Summary
Background
The PI3K-Akt pathway is frequently activated in acute leukemias and represents an important therapeutic target. UCN-01 and perifosine are known to inhibit Akt activation.
Methods
The primary objective of this phase I study was to determine the maximum tolerated dose (MTD) of UCN-01 given in combination with perifosine in patients with advanced acute leukemias and myelodysplastic syndrome. Secondary objectives included safety, pharmacokinetics, pharmacodynamics, and efficacy. Perifosine 150 mg every 6 hours was given orally on day 1 followed by 100 mg once a day continuously in 28-day cycles. UCN-01 was given intravenously over 3 hours on day 4 at three dose levels (DL1=40 mg/m2; DL2=65 mg/m2; DL3=90 mg/m2).
Results
Thirteen patients were treated (DL1, n=6; DL2, n=4; DL3, n=3) according to a traditional “3+3” design. Two patients at the DL3 experienced dose-limiting toxicity including grade 3-4 pericardial effusion, hypotension, hyperglycemia, hyperkalemia, constitutional symptoms and grade 5 pneumonitis. Other frequent toxicities were grade 1-2 nausea, diarrhea, vomiting, fatigue and hyperglycemia. The MTD was determined to be UCN-01 65 mg/m2 with perifosine 100 mg a day. No appreciable direct Akt inhibition could be demonstrated in patients’ mononuclear cells using Western blot, however, reduced phosphorylation of the downstream target ribosomal protein S6 in leukemic blasts was noted by intracellular flow cytometry. No objective responses were observed on this study.
Conclusion
UCN-01 and perifosine can be safely administered, but this regimen lacked clinical efficacy. This approach may have failed because of insufficient Akt inhibition in vivo.
Keywords: Acute leukemia, akt inhibition, UCN-01, perifosine
Introduction
Among the signaling pathways regulating cancer cell proliferation and susceptibility to apoptosis is the phosphatidylinositol-3 kinase (PI3K)-Akt pathway, whose constitutive activation has been implicated in the therapeutic resistance of a variety of malignancies, including acute leukemias [1-3]. Phosphorylation of Akt on either Ser473 or Thr308 or both in leukemia blasts occurs in 50%-80% of acute myeloid leukemia (AML) patients and, in several studies, is associated with worse outcomes [4-9]. Similarly, the frequent activation of the PI3K-Akt pathway seen in both T cell and pre-B cell acute lymphoid leukemia (ALL), whether or not carrying BCR-ABL translocation, confers poor prognosis [10-12]. A variety of intra- and extra-cellular signals appears to regulate this pathway activation in AML, and a number of genetic defects affecting the PI3K-Akt pathway have been detected in T cell ALL [4,13-15]. Thus, the development of strategies to down regulate the activity of PI3K-Akt pathway in acute leukemias represents an important experimental therapeutic goal.
Under the influence of growth factors, cytokines, or oncogenic tyrosine kinases, PI3K is activated and recruited to the plasma membrane, allowing for the phosphorylation of phosphoinositides and recruitment of phosphoinositide-dependent kinase 1 (PDK1) and Akt to the cell membrane. There, Akt undergoes post-translational modification required for its activation: phosphorylation on Thr308 mediated by PDK1 and on Ser473 mediated by mamamalian target of rapamycin (mTOR) complex 2 (mTORC2) [16]. Activated Akt affects multiple downstream targets that control various aspects of cell growth, metabolism, proliferation, and survival [17]. While inhibition of mTORC1, a key downstream target of Akt, by small molecule rapamycin analogues has demonstrated limited clinical activity in acute leukemias [18-21], few studies have examined the anti-leukemic effect of upstream inhibition of the PI3K-Akt pathway.
UCN-01, a staurosporine derivative, and perifosine, an orally available alkylphospholipid, were found to inhibit Akt activation and also demonstrated anti-leukemia activity in preclinical studies [2,22-27]. Among its more potently affected recognized targets, UCN-01 inhibits PDK1 while perifosine blocks the recruitment of the pleckstrin homology (PH) domain of Akt kinase, to prevent its membrane localization and subsequent activation [2,22]. UCN-01 synergized with rapamycin in inducing apoptosis in AML cells [23] and, when given with cytarabine to AML patients, induced Akt dephosphorylation in vivo in leukemia blasts; however, this drug combination was associated with cardiopulmonary toxicities and limited objective responses [28]. Perifosine was shown to induce apoptosis in AML cell lines and primary leukemia cells and to reduce the clonogenic activity of AML, but not normal CD34+ cells [24]. Perifosine also synergized with number of therapeutics, including etoposide,[24] histone deacetylase inhibitors,[25] pro-apoptotic TNF-related apoptosis-inducing ligand (TRAIL),[26] and MEK inhibitors [27] in inducing apoptosis in AML cells. While the described effects correlated with Akt inhibition, perifosine also interfered with MEK/ERK 1/2 pathway and activated c-jun-N-kinase (JNK) in leukemia cells [24-27]. Similarly, UCN-01 is also known to inhibit a number of serine/threonine kinases besides PDK1 [28-30]. Thus, while neither agent is a specific Akt inhibitor, synergistic activity of UCN-01 and perifosine could potentially result from inhibition of Akt activation at pharmacologically achievable concentrations, as has been demonstrated in prostate and lung cancer cell lines [31].
The non-overlapping mechanisms by which these two agents may inhibit Akt signaling led us to hypothesize that perifosine and UCN-01, when combined, may exert more powerful Akt inhibition and anti-leukemia effect. Thus, the present study was initiated to examine whether in acute leukemia patients treated with UCN-01 and perifosine modulation of Akt phosphorylation in leukemia cells can be achieved and whether there would be any convergent toxicity of the two agents. Due to the tight binding of UCN-01 to α1-acid glycoprotein, we elected to dose-escalate UCN-01 in the presence of the recommended phase II dose (RP2D) of perifosine given on a continuous schedule [32].
