-mediated oxidation, began to increase at 12 months of age and 4-hydroxynonenal and 3-nitrotyrosine, products of hydroxyl radical and peroxynitrite action, respectively, were elevated by 18 months, whereas antioxidant proteins AIF and enzymes SOD2 decreased by 18 months (Jiang et al., 2007). Age-related cochlear hair cell loss was enhanced in mice lacking the antioxidant enzyme SOD1 (McFadden et al., 1999), and reduced thickness of the stria vascularis and severe degeneration of spiral ganglion neurons were observed in middle-aged SOD1 knockout mice (Keithley et al., 2005). Similarly, mice lacking senescence marker protein 30 (SMP30)/gluconolactonase (GNL), which could not synthesize vitamin C (VC), showed reduction of VC in the inner ear, increased hearing thresholds, and loss of spiral ganglion cells, suggesting that VC depletion accelerates AHL (Kashio et al., 2009). Conversely overexpression of catalase in the mitochondria reduced oxidative DNA damage in the cochlea and slowed AHL in C57BL/6 mice (Someya et al., 2009). These findings implicate that oxidative damage in the cochlea reflects an age-related decline in the antioxidant defenses and/or an age-related increase in ROS levels and pays a crucial role in the development of AHL.
Several studies have been conducted to examine the effects of antioxidants against AHL. Seidman (2000) conducted a randomized prospective study over a 3-year period, in which Fischer 344 rats were given vitamin E, VC melatonin, or lazaroid, and observed that the antioxidant-treated animals had better auditory sensitivities and a trend for fewer mtDNA deletions compared with placebo subjects.Seidman et al. (2002) also examined the effects of lecithin, a polyunsaturated phosphatidylcholine that plays a rate-limiting role in the activation of numerous membrane-located enzymes including SOD and glutathione, on aging and AHL. When Harlan–Fischer rats aged 18–20 months were divided into controls and experimental group supplemented orally for 6 months with lecithin, lecithin-treated animals showed significantly better hearing sensitivities, higher mitochondrial membrane potentials, and less common ageing mtDNA deletion in the cochlear tissues including stria vascularis and auditory nerve compared to controls. Le and Keithley (2007) demonstrated that aged dogs fed a high antioxidant diet for the last 3 years of their life showed less degeneration of the spiral ganglion cells and stria vascularis compared to dog fed control-diet.
In C57BL/6 mice, supplementation with VC did not increase VC levels in the cochlear tissue or slow AHL (Kashio et al., 2009), but animals fed with diet comprising six antioxidant agents (l-cysteine-glutathione mixed disulfide, ribose-cysteine, NW-nitro-l-arginine methyl ester, vitamin B12, folate, and ascorbic acid) exhibited significantly better hearing sensitivity than controls (Heman-Ackah et al., 2010). When C57BL/6 mice were fed with control diet or diet containing one of 17 antioxidant compounds (acetyl-l-carnitine, α-lipoic acid, carotene, carnosine, coenzyme Q10, curcumin, tocopherol, EGCG, gallic acid, lutein, lycopene, melatonin, poanthocyanidin, quercetin, resveratrol, and tannic acid), AHL was nearly completely prevented by α-lipoic acid and coenzyme Q10 and partially by N-acetyl-l-cysteine, but not by other compounds (Someya et al., 2009). In CBA/J mice, antioxidant-enriched diet containing vitamins A, C, and E, l-carnitine, and α-lipoic acid given from 10 months through 24 months of age significantly increased the antioxidant capacity of the inner ear tissues but did not ameliorate AHL or loss of the hair cells and spiral ganglion cells (Sha et al., 2012). These findings indicate that supplementation with certain antioxidants can slow AHL in animals but that the effects depends on many factors, including the type and dosage of anti-oxidant compounds, timing and duration of the treatment, species, and strains. Defining these factors and those we’ve yet to identify is one of the goals in future research.
