Abstract
Hantaviruses nonlytically infect human endothelial cells (ECs) and cause edematous and hemorrhagic diseases. Andes virus (ANDV) causes hantavirus pulmonary syndrome (HPS), and Hantaan virus (HTNV) causes hemorrhagic fever with renal syndrome (HFRS). Hantaviruses enhance vascular endothelial growth factor directed EC permeability resulting in the disassembly of inter-endothelial cell adherens junctions (AJs). Recent studies demonstrate that Slit2 binding to Robo1/Robo4 receptors on ECs has opposing effects on AJ disassembly and vascular fluid barrier functions. Here we demonstrate that Slit2 inhibits ANDV and HTNV induced permeability and AJ disassembly of pulmonary microvascular ECs (PMECs) by interactions with Robo4. In contrast, Slit2 had no effect on the permeability of ANDV infected human umbilical vein ECs (HUVECs). Analysis of Robo1/Robo4 expression determined that PMECs express Robo4, but not Robo1, while HUVECs expressed both Robo4 and Robo1 receptors. SiRNA knockdown of Robo4 in PMECs prevented Slit2 inhibition of ANDV induced permeability demonstrating that Robo4 receptors determine PMEC responsiveness to Slit2. Collectively, this data demonstrates a selective role for Slit2/Robo4 responses within PMECs that inhibits ANDV induced permeability and AJ disassembly. These findings suggest Slit2s utility as a potential HPS therapeutic that stabilizes the pulmonary endothelium and antagonizes ANDV induced pulmonary edema.
Hantaviruses predominantly infect human ECs and nonlytically cause 2 vascular leakage based diseases hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS)(Bustamante et al., 1997; Chang et al., 2007; Duchin et al., 1994; Enria et al., 1996; Galeno et al., 2002; Hallin et al., 1996; Koster et al., 2001; Levis et al., 1997; Lopez et al., 1996; Nolte et al., 1995; Padula et al., 1998; Schmaljohn and Hjelle, 1997; Zaki et al., 1995). Hantavirus infection alone does not cause EC permeability and instead hantaviruses alter EC responses that regulate fluid barrier properties of capillaries and lymphatic vessels (Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2008; Mackow and Gavrilovskaya, 2009). Currently there are no effective therapeutics for treating symptomatic HPS or HFRS patients (Jonsson et al., 2008).
Infection of ECs by pathogenic hantaviruses results in the hyper-phosphorylation of VEGFR2 and increased EC monolayer permeability in response to vascular endothelial growth factor (VEGF) (Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2011). HPS patients are acutely hypoxic with pulmonary edema fluid accumulating at up to a liter per hour (Koster and Mackow, 2012). VEGF is induced by hypoxic conditions and VEGF was first identified as a permeability factor that potently causes vascular leakage and edema (Dvorak, 2006). Hypoxia induced VEGF causes high altitude induced pulmonary edema, resulting from an auto-amplifying loop of VEGF induction that in turn increases the expression of the hypoxic sensor, HIF-1α (Berger et al., 2005; Dehler et al., 2006; Hopkins et al., 2005; Kosmidou et al., 2008; Manalo et al., 2005).
Vascular integrity is critical to survival and capillary leakage is redundantly regulated by factors, receptors and signaling responses that act in concert to maintain fluid barriers (Gavard and Gutkind, 2006; Kosmidou et al., 2008; Nagy et al., 2012). A growing list of factors counter permeabilizing VEGF-VEGFR2 responses by antagonizing VE-cadherin disassembly and stabilizing AJs (Gavard et al., 2008; Koch et al., 2011; London et al., 2009; Robinson et al., 2004; Xu et al., 2007). Recently Slit2 has been shown to positively or negatively regulate VEGF directed permeability depending on its respective binding Robo1 and Robo4 receptors (Acevedo et al., 2008; Koch et al., 2011; Sheldon et al., 2009). These findings suggest that Slit2 responses are determined by the tissue specific expression of Robo1/Robo4 receptors in ECs (Dickinson and Duncan, 2011; Sheldon et al., 2009).