Patients and methods
Patients
Eligibility for treatment on this phase 1 trial required age ≥ 18 years and relapsed or refractory acute leukemia (AML or ALL), high-risk myelodysplastic syndrome (MDS) failing standard therapy, or chronic myeloid leukemia in accelerated/blast phase despite two prior tyrosine kinase inhibitors. Patients with newly diagnosed AML and ALL were eligible if they were both >60 years old and had poor-risk cytogenetics, therapy–related AML or AML arising from antecedent hematologic disorder (AHD) and were not candidates for curative therapy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, bilirubin ≤1.5 times the upper limit of normal (ULN), alanine aminotransferase or aspartate aminotransferase ≤2.5 times the ULN, and creatinine <2 mg/dl. There was no limitation on the number of prior therapies, provided that the most recent treatment was completed ≥4 weeks prior to enrolment and adverse events recovered to ≤grade 1 according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Patients were to be ≥4 weeks after autologous and ≥90 days after allogeneic hematopoietic stem cell transplant (HSCT), with no active graft versus host disease and off immunosuppression. Exclusion criteria included active CNS disease, a history of allergic reaction to compounds similar to those used in study, uncontrolled illness such as symptomatic heart disease or signs of active ischemia on pre-study electrocardiogram (ECG), poorly-controlled diabetes mellitus, pregnancy/lactation, known HIV infection, and any condition that would limit compliance. Patients with infection controlled with antibiotics were eligible. Treatment with hydroxyurea was not a contraindication to participation provided that the patient had not required an increase in the dose of hydroxyurea for at least 1 week. Study treatment was started once the leukemic blast count was <30 × 109/l. The use of hydroxyurea was allowed for rapidly proliferating disease in the first two weeks of therapy. All hematopoietic growth factors and biologic agents were stopped at the time of enrollment.
All patients provided written, informed consent. The final study protocol, amendments, and informed consent were approved by the Institutional Review Board of the each participating center. The study was conducted in accordance with the Declaration of Helsinki and in compliance with International Conference on Harmonization Good Clinical Practice Guidelines.
Study design and treatment
This was a National Cancer Institute (NCI)-sponsored phase I, dose-escalation study carried out at University of Pennsylvania Abramson Cancer Center, Philadelphia and the University of Maryland Marlene and Stewart Greenebaum Cancer Center, Baltimore (ClinicalTrials.gov identifier: NCT00301938). The primary objective was to define the maximum tolerated dose (MTD) of UCN-01 given in combination with perifosine in acute leukemia and MDS patients. Secondary objectives included safety, pharmacokinetics, pharmacodynamics, and efficacy.
Perifosine was administered at RP2D: 150 mg orally (PO) every 6 hours on day 1 (loading) followed by 100 mg PO daily continuously (maintenance) [32]. UCN-01 was administered at three dose levels (DL1 40 mg/m2; DL2 65 mg/m2; and DL3 90 mg/m2) intravenously (IV) over 3 hours on day 4 [33]. This sequential dose administration was planned to allow for analysis of pharmacodynamics effects of perifosine alone compared to the drug combination. Each treatment cycle lasted 28 days and patients could continue treatment until disease progression, failure to achieve at least hematologic improvement (HI) after 2 cycles, unacceptable toxicity, or withdrawal of consent. In cycle 2 and onward, the perifosine was administered without repeat loading, while the dose of UCN-01 was 50% of the cycle 1 dose due to its known long half-life. The next cycle of therapy could start if non-hematologic toxicity from previous cycle recovered to <CTCAE grade 1 and in patients with no evidence of leukemia in the bone marrow (BM) if absolute neutrophil count (ANC) >0.5 × 109/l and platelets >20 × 109/l. Perifosine was taken with food and prophylactic anti-emetics (5HT3 blockers, dexamethasone, metoclopramide, diphenhydramine) and loperamide were given during loading and as needed during maintenance phase. To prevent hypotension, patients were given one liter normal saline IV over 2 hours prior to and after UCN-01 infusion.
A traditional “3+3” dose-escalation design was used to escalate UCN-01, with provision for cohort expansion to 6 evaluable patients if a dose-limiting toxicity (DLT) was observed among the initial 3 patients. If 2 or more DLTs were observed, dose-escalation was halted and dose finding continued at a lower DL until the MTD was defined. Dose escalation proceeded when none of three or one of six evaluable patients experienced DLT. To be evaluable for dose escalation, a patient had to complete first cycle or experience DLT within the first 28 days. To ensure that the toxicity at MTD is acceptable an additional 6 patients were to be accrued at the MTD to a maximum of 12 patients. Given lack of sufficient evidence of clinical efficacy, the MTD cohort was not expanded.
The toxicity was graded according to NCI CTCAE version 3.0. DLTs for this study were defined as grade 3 or 4 drug-related non-hematologic toxicity; grade 3 diarrhea, nausea, vomiting lasting more than 24 hours despite supportive care; persistent toxicity independent of CTCAE grade that results in >7 days perifosine interruption in first cycle; myelosuppression with BM aplasia on day ≥42 in the absence of leukemia. Dose reduction of UCN-01 and/or perifosine was required for grade 3 and 4 non-hematologic toxicity.
Assessment
Medical history, vital signs, ECOG performance status, physical exam, laboratory tests, BM aspirate/biopsy, ECG, echocardiogram were performed at baseline. Follow-up clinical and laboratory monitoring was performed according to the standards of practice for adults with acute leukemia. Pulse oximetry measurements and vital signs monitoring were performed during and up to 4 hours after each UCN-01 infusion. Glucose levels were measured 24 hours after each UCN-01 infusion. Patients were assessed at least weekly for toxicities. Medication compliance was assessed through diaries and pill counts.
To assess response to therapy, BM examination was repeated on day 21-28 of cycle 1, at any time that progressive disease was suspected or at study conclusion. For patients with acute leukemia and MDS, the response was determined according to the disease-specific International Working Group (IWG) criteria [34,35]. HI in acute leukemia patients was defined according to IWG criteria for MDS.
Pharmacodynamic analysis
Bone marrow (10 cc/time point) and/or peripheral blood (PB) samples (20 cc/time point; if PB blast count was >3 × 109/l) were collected in heparinized tubes pre-treatment, on day 4 prior to UCN-01, on day 21-28 (completion of cycle 1), or at any time patient was coming off study. The samples were processed immediately (U Penn) or within 24 hours of collection (shipped overnight at room temperature, U Maryland). Mononuclear cells were separated by Ficoll/Hypaque density centrifugation and viably frozen in a liquid nitrogen. For Western blot analysis, cells were rapidly thawed and washed with PBS, pelleted by centrifugation, and immediately placed in lysis buffer containing protease and phosphatase inhibitors. 100 g per sample of total cell lysates were loaded on pre-cast 4-12% NuPage gradient gels, separated by electrophoresis, and transferred to nitrocellulose membrane. Following incubation in Li-cor Odyssey blocking solution (Licoln, NE), blots were incubated in TBST (TBS/0.1% Tween 20) / 3% BSA with primary antibodies (Abs) overnight at 4°C, washed with TBST, and then incubated for 1 hour with species-specific secondary Abs tagged to Alexa 680 or IRDye800 fluorescence. A Li-Cor Odyssey fluorescence scanner and associated software (version 3.0) were used to visualize hybridized Abs and quantify signal. Primary antibodies included total Akt, phosphorylated Akt (serine 473; R&D) and total p38α mitogen activated protein kinase (MAPK) (Cell Signaling) and were used at 1:1000 dilution. Secondary Abs were obtained from Rockland Immunochemicals and used at 1:10,000 dilution.