3.3. Effect of calorie restriction against AHL
Caloric restriction (CR) extends the lifespan of most mammalian species and is the only intervention shown to slow the rate of aging in mammals. Maximum lifespan is thought to be increased by reducing the rate of aging, while the average lifespan can be increased by improving environmental conditions. In laboratory rodents, CR delays the onset of age-related diseases such as lymphomas, prostate cancer, nephropathy, cataracts, diabetes, hypertension, and hyperlipidemia, and autoimmune diseases (see Sohal and Weindruch, 1996; Mair and Dillin, 2008). Despite such evidence, the question remains whether CR also acts to retard aging and disease in higher species such as non-human primates and humans. In monkeys, CR has been reported to result in signs of improved health including reduced body fat, higher insulin sensitivity, increase in high-density lipoprotein and reduction in very low-density lipoprotein levels (Rezzi et al., 2009). Twenty-year longitudinal adult-onset CR study in rhesus macaques maintained at the Wisconsin National Primate Research Center (WNPRC) demonstrated that moderate CR lowered the incidence of aging-related deaths and delayed the onset of age-associated pathologies, such as diabetes, cancer, cardiovascular disease, and brain atrophy (Colman et al., 2009). Very recently, a CR regimen implemented in young and older age rhesus monkeys at the National Institute on Aging (NIA) has been shown not to improve survival outcomes, contrast with an ongoing study at WNPRC, suggesting a separation between health effects, morbidity and mortality (Mattison et al., 2012).
It is difficult to determine whether CR has beneficial effects on longevity and age-related diseases in humans because there are no validated biomarkers that can serve as surrogate markers of aging and because it is impractical to conduct randomized, diet-controlled, long-term survival studies in humans. Nonetheless, data from epidemiologic studies suggest that CR may have beneficial effects on the factors involved in the pathogenesis of primary and secondary aging and life expectancy in humans. Food shortages during World War in European countries were associated with a sharp decrease in coronary heart disease mortality, which increased again after the war ended (Hindhede, 1921; Strom and Jensen, 1951). Another study among Spanish nursing home residents undergoing long-term alternate day feeding regimen also demonstrated decreased morbidity and mortality (Vallejo, 1957). In addition, inhabitants of Okinawa island, who ate ≈30% fewer calories than the rest of Japanese residents, had ≈35% lower rates of cardiovascular disease and cancer mortality than the average Japanese population and had one of the highest numbers of centenarians in the world (Kagawa, 1978). Due to the Westernization on the nutrition, resulting in increased meat intake and fat energy ratio and decreased intake of beans and vegetables, the longest life expectancy at birth for men in Okinawa is now no higher than the national average in Japan, reflecting increased mortality ratio due to heart disease and cerebrovascular disease (Miyagi et al., 2003). It should be noted, however, that these associations do not prove causality between decreased calorie intake and increased survival and that CR studies in humans did not always show influence on age-related changes.
The preventive effect of CR against AHL has been inconsistent across reports (see review by Someya et al., 2010a). Fischer rats that were calorie restricted to 70% of the control intake beginning at one month of age and then housed for 24–25 months showed significantly better hearing thresholds, reduced hair cell loss, and decreased mtDNA common deletion in the auditory nerve and stria vascularis of the cochlea compared to controls (Seidman, 2000). CR also delayed the onset of AHL in the AU, CBA and B6 strains of mice, but not in the DBA, WB, or BALB strains. Beneficial effects by CR have been reported in monkeys maintained at WNPRC, but not in those at NIA. Interestingly, high fat diet given for 12 month, which is opposite to CR, elevated hearing thresholds at high-frequency region and increased ROS generation, expressions of NADPH oxidase and UCP, accumulation of mtDNA common deletion, and cleaved caspase-3 and TUNEL-positive cells in the inner ear of Sprague–Dawley rats (Du et al., 2012).