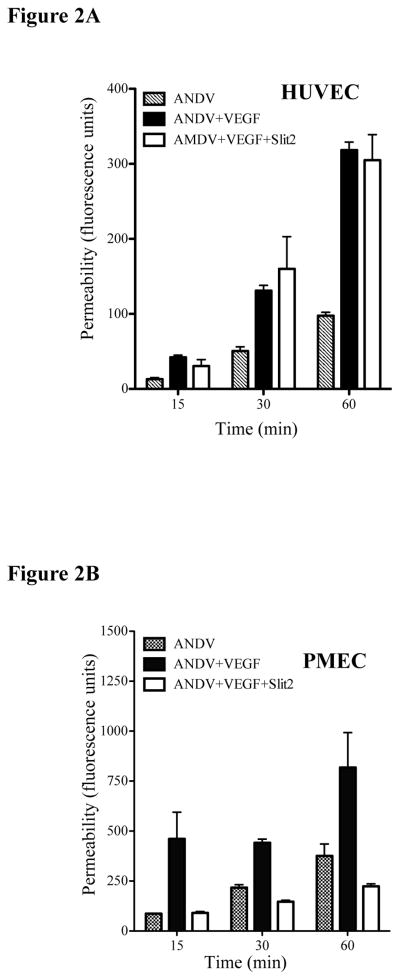
Robo4 is expressed in pulmonary tissues suggesting the potential for Slit2 responses to inhibit hantavirus induced permeability in pulmonary settings (Jones et al., 2009; London and Li, 2011). The role of Slit-2 stabilizing fluid barrier functions of the vasculature (Jones et al., 2009) prompted us to determine if Slit2 inhibits hantavirus induced EC responses. We initially monitored the permeability of ANDV (HPS), HTNV (HFRS) and TULV (no associated human disease) infected PMECs in response to Slit2 addition (London and Li, 2011). PMECs were comparably infected by ANDV, HTNV and TULV as demonstrated by expression of the viral nucleocapsid protein within monolayers (Fig. 1A)(Gavrilovskaya et al., 1998). Pathogenic ANDV and HTNV enhanced PMEC permeability in response to VEGF (Gavrilovskaya et al., 2008; Raymond et al., 2005), however the addition of Slit2 to infected PMECs abrogated ANDV or HTNV monolayer permeability (Fig. 1A). To demonstrate that the effects were the result of changes in AJs we subsequently monitored the effect of Slit2 on VE-cadherin internalization (Gavard and Gutkind, 2006; Gorbunova et al., 2010). ANDV infection resulted in the internalization of VE-cadherin in 80–90% of PMECs. However, addition of Slit2 to PMECs reduced VE-cadherin internalization to background levels (Fig. 1B). These findings demonstrate that Slit2 regulates ANDV induced VE-cadherin disassembly within AJs of infected PMECs. In contrast, we found that ANDV induced HUVEC permeability responses were insensitive to Slit2 addition (Fig. 2A) and unique from Slit2 inhibition of permeability responses observed in PMECs at all time points after VEGF addition (Fig. 2B). This indicated that Slit2 regulates PMEC, but not HUVEC, permeability responses induced by ANDV, and suggests the importance of analyzing tissue specific EC responses.
Figure 1.
Permeability and VE-cadherin internalization assays were performed 3 days post-infection as described (Gavrilovskaya et al., 2008). (A) Pulmonary Microvascular ECs (PMECs/Human microvascular ECs-lung-Cambrex) were mock, ANDV (CHI-7913), Hantaan virus (HTNV 76–118) or TULV (Tula/Moravia/MA 5302V/94) infected in BSL3 at an MOI of 0.5. In lower panel infected cells were immunoperoxidase stained for the hantavirus nucleocapsid protein (Geimonen et al., 2002). Three days p.i. monolayers were treated as indicated with VEGF (100 ng/ml) or Slit2 (N-terminal residues 1–1093 of human Slit2 homolog, Slit2-N; PeproTech, >98% pure) (10 nM) prior to assessing monolayer permeability to FITC-dextran (40,000; 0.5 mg/ml) as previously described. (B) PMECs were infected for 3 days and subsequently analysis of ANDV induced VE-cadherin internalization was performed as previously described on PMECs with or without Slit2 (Gavard and Gutkind, 2006; Gorbunova et al., 2010). Experiments were performed four times and error bars represent s.e.m.
Figure 2.
PMECs or HYVECs were infected for three days at an MOI of 0.5 and subsequently assayed for the effect of Slit2 on ANDV permeability in (A) human umbilical vein ECs (HUVECs-Cambrex); or (B) PMECs 15, 30 and 60 minutes post-VEGF addition, was performed as in Fig. 1 and previously described (Gavrilovskaya et al., 2008).