Single-cell pharmacodynamic monitoring of S6 ribosomal protein phosphorylation in leukemia blasts by flow cytometry was conducted as previously described [36]. Briefly, unfractionated marrow or peripheral blood was passed through a 40 micron mesh filter and multiple 100 μL aliquots were prepared. To these aliquots, sample conditions included unmanipulated aliquots as well as ex vivo activation of S6 phosphorylation by phorbol myristate acetate (PMA, Sigma-Aldrich) 400 nM for 10 minutes, and inhibition by rapamycin (Sigma-Aldrich) 1000 nM for 30 minutes to establish dynamic controls. These conditions were carried out in a dry bath (humidified incubator at 37°C, 21% O2, 5% CO2). Subsequently, samples were fixed with ultrapure formaldehyde (4%, methanol-free, Polysciences) and red cells lysed with Triton X-100 (0.1%, Sigma-Aldrich) prior to protein denaturation with ice cold methanol (90%), as previously described [36]. Following methanol incubation, samples were washed twice at 4°C and stained with antibodies (30 minutes in the dark), then washed once and subjected to flow cytometric analysis. Abs used included murine anti-human CD45 PerCP, CD34 APC, CD19 PE, CD33 PE (all from Becton-Dickinson), and rabbit monoclonal phospho-S6 (serine 235/6) conjugated to Alexa 488 or Alexa 647 (Cell Signaling). A fluorescence minus one (FMO) control containing antibodies directed against cell surface markers but not phospho-S6 was included for all subjects to estimate autofluorescence and facilitate definition of a phospho-S6 (p-S6) negative gates. Singly-stained compensation controls were created using the same fluorophores bound to polystyrene beads (Spherotech) and acquired individually. Data were acquired on a FACS-Calibur using Cell Quest software or an FACSCanto using DiVA software (BD Biosciences) and analyzed using FlowJo software version 8 and 9 (TreeStar). At least 10,000 total cell events were collected for all samples. Samples with fewer than 1000 cell events in a blast gate defined by CD45 × SSC were considered insufficient for further analysis. Constitutive phosphorylation of S6 required the finding of >5% of blast gate events falling in the positive phospho-S6 region defined by comparing PMA and FMO controls.
Pharmacokinetics
Blood samples (10 mL each) were collected into heparinized tubes in cycle 1 on day 1, on day 4 prior to perifosine and UCN-01 administration and immediately after completion of UCN-01 infusion, and on day 5 24 hours after UCN-01 infusion. Additional samples were collected in cycle 2 and 3 prior and immediately after the end of UCN-01 infusion. Samples were immediately centrifuged at 10,000g/4°C for 10 minutes, separated plasma was aliquoted in cryogenic vials and fresh frozen in liquid nitrogen until analysis. Perifosine and UCN-01 plasma concentrations were determined using previously published reverse-phase validated high-performance liquid chromatography assays with tandem mass spectrometry detection [37,38]. The PK variables of UCN-01 and perifosine were determined by a model independent approach using WinNonlinv 5.2. Pharmacokinetic variables estimated included the maximum observed plasma concentrations (Cmax) for UCN-01 and the steady-state plasma concentrations (Css) for perifosine. The Css was determined by averaging through concentrations obtained on days 4 and 5.
Statistical analysis
Statistical analyses were restricted to summaries of all data from all treated patients to assess safety, pharmacokinetics, pharmacodynamics and preliminary responses.
Results
Patient characteristics
Thirteen patients with AML (n=11), ALL (n=1), and MDS (n=1) were treated across three dose levels (DL1, n=6; DL2, n=4; DL3, n=3). Their characteristics are depicted in Table 1. The median age of patients was 69 (range, 20-85) years, 10 patients (77%) were white, and 10 patients (77%) were male. They had received median 3 (range, 1-6) prior treatments and, at the time of enrollment, 11 patients were refractory to the last treatment, including 5 with primary refractory disease. Except for a single ALL patient with first remission of 15 months, all other patients had first remission of <12 months. Seven patients had AML arising from AHD and 2 therapy-related AML; six had adverse and 5 intermediate karyotypes.
Table 1.
Patient characteristics
| Pt # | Dose Level |
Age | Sex | Race | ECOG PS |
Disease | Karyotype | Prior Rx (n) |
Disease Status |
First Remission (mos) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 75 | M | White | 1 | MDS→2°AML | 47, XY, +15 | 3 | 2nd relapse | 7 |
|
| ||||||||||
| 2 | 1 | 69 | M | White | 2 | MDS→2°AML | 46, XY, t(3;21), del(7q) |
1 | primary refractory |
n/a |
|
| ||||||||||
| 3 | 1 | 68 | M | White | 0 | MDS→2°AML | 47, XY, +8 | 2 | refractory relapse |
7 |
|
| ||||||||||
| 4 | 1 | 85 | F | White | 0 | MPN→2°AML | 46, XX | 1 | primary refractory |
n/a |
|
| ||||||||||
| 5 | 1 | 20 | M | American Indian |
0 | de novo AML | 46, XY, t(8;21), del(9p) |
4 | refractory relapse |
4.5 |
|
| ||||||||||
| 6 | 1 | 74 | F | White | 1 | t-AML | 46, XX,add(4q35), inv(16) |
6 | refractory relapse |
9 |
|
| ||||||||||
| 7 | 2 | 74 | M | White | 1 | RAEB/IPSS int-2 | 46, XY | 1 | primary refractory |
n/a |
|
| ||||||||||
| 8 | 2 | 76 | M | White | 1 | MDS→2°AML | 46, XY, del(9q13) | 2 | primary refractory |
n/a |
|
| ||||||||||
| 9 | 2 | 61 | M | Black | 1 | MDS→2°AML | 46, XY | 3 | refractory relapse |
5 |
|
| ||||||||||
| 10 | 2 | 67 | M | White | 0 | t-AML | 46, XY, t(9;11), del(11q23) |
1 | 1st relapse | 6 |
|
| ||||||||||
| 11 | 3 | 68 | F | White | 0 | de novo AML | 45, XX, −7 | 4 (allo) |
refractory relapse |
5 |
|
| ||||||||||
| 12 | 3 | 76 | M | Native Hawaiian |
1 | MDS→2°AML | 47, XY, del(7q22), del(12p), +13, i(17q10) |
3 | primary refractory |
n/a |
| 13 | 3 | 38 | M | White, Hispanic |
2 | Pre-B ALL | 46, XY | 4 | refractory relapse |
15 |
Patients received median 1 (range, 1-3) cycle of therapy. Five patients did not complete first cycle because of progressive disease (3 patients DL1; days 11, 14, 22), drug–related toxicity (DLT; 1 patient DL3; day 4) and drug-unrelated toxicity (cholecystitis/sepsis; 1 patient DL3; day 13). To achieve temporary count control, five patients received hydroxyurea for median 5 (range, 3-14) days in the first two weeks of treatment.