The underlying mechanisms for the CR-associated benefits remain unclear.Someya et al. (2007b) observed that C57B/6 mice that received CR by 15 months of age retained normal hearing and showed no obvious cochlear degeneration and a significant reduction in the number of TUNEL-positive cells and cleaved caspase-3-positive cells in the spiral ganglion cells compared to age-matched controls; microarray analysis also revealed that CR down-regulated the expression of 24 apoptotic genes, including Bak (BCL2-antagonist/killer 1) and Bim (BCL2-like 11), suggesting that CR could prevent apoptosis of the cochlear cells. In addition, oxidative stress by paraquat induced Bak expression and apoptosis in primary cochlear cells, which was ameliorated in Bak-deficient cells (Someya et al., 2009). Furthermore, a mitochondrially targeted catalase transgene and oral supplementation with α-lipoic acid and coenzyme Q10 suppressed Bak expression in the cochlea, reduced cochlear cell death, and prevented AHL, suggesting that oxidative stress induces Bak-dependent apoptosis in the cochlear cells (Someya et al., 2009). It has recently been reported that CR failed to reduce oxidative DNA damage and prevent AHL in C57B/6 mice lacking the mitochondrial deacetylase Sirt3, a member of the sirtuin family (Someya et al., 2010b). In response to CR, Sirt3 directly deacetylated and activated mitochondrial isocitrate dehydrogenase 2 (Idh2), leading to increased NADPH levels and an increased ratio of reduced-to-oxidized glutathione in mitochondria. In cultured cells, overexpression of Sirt3 and/or Idh2 increased NADPH levels and protected from oxidative stress-induced cell death. These findings strongly suggest that at least a primary mechanism underlying the beneficial effects of CR is mediated by ROS-antioxidant systems and that Sirt3 is essential in enhancing the mitochondrial glutathione antioxidant defense system in the cochlea during CR.
3.4. Mitochondrial dysfunction and mitochondrial DNA mutations in AHL
Recent development of DNA microarray analysis has provided a global analysis of gene expression in the aging tissues. Someya et al. (2007a) compared gene expression profiles in the cochlea between 2-month-old and 8-month-old DBA/2J and found that AHL was associated with profound down-regulation of genes involved in the mitochondrial respiratory chain complexes in the cochlea of aged DBA/2J mice. A comparison of cochleae from middle aged C57B/6 mice under CR and normal control diet revealed that genes involved in apoptosis were down-regulated whereas those involved in mitochondrial function and DNA repair were up-regulated as a result of CR (Someya et al., 2007b).
As discussed before, mtDNA mutations and common ageing deletions have been reported to increase with aging in human temporal bones (Bai et al., 1997; Markaryan et al., 2009, 2010; Fischel-Ghodsian et al., 1997). It has been shown that accumulation of mtDNA mutations leads to premature aging in mitochondrial mutator mice (Polg knockin mice), indicating a causal role of mtDNA mutations in mammalian aging (Kujoth et al., 2005; Trifunovic et al., 2004). The Polg knockin mice were created by introducing a two base substitution, which results in a defect in mtDNA proof-reading ability. Young Polg mutator mice were indistinguishable from wild-type WT littermates, but 9–10 months old mutator mice displayed a variety of premature aging phenotypes, including early onset of AHL, severe loss of the spiral ganglion neurons, degeneration of the stria vascularis, and increase of TUNEL-positive spiral ganglion cells, while age-matched wild-type mice displayed only minor loss/degeneration of the cochlear cells (Someya et al., 2008). DNA microarray analysis revealed that mtDNA mutations were associated with transcriptional alterations consistent with impairment of energy metabolism, induction of apoptosis, cytoskeletal dysfunction, and hearing dysfunction in the cochlea of aged Polg mutator mice.Niu et al. (2007) also reported that the mtDNA mutator mice showed progressive apoptotic cell loss in the spiral ganglion, increased pathology in the stria vascularis, and accelerated progressive degeneration in the neurons in the cochlear nucleus compared to wild-type mice. These findings imply that accumulation of mtDNA mutations lead to mitochondrial dysfunction, an associated impairment of energy metabolism, and the induction of an apoptotic program in the cochlea.