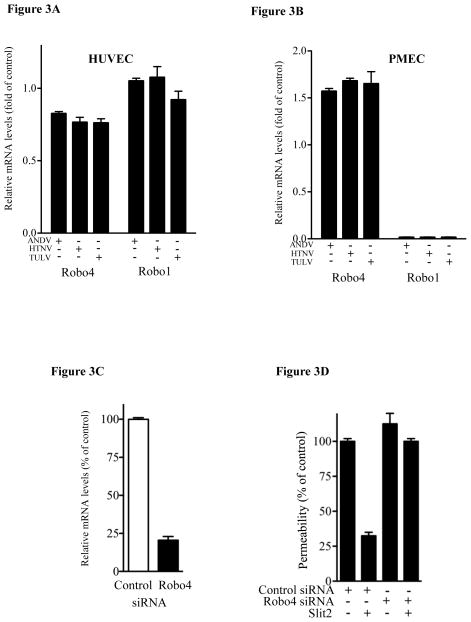
Robo1 and Robo4 receptors elicit opposing permeability responses following Slit2 binding (Marlow et al., 2010). This suggested a role for discrete Robo1/Robo4 levels in the differential Slit-2 responsiveness of HUVECs and PMECs. We determined the relative mRNA levels of Robo1 and Robo4 in HUVEC and PMECs infected with ANDV, HTNV or TULV and found that HUVECs contained high levels of both Robo1 and Robo4 mRNAs (Fig. 3A), while Robo4 mRNA levels were >10 fold higher than Robo1 in hantavirus infected PMECs (Fig. 3B). These findings suggest that PMECs predominantly express Robo4 receptors which regulate EC permeability in response to Slit2. We probed the role of Robo4 expression in the Slit2 responsiveness of PMECs using an siRNA knockdown approach (Kaur et al., 2008). Compared to control siRNAs, Robo4 siRNAs reduced Robo4 mRNA levels >80% (Fig. 3C). Control siRNAs had no effect on the Slit2 responsiveness of ANDV infected PMECs (Fig. 3D). In contrast, PMECs transfected with Robo4-specific siRNAs failed to regulate ANDV directed permeability following Slit2 addition. These findings indicate that Slit2 inhibits ANDV-induced hyperpermeability of PMECs via a Robo4 dependent mechanism.
Figure 3.
Role of Robo1/4 in Slit2 regulation of PMECs. HUVECs (Pepini et al., 2010) (3A) or PMECs (3B) were infected as above and analyzed by qRT-PCR for Robo1 and Robo4 mRNAs relative to GAPDH mRNA levels (Pepini et al., 2010). (3C) PMECs were transfected with negative-control (si-NEG2) or Robo4-specific siRNA and assayed by qRT-PCR for Robo4 mRNA levels as in 3B (Pepini et al., 2010). (3D) PMECs were ANDV infected as in Fig 1, Robo4 or control siRNA transfected 1 day post-infection, as in 3C, and assayed for PMEC permeability in response to VEGF as in Fig 1.
The ability of Slit2 to inhibit hantavirus permeability suggested that Slit2 effects might be additive to the effects of chemical inhibitors which block VE-cadherin internalization and PMEC permeability responses through unique mechanisms. Figure 4 compares Slit2 regulated permeability of ANDV infected PMECs to regulation by the Src inhibitor dasatinib and the S1P analog FTY720 (Gavrilovskaya et al., 2008; Gorbunova et al., 2011). Inhibitors were used individually or additively and assayed for their effect on ANDV induced monolayer induced permeability. Slit2 alone inhibited ANDV induced PMEC permeability similarly to dasatinib or FTY720 (Figure 4). Slit2 addition in combination with dasatinib or FTY720 resulted in more effective inhibition of ANDV induced permeability responses than any compound applied individually (Figure 4). These findings suggest that Slit2, alone or in conjunction with other compounds, may be useful in enhancing fluid barrier functions of the pulmonary endothelium during hantavirus infection.
Figure 4.
PMECs were ANDV infected and assayed for changes in permeability as in Fig. 1 in the presence or absence of Slit2 (effective range 5–50nM), dasatinib or FTY720 (Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2011).