Safety profile
Cycle 1 toxicities of UCN-01 and perifosine are listed in Table 2. The most frequent toxicities were grade 1-2 nausea and diarrhea (62%), vomiting (54%), fatigue and hyperglycemia (38%), and anorexia (23%). One grade 3 fatigue and one grade 4 hyperglycemia were observed in the same patient on DL3 who experienced other DLTs. No ≥grade 3 toxicities were observed at the DLs 1 and 2. Among 6 patients treated at the DL1, 3 patients completed the first cycle and were evaluable for dose escalation. One of those patients completed almost 3 cycles of therapy before being taken off study due to lower extremity deep venous thrombosis related to preexisting co-morbidity, while the other two patients developed progressive disease after cycle 1 and on day 12 of cycle 2, respectively. No ≥grade 3 drug-related toxicities were observed in subsequent cycles. Four patients were treated at DL2. One patient had a delay in UCN-01 administration in cycle 1 (day 10) because of infection but ultimately completed all therapy. This patient was not evaluable for dose-escalation, however, the remaining 3 patients completed first cycle of therapy without any DLTs, including the patient who went on to receive total 3 cycles of therapy. Later one developed unilateral periorbital inflammation and, in the process of evaluation, died from intracranial hemorrhage unrelated to study drugs. The other 3 patients progressed after cycle 1. Among 3 patients treated at DL3, 2 experienced DLTs and the third patient stopped taking perifosine on day 13 because of sepsis unrelated to study drugs. One patient experienced a DLT on day 4 (after UCN-01 administration) consisting of grade 4 hyperglycemia, pericardial effusion and tamponade, and grade 3 hyperkalemia, hypotension and constitutional symptoms such as muscle aches, abdominal pain and fatigue. This patient recovered but was not given further therapy. While hyperglycemia was attributed to UCN-01 based on known clinical experiences (43), the rest of toxicities were attributed to both drugs. A second patient developed grade 4 dyspnea 48-72 hours after UCN-01 administration requiring brief intubation but his clinical picture was confounded by simultaneous COPD exacerbation. He went on to complete the first cycle but within 24 hours of stopping the treatment for disease progression, he developed dyspnea and grade 5 pneumonitis, possibly perifosine-related.
Table 2.
Treatment-related adverse events (CTCAE version 3.0)
| Adverse events | DL1 (N=6) | DL2 (N=4) | DL3 (N=3) | All DLs (N=13) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gr 1-2 | Gr 3-4 | Gr 1-2 | Gr 3-4 | Gr 1-2 | Gr 3-4 | Gr 5 | All toxicities | |
|
| ||||||||
| Nausea | 6 (100%) | - | 1 (25%) | - | 1 (33%) | - | - | 8 (62%) |
| Diarrhea | 6 (100%) | - | 1 (25%) | - | 1 (33%) | - | - | 8 (62%) |
| Vomiting | 5 (83%) | - | 1 (25%) | - | 1 (33%) | - | - | 7 (54%) |
| Fatigue/Weakness | 3 (50%) | - | 1 (25%) | - | - | 1 (33%) | - | 5 (38%) |
| Hyperglycemia | 1 (17%) | - | 2 (50%) | - | 1 (33%) | 1 (33%) | - | 5 (38%) |
| Anorexia | 2 (33%) | - | 1 (25%) | - | - | - | - | 3 (23%) |
| Constipation | 1 (17%) | - | 1 (25%) | - | - | - | - | 2 (15%) |
| Hypotension | - | - | 1 (25%) | - | - | 1 (33%) | - | 2 (15%) |
| Hypokalemia | 1 (17%) | - | - | - | - | 1 (33%) | - | 2 (15%) |
| Elevated AST | 1 (17%) | - | - | - | 1 (33%) | - | - | 2 (15%) |
| Elevated ALT | 1 (17%) | - | - | - | 1 (33%) | - | - | 2 (15%) |
| Pneumonitis | - | - | - | - | - | - | 1 (33%) | 1 (8%) |
| Dyspnea | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Pericardial | ||||||||
| effusion/tamponade | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Sepsis | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Feeling poorly | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Muscle aches | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Abdominal pain | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Hyperkalemia | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Leukopenia | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Thrombocytopenia | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Anemia | - | - | - | - | - | 1 (33%) | - | 1 (8%) |
| Lethargy | - | - | 1 (25%) | - | - | - | - | 1 (8%) |
| Weight loss | - | - | 1 (25%) | - | - | - | - | 1 (8%) |
| Mucositis | 1 (17%) | - | - | - | - | - | - | 1 (8%) |
| Hypoalbuminemia | 1 (17%) | - | - | - | - | - | - | 1 (8%) |
| Dizziness | - | - | - | - | 1 (33%) | - | - | 1 (8%) |
| Rigors/chills | - | - | - | - | 1 (33%) | - | - | 1 (8%) |
| Fever | - | - | - | - | 1 (33%) | - | - | 1 (8%) |
Pharmacokinetics, pharmacodynamics and efficacy
Adequate samples for pharmacokinetic analysis on day 4 and 5 were available from 9 of 13 patients. Unfortunately, the majority of patients did not have sampling in cycle 2 and onward due to early withdrawal for either disease progression or toxicity. The average maximum plasma concentrations of UCN-01 observed in cycle 1 were 15.8 ± 4.1 μg/mL (n = 5) at 40 mg/m2, 9.3 ± 2 μg/mL (n = 2) at 65 mg/m2, and 13. 2 ± 0.7 μg/mL (n = 2) at 90 mg/m2. Due to small sample size and high variability, dose proportionality was not assessed. Maximum UCN-01 plasma concentrations achieved were in the range of 7.906 to 21.83 μg/mL (16.4 to 45.27 μM/L), which is consistent with published data [33]. The average steady state concentration (Css) of perifosine was 4.39 ± 2.59 μg/mL (range, 1.682-9.722 μg/mL) (n = 9), consistent with previous reports [39,32]. The perifosine concentration on day 1 of cycle 2 in 3 patients was 6.178 ± 1.912 μg/mL.