4. Putative mechanisms of AHL
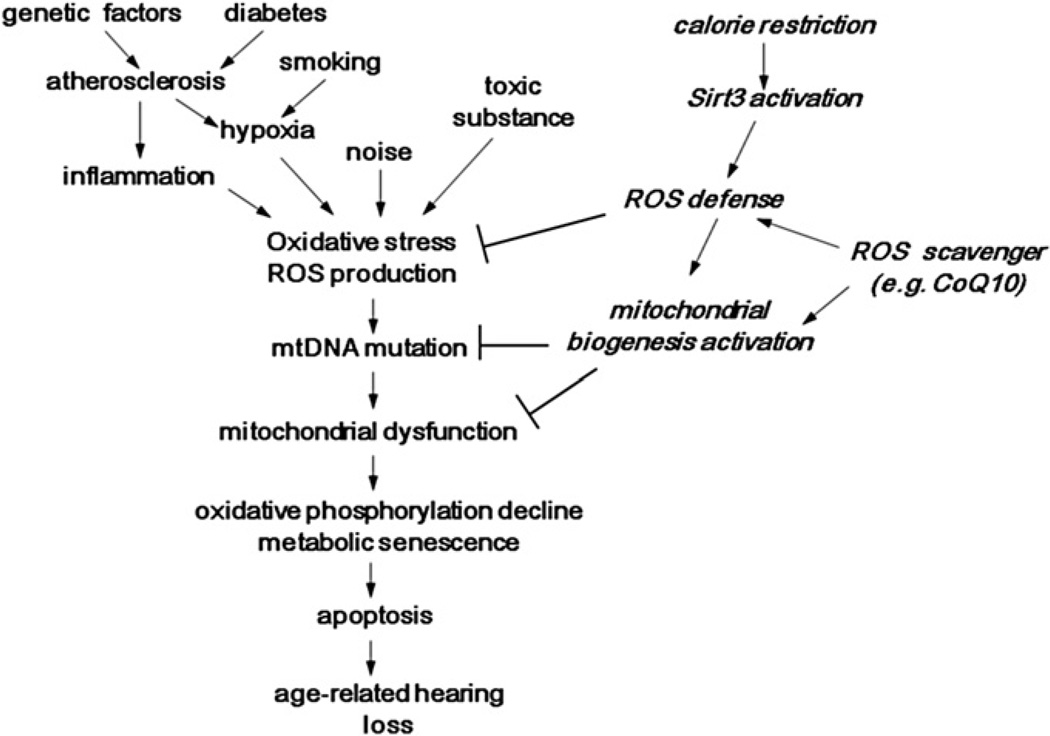
As discussed above by reviewing recent human and animal studies, it is now well established that oxidative stress and mtDNA mutations/deletions play a crucial role in the development of AHL. Substantial evidence has accumulated from animal studies that cumulative effect of oxidative stress could induce damage to macromolecules such as mtDNA in the cochlea and that the resulting accumulation of mtDNA mutations/deletions and decline of mitochondrial function over time progressively induce (Bak-dependent) apoptosis of the cochlear cells. Epidemiological human studies have demonstrated four categories of risk factors of AHL, i.e., cochlear aging, environment such as noise exposure, genetic predisposition, and health co-morbidities such as cigarette smoking and atherosclerosis. Genetic investigation has identified several putative associating genes, including those related to antioxidant defense system and atherosclerosis. Exposure to noise is known to induce excess generation of reactive oxygen species (ROS) in the cochlea, and cumulative oxidative stress can be enhanced by relatively hypoxic situations resulting from the impaired homeostasis of cochlear blood supply due to atherosclerosis, which could be accelerated by genetic and co-morbidity factors. Antioxidant defense system may also be influenced by genetic backgrounds including race. The conceptual figure of the model for the development of AHL has been shown in Fig. 2. This may explain the large variations of the onset and extent of AHL among elderly subjects. AHL has been shown to be slowed by certain interventions, such as CR and supplementation with antioxidants, in laboratory animals. Large clinical trials are needed to investigate if AHL can be delayed or prevented in humans and gain insights into the molecular mechanisms of AHL. Given the social value, quality of life and economic costs of AHL and the safety of many of the potentially effective interventions, we hope that such trials will begin in the near future.
Fig. 2.
Conceptual model of the development of age-related hearing loss.
Acknowledgements
This work was supported by grants from 1) The University of Tokyo, Global Center of Education and Research for Chemical Biology of the Diseases, 2) Ministry of Education, Culture, Sports, Science & Technology, and 3) Ministry of Health, Labour and Welfare in Japan to T.Y., NIH/NIDCD 1R03DC011840-01, American Federation for Aging Research to S.S, NIH K23DC011279, Triological Society/American College of Surgeons Clinician Scientist Award, and Eleanor Schwartz Charitable Foundation to F.R.L.