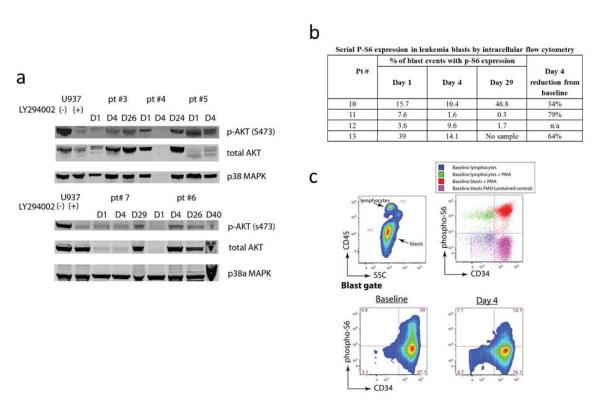
Paired baseline, day 4 (perifosine effect, n=5) and day 21-29 or off study (UCN-01 and perifosine effect, n=4) samples were analyzed by Western blot for inhibition of Akt phosphorylation on Ser473, total Akt and p38α MAPK expression. As total Akt levels varied over time in samples analyzed, Akt phosphorylation alone (or phosphorylation relative to total Akt) gave misleading results. Given that the expression of total p38α MAPK was essentially static over time, we selected it as a loading control and thereby used it to determine relative changes in either total or phosphorylated Akt. As demonstrated in Figure 1a, 4 of 5 baseline samples showed Akt phosphorylation at Ser473. The sample with absent baseline Akt phosphorylation was obtained from a patient with AML who had 75% blasts in the BM but aspirate was hemodilute which may affect the results. All other patients had 60-95% blasts in the bone marrow at all the time points with the exception of patient #7 with MDS and less than 30% marrow blasts. We could not demonstrate any appreciable phospho-Ser473 Akt inhibition following perifosine (day 4) or perifosine/UCN-01 treatment. In fact, two patients demonstrated up-regulation of Akt phospho-Ser473 on day 4 (patient #6) and day 29 (patient #7).
Fig 1. The in vivo effects of perifosine and UCN-01 in leukemia cells.
a Western blot shows phospho-Ser473 Akt and total Akt expression among purified mononuclear cells collected from marrow or blood on indicated treatment days. P38 MAPK is shown to ensure equal protein loading. b Table demonstrating the % of leukemia blasts with constitutive p-S6 expression on day 1 and following treatment on days 4 and 29 as measured by intracellular flow cytometry. c Fixed, unfractionated peripheral blood sample from subject #13 shows alterations in S6 phosphorylation downstream of Akt induced by perifosine monotherapy. Lymphoblasts and lymphocytes are discriminated by CD45 antigen density and granulocytes by side scatter. In top right, controls for S6 basal phosphorylation are established by comparing fully stained samples to that of samples lacking pS6 antibody (fluorescence minus one, FMO). PMA stimulated blasts occupy the positive gate, while unstimulated lymphocytes serve as an internal negative control for both CD34 and p-S6 staining. The two bottom panels show blast gate events at baseline and on day 4 of therapy. The percentage of gated events in each region is indicated, illustrating a >50% reduction in +p-S6 events by day 4.
Given the apparent lack of pharmacodynamic effect when measured by Western blot, we subsequently analyzed samples by fixed blood/marrow phospho-flow cytometry for inhibition of the ribosomal protein S6, which is a downstream target of Akt. We chose p-S6 as readout due to its greater signal to noise ratio than phospho-Akt in clinical phospho-flow specimens and its robustness as a surrogate of Akt/mTOR kinase activity. As is typical when p-S6 is evaluated in normal or malignant blasts, a subset showed constitutive phosphorylation [40,36]. Baseline percentages of blast events with constitutive p-S6 ranged from 3.6% to 39%. Among the three subjects with baseline constitutive p-S6 >5%, two showed a >50% reduction in the percentage of p-S6+ blasts (patients #11 and #13), and the third a <50% reduction on day 4 that was not sustained at day 29 (patient #10), Figure 1b. Representative data from a subject showing inhibition of p-S6 signal downstream of Akt are shown in Figure 1c (patient #13).
Among the 11 patients evaluable for response, 3 had early disease progression before completion of first cycle, 6 had progressive disease after cycle 1, and 2 patients had stable disease for several months. Of these 2 patients with disease stabilization, one patient (patient #1) had AML arising from MDS and remained platelet transfusion independent with stable blasts (30-40%) in the marrow and rare red blood cell (RBC) transfusion requirements, and the second (patient #7) with MDS achieved temporary improvement in neutrophil count >0.5 × 109/l, BM remission (blasts 11% pre-treatment declined to 2% after cycle 1) and remained with stable RBC transfusion requirements and stable un-transfused platelet count. However, both patients were ultimately removed from study after cycle 3 due to drug-unrelated toxicities. Patient #7, who had sufficient specimen for pharmacodynamic analysis, demonstrated up-regulation of phospho-Ser473 Akt expression on day 29 by Western blot.
Discussion
Perifosine and UCN-01 affect multiple targets, including inhibiting PI3K-Akt pathway in a variety of malignant cell types, and such inhibition correlates with cytotoxicity in vitro and in vivo in animal models. We report here the results of a phase I trial of UCN-01 and perifosine in patients with advanced acute leukemias. We defined the MTD of UCN-01 to be 65 mg/m2 when given over 3 hours with perifosine 600 mg loading followed by 100 mg maintenance dose. However, this regimen did not demonstrate significant anti-leukemia activity. In addition to clinical results, we provide pharmacodynamic data suggesting that Western blot assay may not be sufficiently sensitive method to monitor changes in phospho-Ser473 Akt inhibition in BM specimens upon treatment. In contrast, direct monitoring of p-S6 inhibition in leukemia blasts by intra-cellular flow cytometry appears to be more informative and allows direct examination of malignant cell population. This should be taken into consideration when new studies with agents targeting this pathway are designed.