Footnotes
Disclosures
Dr. Lin has served as a consultant to Pfizer, Autifony, and Cochlear Corp. Dr. Lin is on the scientific advisory board of Autifony.
References
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch. Intern. Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bai U, Seidman MD, Hinojosa R, Quirk WS. Mitochondrial DNA deletions associated with aging and possibly presbycusis: a human archival temporal bone study. Am. J. Otol. 1997;18:449–453. [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Brant LJ, Fozard JL. Age changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. J. Acoust. Soc. Am. 1990;88:813–820. doi: 10.1121/1.399731. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr. Opin. Otolaryngol. Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Christensen K, Frederiksen H, Hoffman HJ. Genetic and environmental influences on self-reported reduced hearing in the old and oldest old. J. Am. Geriatr. Soc. 2001;49:1512–1517. doi: 10.1046/j.1532-5415.2001.4911245.x. [DOI] [PubMed] [Google Scholar]
- Coling D, Chen S, Chi LH, Jamesdaniel S, Henderson D. Age-related changes in antioxidant enzymes related to hydrogen peroxide metabolism in rat inner ear. Neurosci. Lett. 2009;464:22–25. doi: 10.1016/j.neulet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC., Jr Health and Nutrition Examination Survey of 1971–75: part I. Ear and race effects in hearing. J. Am. Acad. Audiol. 1994;5:30–36. [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA. 1998a;279:1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The epidemiology of hearing loss study. Am. J. Epidemiol. 1998b;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BE, Klein R, Chappell R, Nondahl DM, Dalton DS. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch. Otolaryngol. Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein R, Klein BE, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998;21:1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- Du Z, Yang Y, Hu Y, Sun Y, Zhang S, Peng W, Zhong Y, Huang X, Kong W. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of D-galactose-induced aging rats. Hear. Res. 2012;287:15–24. doi: 10.1016/j.heares.2012.04.012. [DOI] [PubMed] [Google Scholar]
- el Barbary A, Altschuler RA, Schacht J. Glutathione S-transferases in the organ of Corti of the rat: enzymatic activity, subunit composition and immunohistochemical localization. Hear. Res. 1993;71:80–90. doi: 10.1016/0378-5955(93)90023-t. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Picciotti PM, Paludetti G, Troiani D. Pathogenesis of presbycusis in animal models: a review. Exp. Gerontol. 2011;46:413–425. doi: 10.1016/j.exger.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N, Bykhovskaya Y, Taylor K, Kahen T, Cantor R, Ehrenman K, Smith R, Keithley E. Temporal bone analysis of patients with presbycusis reveals high frequency of mitochondrial mutations. Hear. Res. 1997;110:147–154. doi: 10.1016/s0378-5955(97)00077-4. [DOI] [PubMed] [Google Scholar]
- Fransen E, Topsakal V, Hendrickx JJ, Van Laer L, Huyghe JR, Van Eyken E, Lemkens N, Hannula S, Mäki-Torkko E, Jensen M, Demeester K, Tropitzsch A, Bonaconsa A, Mazzoli M, Espeso A, Verbruggen K, Huyghe J, Huygen PL, Kunst S, Manninen M, Diaz-Lacava A, Steffens M, Wienker TF, Pyykkö I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning P, Van Camp G. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter studyJAssoc. Res. Otolaryngol. 2008;9:264–276. doi: 10.1007/s10162-008-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, Tembe WD, Halperin RF, Thorburn AQ, Thys S, Bonneux S, Fransen E, Huyghe J, Pyykkö I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Makmura L, Ohmen JD, Linthicum FH, Jr, Fayad JN, Pearson JV, Craig DW, Stephan DA, Van Camp G. GRM7 variants confer susceptibility to age-related hearing impairment. Hum. Mol. Genet. 2009;18:785–796. doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch. Otolaryngol. Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch. Otolaryngol. Head Neck Surg. 1999;125:654–659. doi: 10.1001/archotol.125.6.654. [DOI] [PubMed] [Google Scholar]
- Girotto G, Pirastu N, Sorice R, Biino G, Campbell H, d’Adamo AP, Hastie ND, Nutile T, Polasek O, Portas L, Rudan I, Ulivi S, Zemunik T, Wright AF, Ciullo M, Hayward C, Pirastu M, Gasparini P. Hearing function and thresholds: a genome-wide association study in European isolated populations identifies new loci and pathways. J. Med. Genet. 2011;48:369–374. doi: 10.1136/jmg.2010.088310. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Rochtchina E, Wang JJ, Schneider J, Leeder SR, Mitchell P. Prevalence of age-related hearing loss in older adults: Blue Mountains Study. Arch. Invest. Med. 2009;169:415–416. doi: 10.1001/archinternmed.2008.597. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schulte BA. Alterations in microvasculature are associated with atrophy of the stria vascularis in quiet-aged gerbils. Hear. Res. 1995;82:44–52. doi: 10.1016/0378-5955(94)00161-i. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. fourth ed. Oxford, New York: Oxford University Press; 2007. [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, de RN, Harris TB, Rubin SM, Simonsick EM, Tylavsky FA, Newman AB. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Heman-Ackah SE, Juhn SK, Huang TC, Wiedmann TS. A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol. Head Neck Surg. 2010;143:429–434. doi: 10.1016/j.otohns.2010.04.266. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J. Assoc. Res. Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindhede M. The effects of food restriction during war on mortality in Copenhagen. JAMA. 1921;74:381–382. [Google Scholar]
- Huyghe JR, Van LL, Hendrickx JJ, Fransen E, Demeester K, Topsakal V, Kunst S, Manninen M, Jensen M, Bonaconsa A, Mazzoli M, Baur M, Hannula S, Maki-Torkko E, Espeso A, Van EE, Flaquer A, Becker C, Stephens D, Sorri M, Orzan E, Bille M, Parving A, Pyykko I, Cremers CW, Kremer H, Van de Heyning PH, Wienker TF, Nurnberg P, Pfister M, Van CG. Genome-wide SNP-based linkage scan identifies a locus on 8q24 for an age-related hearing impairment trait. Am. J. Hum. Genet. 2008;83:401–407. doi: 10.1016/j.ajhg.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya I, Suzuki M, Mogi G. Age-related changes in the murine cochlear lateral wall. Hear. Res. 2000;139:116–122. doi: 10.1016/s0378-5955(99)00170-7. [DOI] [PubMed] [Google Scholar]
- Ishii EK, Talbott EO. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. J. Occup. Environ. Med. 1998;40:661–666. doi: 10.1097/00043764-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Jerger J, Jerger S, Pepe P, Miller R. Race difference in susceptibility to noise-induced hearing loss. Am. J. Otol. 1986;7:425–429. [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol. Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev. Med. 1978;7:205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Karlsson KK, Harris JR, Svartengren M. Description and primary results from an audiometric study of male twins. Ear Hear. 1997;18:114–120. doi: 10.1097/00003446-199704000-00003. [DOI] [PubMed] [Google Scholar]
- Kashio A, Amano A, Kondo Y, Sakamoto T, Iwamura H, Suzuki M, Ishigami A, Yamasoba T. Effect of vitamin C depletion on age-related hearing loss in SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 2009;390:394–398. doi: 10.1016/j.bbrc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear. Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A, Van LL, Van CG. Genetic studies on noise-induced hearing loss: a review. Ear Hear. 2009;30:151–159. doi: 10.1097/AUD.0b013e3181987080. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J. Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Endocochlear potentials and compound actionpotential recovery: functions in the C57BL/6J mouse. Hear. Res. 2002;172:118–126. doi: 10.1016/s0378-5955(02)00552-x. [DOI] [PubMed] [Google Scholar]
- Lautermann J, McLaren J, Schacht J. Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hear. Res. 1995;86:15–24. doi: 10.1016/0378-5955(95)00049-a. [DOI] [PubMed] [Google Scholar]
- Le T, Keithley EM. Effects of antioxidants on the aging inner ear. Hear. Res. 2007;226:194–202. doi: 10.1016/j.heares.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United StatesJGerontol. A Biol. Sci. Med. Sci. 2011a;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Maas P, Chien W, Carey JP, Ferrucci L, Thorpe R. Association of skin color, race/ethnicity, and hearing loss among adults in the USA. J. Assoc. Res. Otolaryngol. 2011b;13:109–117. doi: 10.1007/s10162-011-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J. Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Markaryan A, Nelson EG, Hinojosa R. Quantification of the mitochondrial DNA common deletion in presbycusis. Laryngoscope. 2009;119:1184–1189. doi: 10.1002/lary.20218. [DOI] [PubMed] [Google Scholar]