Overall, the combination of perifosine and UCN-01 was tolerable until the dose of UCN-01 was escalated to 90 mg/m2 when 2 of 3 patients developed DLTs including grade 3/4 cardiac and metabolic disturbances and grade 5 pneumonitis. The pharmacokinetic parameters in both of these patients (Cmax UCN-01 of 13.69 and 12.67 μg/ml and Css perifosine of 2.632 and 2.884 μg/ml) did not differ from those observed among other patients treated on this or previous studies of UCN-01 or perifosine [39,33,32]. Although UCN-01 90 mg/m2 given over 3 hours in combination with carboplatin was found to be safe in patients with solid tumors [33], patients with advanced leukemia represent a different patient population that is likely to be more susceptible to hypotension, cytokine release, and cardiopulmonary toxicity associated with UCN-01 administration. Similar toxicities were also described when AML patients were treated with continuous infusion of UCN-01 and cytarabine [28]. Thus, the MTD of UCN-01 given in combination with perifosine (600 mg/100 mg) was determined to be 65 mg/m2 with no DLT observed in 4 patients or overall 6 cycles. The most common toxicities associated with this regimen were grade 1-2 gastrointestinal and constitutional symptoms occurring at frequency previously reported for perifosine alone [39,32]. Hyperglycemia was manageable on this study with the exception of a single patient developing dose-limiting hyperglycemia among other DLTs.
This regimen was not myelosuppressive, leading to frequent disease progression prior to completion of cycle 1; thus, we did not expand the cohort at MTD as initially planned. Stable disease for 3 cycles was observed only in 2 patients, both of whom had low blast percentage in the marrow in the setting of MDS and secondary AML, and in one of whom temporary improvement in neutrophil count and transient marrow remission was achieved. Frequent disease progression was also observed when single agent perifosine was given to 19 patients with acute and chronic leukemias in a phase II study, leading to early study termination (unpublished data; source Investigator’s Brochure; September 24, 2010; Keryx/AOI Pharmaceuticals, Inc.). Similarly, only 1 of 13 relapsed/refractory AML patients achieved remission when treated with UCN-01 and cytarabine [28]. Our results suggest that combined administration of UCN-01 and perifosine does not have synergistic clinical anti-leukemia activity.
The pharmacokinetics of perifosine and UCN-01 had been extensively characterized in a number of clinical studies. In our study, we were able to demonstrate that mean Css of perifosine (4.39 ± 2.59 μg/mL) and Cmax of UCN-01 (range, 7.906 to 21.83 μg/mL) are similar to values reported previously for these agents and within their in vitro bioactive range [39,33,41,32] Given the small number of patients and high variability, we could not address dose proportionality of UCN-01. One of the study objectives was to examine whether perifosine alone or perifosine plus UCN-01 inhibit Akt phosphorylation in clinical specimens during treatment. We examined changes in phospho-Ser473 Akt which should be inhibited by both drugs. Although UCN-01 directly targets PDK1 and inhibits phospho-Thr308 Akt, previous studies on tumor cell lines [42,22] as well as analysis of primary AML specimens from patients treated on UCN-01 and cytarabine study demonstrated Ser473 Akt inhibition upon UCN-01 treatment [28]. Unfortunately, we could not consistently document Ser473 Akt inhibition in marrow specimens obtained from patients after perifosine alone and perifosine plus UCN-01 using quantitative Western blot measurements (Li-cor Odyssey). In UCN-01 plus cytarabine study the changes in phospho-Ser473 Akt in leukemia specimens were expressed relative to total Akt; however, we observed substantial variation in total Akt levels over time, suggesting that phospho-Ser473 to total Akt ratio may be misleading. This can potentially explain disparate observations among studies. Given these results which could in part reflect heterogeneity of the sample cell population and/or lack of sensitivity of the assay and in an attempt to obtain more consistent results, we next examined the phosphorylation status of the ribosomal protein S6 using phospho-specific flow cytometry. The validity of this assay in monitoring the in vivo effects of mTOR inhibition by rapamycin in primary AML blasts has been recently demonstrated by our group [36]. S6 is abundantly expressed in normal and malignant cells and its phosphorylation is mediated by the p70S6 kinase, a downstream target of Akt via mTOR. Inhibition of p70S6K has been demonstrated upon perifosine (2-10 μM) treatment of leukemia cell lines [24]. Two of the three subjects with baseline constitutive signaling through S6 showed down regulation of this downstream Akt target and one more modest reduction in phosphorylation. Although technical limitations cannot be excluded, the observation that 1 in 4 patients did not show demonstrable baseline p-S6 is consistent with observations in relapsed/refractory AML (n=50) using identical flow cytometric cell processing methods (AE Perl, unpublished). This finding may ultimately lead to selection for signal transduction inhibitors therapy based upon demonstration of target activation. The lack of clinical efficacy of the regimen made it impossible to correlate the magnitude of signal disruption with response. Still, target inhibition was submaximal in all but one subject at the dose level that is not clinically tolerable for this drug combination. It is therefore possible that drugs targeting Akt could achieve more marked efficacy were they to achieve more substantial degrees of target inhibition and/or reductions in downstream signaling. Given that both perifosine and UCN-01 affect multiple intracellular targets beside Akt, it is possible that novel more selective Akt inhibitors [43-46] might exhibit a wider therapeutic index in this setting.
In summary, perifosine and UCN-01 (≤65 mg/m2/3 hours) are tolerable but not effective in patients with acute leukemia. Our approach may have failed because of insufficient Akt inhibition in vivo and/or need to simultaneously inhibit multiple pathways to achieve anti-leukemia effect. Response to perifosine in clinical studies appears to correlate with high baseline phospho-Akt expression or with the presence of PI3K-Akt pathway mutation in tumor cells [47-49,27]; thus, future studies should aim to select for this patient population. However, given the lack of efficacy of perifosine alone in leukemia patients, this approach should be explored using novel more selective Akt pathway inhibitors whether alone or in combination with chemotherapy or agents targeting other key survival pathways in leukemia cells. More sensitive assays to monitor in vivo effects such as intracellular phospho-flow cytometry or protein arrays are warranted.
Acknowledgments and conflict of interest
This work was supported in part by National Cancer Institute Cooperative Agreement UO-1 CA062487 (I.G. and E.A.S.). Keryx/AOI Pharmaceuticals, Inc. provided support for pharmacokinetic and pharmacodynamics studies (M.C. and K.S.B.).
Footnotes
The authors declare that they do not have further conflict of interest.
The results of this study were in part presented at the American Society of Hematology meeting 2011 (abstract #1557)
Ethical standards All patients provided written, informed consent. The final study protocol, amendments, and informed consent were approved by the Institutional Review Board of the each participating center. The study was conducted in accordance with the Declaration of Helsinki and in compliance with International Conference on Harmonization Good Clinical Practice Guidelines.