- Markaryan A, Nelson EG, Hinojosa R. Major arc mitochondrial DNA deletions in cytochrome c oxidase-deficient human cochlear spiral ganglion cells. Acta Otolaryngol. 2010;130:780–787. doi: 10.3109/00016480903397702. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol. Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Miller RA, Austad S, Burke D, Chrisp C, Dysko R, Galecki A, Jackson A, Monnier V. Exotic mice as models for aging research: polemic and prospectus. Neurobiol. Aging. 1999;20:217–231. doi: 10.1016/s0197-4580(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Miyagi S, Iwama N, Kawabata T, Hasegawa K. Longevity and diet in Okinawa, Japan: the past, present and future. Asia Pac. J. Public Health. 2003;15(Suppl.):S3–S9. doi: 10.1177/101053950301500S03. [DOI] [PubMed] [Google Scholar]
- Murillo-Cuesta S, Contreras J, Zurita E, Cediel R, Cantero M, Varela-Nieto I, Montoliu L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment. Cell Melanoma Res. 2010;23:72–83. doi: 10.1111/j.1755-148X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- Niu X, Trifunovic A, Larsson NG, Canlon B. Somatic mtDNA mutations cause progressive hearing loss in the mouse. Exp. Cell Res. 2007;313:3924–3934. doi: 10.1016/j.yexcr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Age-related hearing loss: the status of Schuknecht’s typology. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:439–443. doi: 10.1097/01.moo.0000134450.99615.22. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rice ME, Lett JM, Gagnon PM. Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear. Res. 2009;249:1–14. doi: 10.1016/j.heares.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Pickles JO. An Introduction to the Physiology of Hearing. Bingley, UK: Emerald Group Publishing; 2008. [Google Scholar]
- Rezzi S, Martin FP, Shanmuganayagam D, Colman RJ, Nicholson JK, Weindruch R. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp. Gerontol. 2009;44:356–362. doi: 10.1016/j.exger.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley PA. Melanin. Int. J. Biochem. Cell Biol. 1997;29:1235–1239. doi: 10.1016/s1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Schacht J, Altschuler R, Burke DT, Chen S, Dolan D, Galecki AT, Kohrman D, Miller RA. Alleles that modulate late life hearing in genetically heterogeneous mice. Neurobiol. Aging. 2012;33(1842):e15–e29. doi: 10.1016/j.neurobiolaging.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer EQ, Arnold W, Wangemann P. Pharmacological reversal of endothelin-1 mediated constriction of the spiral modiolar artery: a potential new treatment for sudden sensorineural hearing loss. BMC Ear Nose Throat Disord. 2005;5:10. doi: 10.1186/1472-6815-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear. Res. 1996;102:125–132. doi: 10.1016/s0378-5955(96)00154-2. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura HO, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J. Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF. Presbycusis. Laryngoscope. 1955;65:402–419. doi: 10.1097/00005537-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann. Otol. Rhinol. Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Watanuki K, Takahashi T, Belal AA, Jr, Kimura RS, Jones DD, Ota CY. Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope. 1974;84:1777–1821. doi: 10.1288/00005537-197410000-00012. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear. Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol. Head Neck Surg. 2002;127:138–144. doi: 10.1067/mhn.2002.127627. [DOI] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear. Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Halsey K, Wearne KA, Schacht J. Antioxidant-enriched diet does not delay the progression of age-related hearing loss. Neurobiol. Aging. 2012;33(1010):e15–e16. doi: 10.1016/j.neurobiolaging.