References
- 1.Kim D, Dan HC, Park S, Yang L, Liu Q, Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV, Cheng JQ. AKT/PKB signaling mechanisms in cancer and chemoresistance. Frontiersin bioscience : a journal and virtual library. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 2.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert opinion on investigational drugs. 2009;18(9):1333–1349. doi: 10.1517/14728220903136775. doi:10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 3.Martelli AM, Tabellini G, Bortul R, Tazzari PL, Cappellini A, Billi AM, Cocco L. Involvement of the phosphoinositide 3-kinase/Akt signaling pathway in the resistance to therapeutic treatments of human leukemias. Histology and histopathology. 2005;20(1):239–252. doi: 10.14670/HH-20.239. [DOI] [PubMed] [Google Scholar]
- 4.Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, Buerger H, Muller-Tidow C, Choudhary C, McMahon M, Berdel WE, Serve H. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer research. 2005;65(21):9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. doi:10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 5.Gallay N, Dos Santos C, Cuzin L, Bousquet M, Simmonet Gouy V, Chaussade C, Attal M, Payrastre B, Demur C, Recher C. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(6):1029–1038. doi: 10.1038/leu.2008.395. doi:10.1038/leu.2008.395. [DOI] [PubMed] [Google Scholar]
- 6.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108(7):2358–2365. doi: 10.1182/blood-2006-02-003475. doi:10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min YH, Cheong JW, Kim JY, Eom JI, Lee ST, Hahn JS, Ko YW, Lee MH. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer research. 2004;64(15):5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. doi:10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- 8.Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broet P, Cornillet-Lefebvre P, Lioure B, Ugo V, Blanchet O, Ifrah N, Witz F, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood. 2007;110(3):1025–1028. doi: 10.1182/blood-2006-12-061283. doi:10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102(3):972–980. doi: 10.1182/blood-2002-11-3429. doi:10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 10.Jotta PY, Ganazza MA, Silva A, Viana MB, da Silva MJ, Zambaldi LJ, Barata JT, Brandalise SR, Yunes JA. Negative prognostic impact of PTEN mutation in pediatric T-cell acute lymphoblastic leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(1):239–242. doi: 10.1038/leu.2009.209. doi:10.1038/leu.2009.209. [DOI] [PubMed] [Google Scholar]
- 11.Larson Gedman A, Chen Q, Kugel Desmoulin S, Ge Y, LaFiura K, Haska CL, Cherian C, Devidas M, Linda SB, Taub JW, Matherly LH. The impact of NOTCH1, FBW7 and 20 PTEN mutations on prognosis and downstream signaling in pediatric T-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(8):1417–1425. doi: 10.1038/leu.2009.64. doi:10.1038/leu.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morishita N, Tsukahara H, Chayama K, Ishida T, Washio K, Miyamura T, Yamashita N, Oda M, Morishima T. Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatric blood & cancer. 2011 doi: 10.1002/pbc.24034. doi:10.1002/pbc.24034. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Pedersen M, Ronnstrand L. Gab2 is involved in differential phosphoinositide 3-kinase signaling by two splice forms of c-Kit. The Journal of biological chemistry. 2008;283(41):27444–27451. doi: 10.1074/jbc.M709703200. doi:10.1074/jbc.M709703200. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, Green A, Mayeux P, Lacombe C, Bouscary D. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95(5):819–828. doi: 10.3324/haematol.2009.013797. doi:10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA, Winter SS, Larson R, Zhang J, Protopopov A, Chin L, Pandolfi PP, Silverman LB, Hunger SP, Sallan SE, Look AT. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647–650. doi: 10.1182/blood-2009-02-206722. doi:10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. doi:10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. doi:10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, Porter DL, Stadtmauer EA, Goldstein SC, Frey NV, Nasta SD, Hexner EO, Dierov JK, Swider CR, Bagg A, Gewirtz AM, Carroll M, Luger SM. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(21):6732–6739. doi: 10.1158/1078-0432.CCR-09-0842. doi:10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 19.Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, Benzaquen D, Laurent G, Huguet F, Payrastre B. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–2534. doi: 10.1182/blood-2004-06-2494. doi:10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 20.Rizzieri DA, Feldman E, Dipersio JF, Gabrail N, Stock W, Strair R, Rivera VM, Albitar M, Bedrosian CL, Giles FJ. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(9):2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. doi:10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 21.Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A, Thomas D, Wierda W, Apostolidou E, Albitar M, O’Brien S, Andreeff M, Giles FJ. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(17):5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. doi:10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine) Oncogene. 2002;21(11):1727–1738. doi: 10.1038/sj.onc.1205225. doi:10.1038/sj.onc.1205225. [DOI] [PubMed] [Google Scholar]
- 23.Hahn M, Li W, Yu C, Rahmani M, Dent P, Grant S. Rapamycin and UCN-01 synergistically induce apoptosis in human leukemia cells through a process that is regulated by the Raf-1/MEK/ERK, Akt, and JNK signal transduction pathways. Molecular cancer therapeutics. 2005;4(3):457–470. doi: 10.1158/1535-7163.MCT-04-0137. doi:10.1158/1535-7163.MCT-04-0137. [DOI] [PubMed] [Google Scholar]
- 24.Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, Evangelisti C, Ottaviani E, Martinelli G, Testoni N, McCubrey JA, Martelli AM. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22(1):147–160. doi: 10.1038/sj.leu.2404980. doi:10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- 25.Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, Spiegel S, Grant S. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer research. 2005;65(6):2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. doi:10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 26.Tazzari PL, Tabellini G, Ricci F, Papa V, Bortul R, Chiarini F, Evangelisti C, Martinelli G, Bontadini A, Cocco L, McCubrey JA, Martelli AM. Synergistic proapoptotic activity of recombinant TRAIL plus the Akt inhibitor Perifosine in acute myelogenous leukemia cells. Cancer research. 2008;68(22):9394–9403. doi: 10.1158/0008-5472.CAN-08-2815. doi:10.1158/0008-5472.CAN-08-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, Ferreira-Gonzalez A, Dent P, Grant S. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood. 2009;114(20):4507–4516. doi: 10.1182/blood-2008-09-177881. doi:10.1182/blood-2008-09-177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, Gandhi V, Plunkett W. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107(6):2517–2524. doi: 10.1182/blood-2005-08-3351. doi:10.1182/blood-2005-08-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi I, Saitoh Y, Yoshida M, Sano H, Nakano H, Morimoto M, Tamaoki T. UCN-01 and UCN-02, new selective inhibitors of protein kinase C. II. Purification, physico-chemical properties, structural determination and biological activities. The Journal of antibiotics. 1989;42(4):571–576. doi: 10.7164/antibiotics.42.571. [DOI] [PubMed] [Google Scholar]