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokata H. Why are individual differences in the hearing ability so great in the elderly? Relationship of hearing with systemic aging. Audiol. Jpn. 2008;51:177–184. [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Prolla TA, Tanokura M. Genes encoding mitochondrial respiratory chain components are profoundly down-regulated with aging in the cochlea of DBA/2J mice. Brain Res. 2007a;1182:26–33. doi: 10.1016/j.brainres.2007.08.090. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol. Aging. 2007b;28:1613–1622. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, Prolla TA. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol. Aging. 2008;29:1080–1092. doi: 10.1016/j.neurobiolaging.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. SciU.SA. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Tanokura M, Weindruch R, Prolla TA, Yamasoba T. Effects of caloric restriction on age-related hearing loss in rodents and rhesus monkeys. Curr. Aging Sci. 2010a;3:20–25. doi: 10.2174/1874609811003010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010b;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Pathologic changes of presbycusis begin in secondary processes and spread to primary processes of strial marginal cells. Hear. Res. 2005;205:225–240. doi: 10.1016/j.heares.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Strom A, Jensen RA. Mortality from circulatory diseases in Norway 1940–1945. Lancet. 1951;258:126–129. doi: 10.1016/s0140-6736(51)91210-x. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Uchida Y, Nakashima T, Ando F, Shimokata H. The association between gene polymorphisms in uncoupling proteins and hearing impairment in Japanese elderly. Acta Otolaryngol. 2010;130:487–492. doi: 10.3109/00016480903283758. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Sugiura S, Nakashima T, Ando F, Shimokata H. Endothelin-1 gene polymorphism and hearing impairment in elderly Japanese. Laryngoscope. 2009;119:938–943. doi: 10.1002/lary.20181. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Sugiura S, Ando F, Nakashima T, Shimokata H. Molecular genetic epidemiology of age-related hearing impairment. Auris Nasus Larynx. 2011;38:657–665. doi: 10.1016/j.anl.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Vallejo EA. Hunger diet on alternate days in the nutrition of the aged. Prensa Med. Argent. 1957;44:119–120. [PubMed] [Google Scholar]
- Van EE, Van CG, Van LL. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol. Neurootol. 2007;12:345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- Van LL, Van EE, Fransen E, Huyghe JR, Topsakal V, Hendrickx JJ, Hannula S, Maki-Torkko E, Jensen M, Demeester K, Baur M, Bonaconsa A, Mazzoli M, Espeso A, Verbruggen K, Huyghe J, Huygen P, Kunst S, Manninen M, Konings A, Diaz-Lacava AN, Steffens M, Wienker TF, Pyykko I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Van CG. The grainyhead like 2 gene (GRHL2), alias TFCP2L3, is associated with age-related hearing impairment. Hum. Mol. Genet. 2008;17:159–169. doi: 10.1093/hmg/ddm292. [DOI] [PubMed] [Google Scholar]
- Van LL, Huyghe JR, Hannula S, Van Eyken E, Stephan DA, Mäki-Torkko E, Aikio P, Fransen E, Lysholm-Bernacchi A, Sorri M, Huentelman MJ, Van Camp G. A genome-wide association study for age-related hearing impairment in the Saami. Eur. J. Hum. Genet. 2010;18:685–693. doi: 10.1038/ejhg.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken E, Van Camp G, Fransen E, Topsakal V, Hendrickx JJ, Demeester K, Van de Heyning P, Mäki-Torkko E, Hannula S, Sorri M, Jensen M, Parving A, Bille M, Baur M, Pfister M, Bonaconsa A, Mazzoli M, Orzan E, Espeso A, Stephens D, Verbruggen K, Huyghe J, Dhooge I, Huygen P, Kremer H, Cremers CW, Kunst S, Manninen M, Pyykkö I, Lacava A, Steffens M, Wienker TF, Van Laer L. Contribution of the N-acetyl-transferase 2 polymorphism NAT2*6A to age-related hearing impairment. J. Med. Genet. 2007;44:570–578. doi: 10.1136/jmg.2007.049205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82–90. doi: 10.1016/s0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Kamide K, Takiuchi S, Matayoshi T, Hanada H, Kada A, Yang J, Miwa Y, Yoshii M, Horio T, Yoshihara F, Nakamura S, Nakahama H, Tei C, Miyata T, Kawano Y. Association of single nucleotide polymorphisms in endothelin family genes with the progression of atherosclerosis in patients with essential hypertension. J. Hum. Hypertens. 2007;21:883–892. doi: 10.1038/sj.jhh.1002234. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]