- 30.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(7):1955–1960. doi: 10.1158/1078-0432.CCR-06-2793. doi:10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 31.Dasmahapatra GP, Didolkar P, Alley MC, Ghosh S, Sausville EA, Roy KK. In vitro combination treatment with perifosine and UCN-01 demonstrates synergism against prostate (PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(15):5242–5252. doi: 10.1158/1078-0432.CCR-03-0534. doi:10.1158/1078-0432.CCR-03-0534. [DOI] [PubMed] [Google Scholar]
- 32.Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, Arzoomanian R, Alberti D, Wilding G. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(22):7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. doi:10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 33.Edelman MJ, Bauer KS, Jr., Wu S, Smith R, Bisacia S, Dancey J. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(9):2667–2674. doi: 10.1158/1078-0432.CCR-06-1832. doi:10.1158/1078-0432.CCR-06-1832. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, Lowenberg B, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Wijermans PW, Gore S, Greenberg PL, World Health, Organization international working g Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–3674. [PubMed] [Google Scholar]
- 35.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis SoRCTO, Reporting Standards for Therapeutic Trials in Acute Myeloid L Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. doi:10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Perl AE, Kasner MT, Shank D, Luger SM, Carroll M. Single-cell pharmacodynamic monitoring of S6 ribosomal protein phosphorylation in AML blasts during a clinical trial combining the mTOR inhibitor sirolimus and intensive chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(6):1716–1725. doi: 10.1158/1078-0432.CCR-11-2346. doi:10.1158/1078-0432.CCR-11-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer KS, Lush RM, Rudek MA, Shih C, Sausville E, Figg WD. A high-performance liquid chromatography method using ultraviolet and fluorescence detection for the quantitation of UCN-01, 7-hydroxystaurosporine, from human plasma and saliva. Biomedical chromatography : BMC. 2000;14(5):338–343. doi: 10.1002/1099-0801(200008)14:5<338::AID-BMC993>3.0.CO;2-6. doi:10.1002/1099-0801(200008)14:5<338::AID-BMC993>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Woo EW, Messmann R, Sausville EA, Figg WD. Quantitative determination of perifosine, a novel alkylphosphocholine anticancer agent, in human plasma by reversed-phase liquid chromatography-electrospray mass spectrometry. Journal of chromatography B, Biomedical sciences and applications. 2001;759(2):247–257. doi: 10.1016/s0378-4347(01)00231-6. [DOI] [PubMed] [Google Scholar]
- 39.Crul M, Rosing H, de Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JH, Beijnen JH, ten Bokkel Huinink WW. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. European journal of cancer. 2002;38(12):1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 40.Marvin J, Swaminathan S, Kraker G, Chadburn A, Jacobberger J, Goolsby C. Normal bone marrow signal-transduction profiles: a requisite for enhanced detection of signaling dysregulations in AML. Blood. 2011;117(15):e120–130. doi: 10.1182/blood-2010-10-316026. doi:10.1182/blood-2010-10-316026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. European journal of cancer. 1997;33(3):442–446. doi: 10.1016/s0959-8049(97)89020-x. [DOI] [PubMed] [Google Scholar]
- 42.Pei XY, Dai Y, Rahmani M, Li W, Dent P, Grant S. The farnesyltransferase inhibitor L744832 potentiates UCN-01-induced apoptosis in human multiple myeloma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(12):4589–4600. doi: 10.1158/1078-0432.CCR-04-2346. doi:10.1158/1078-0432.CCR-04-2346. [DOI] [PubMed] [Google Scholar]
- 43.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2(6):510–517. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapuis N, Tamburini J, Green AS, Vignon C, Bardet V, Neyret A, Pannetier M, Willems L, Park S, Macone A, Maira SM, Ifrah N, Dreyfus F, Herault O, Lacombe C, Mayeux P, Bouscary D. Dual inhibition of PI3K and mTORC1/2 signaling by NVP-BEZ235 as a new therapeutic strategy for acute myeloid leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(22):5424–5435. doi: 10.1158/1078-0432.CCR-10-1102. doi:10.1158/1078-0432.CCR-10-1102. [DOI] [PubMed] [Google Scholar]
- 45.Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, Fini M, McCubrey JA. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 2012;3(4):371–394. doi: 10.18632/oncotarget.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N, Vignon C, Park S, Guichard S, Herault O, Fricot A, Hermine O, Moura IC, Auberger P, Ifrah N, Dreyfus F, Bonnet D, Lacombe C, Mayeux P, Bouscary D, Tamburini J. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26(6):1195–1202. doi: 10.1038/leu.2011.339. doi:10.1038/leu.2011.339. [DOI] [PubMed] [Google Scholar]
- 47.Fu S, Hennessy BT, Ng CS, Ju Z, Coombes KR, Wolf JK, Sood AK, Levenback CF, Coleman RL, Kavanagh JJ, Gershenson DM, Markman M, Dice K, Howard A, Li J, Li Y, Stemke-Hale K, Dyer M, Atkinson E, Jackson E, Kundra V, Kurzrock R, Bast RC, Jr., Mills GB. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecologic oncology. 2012 doi: 10.1016/j.ygyno.2012.04.006. doi:10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guidetti A, Locatelli S, Viviani S, Dodero A, Farina L, Russo D, Bulian P, Sorasio R, Nicola MD, Corradini P, Anichini A, Gianni AM, Carlo-Stella C. Phosphorylation Levels of Extracellular-Signal Regulated Kinase (ERK) and AKT in Circulating Lymphocytes Predict Response to Targeted Therapy with Kinase Inhibitors in Refractory/Relapsed Hodgkin Lymphoma Patients. ASH Annual Meeting Abstracts. 2011;118(21):3705. [Google Scholar]
- 49.Jakubowiak AJ, Richardson PG, Zimmerman T, Alsina M, Kaufman JL, Kandarpa M, Kraftson S, Ross CW, Harvey C, Hideshima T, Sportelli P, Poradosu E, Gardner L, Giusti K, Anderson KC. Perifosine plus lenalidomide and dexamethasone in relapsed and relapsed/refractory multiple myeloma: a Phase I Multiple Myeloma Research Consortium study. British journal of haematology. 2012 doi: 10.1111/j.1365-2141.2012.09173.x. doi:10.1111/j.1365-2141.2012.09173.x. [DOI] [PubMed] [Google Scholar]