Hypoxia induced VEGF reflects an apparent short term pulmonary repair mechanism that when sustained causes vascular dysfunction and edema (Berger et al., 2005; Stenmark et al., 2006; Tang et al., 2004). VEGF is an EC specific growth and permeability factor that acts on cells within 0.5 mm from its release and both directs EC migration and localized permeability of the endothelium (Dvorak, 2006; Dvorak et al., 1991). HPS patients are acutely hypoxic, have elevated levels of VEGF in their pulmonary edema fluid and patient hyper-oxygenation has contributed to a substantial reduction in HPS mortality rates (~85% to~35%) (Gavrilovskaya et al., 2012a; Koster and Mackow, 2012; Zaki et al., 1995). HPS patients often present in respiratory distress weeks after initial infection and rapidly progress to a critical disease period in which they accumulate pulmonary edema fluid at up to 1 liter per hour (Koster and Mackow, 2012). Both increased capillary permeability and decreased fluid clearance may contribute to HPS and hantaviruses infect the EC lining of vascular and lymphatic vessels (Koster and Mackow, 2012).
In vitro, pathogenic hantavirus infection of microvascular and lymphatic ECs enhances VEGF directed VEGFR2 phosphorylation, EC permeability and AJ disassembly (Gavrilovskaya et al., 2010, 2012b; Gavrilovskaya et al., 2008; Gorbunova et al., 2010; Gorbunova et al., 2011; Macneil et al., 2011; Shrivastava-Ranjan et al., 2010). These findings and the acute state of symptomatic hantavirus patients suggest targeting the endothelium, rather than virus, in order to stabilize and restore normal vascular barrier and clearance functions(Gavrilovskaya et al., 2012b; Gavrilovskaya et al., 2008; Gorbunova et al., 2011). Compounds that directly target VEGFR2 induced changes in AJs (ie. pazopanib/dasatinib) as well as growth factors and lipid mediators (ie. Ang-1, S1P/FTY720), that act through unique EC receptors, have the potential to restrict hantavirus directed EC permeability (Gavrilovskaya et al., 2008; Gorbunova et al., 2011). Studies presented here demonstrate that Slit2, a naturally secreted protein, restricts ANDV induced permeability of pulmonary ECs by binding Robo4 receptors. Our findings suggest that Slit2, alone or in conjunction with additional vascular barrier enhancers, may serve to prevent ANDV induced vascular permeability by stabilizing VE-cadherin within inter-EC junctions (Gavrilovskaya et al., 2008). These existing compounds and additional receptor and pathway specific inhibitors may provide therapeutic approaches for reducing the severity of hantavirus diseases at late times after infection when antivirals are ineffective (Jonsson et al., 2008).
Highlights.
Slit2 inhibits ANDV induced permeability of pulmonary endothelial cells.
Robo4 receptors determined Pulmonary MEC responsiveness to Slit2.
Slit2 enhances dasatinib and FTY720 inhibition of ANDV induced permeability.
Acknowledgments
Studies were supported by NIH grants R01AI47873, PO1AI055621, R21AI1080984, and U54AI57158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Acevedo LM, Weis SM, Cheresh DA. Robo4 counteracts VEGF signaling. Nat Med. 2008;14:372–373. doi: 10.1038/nm0408-372. [DOI] [PubMed] [Google Scholar]
- Berger MM, Hesse C, Dehnert C, Siedler H, Kleinbongard P, Bardenheuer HJ, Kelm M, Bartsch P, Haefeli WE. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;172:763–767. doi: 10.1164/rccm.200504-654OC. [DOI] [PubMed] [Google Scholar]
- Bustamante EA, Levy H, Simpson SQ. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest. 1997;112:1133–1136. doi: 10.1378/chest.112.4.1133. [DOI] [PubMed] [Google Scholar]
- Chang B, Crowley M, Campen M, Koster F. Hantavirus cardiopulmonary syndrome. Semin Respir Crit Care Med. 2007;28:193–200. doi: 10.1055/s-2007-976491. [DOI] [PubMed] [Google Scholar]
- Dehler M, Zessin E, Bartsch P, Mairbaurl H. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur Respir J. 2006;27:600–606. doi: 10.1183/09031936.06.00061505. [DOI] [PubMed] [Google Scholar]
- Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2011;139:697–704. doi: 10.1530/REP-10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Moolenaar RL, Reef SE, Nolte KB, Gallaher MM, Butler JC, Breiman RF, Group HS. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group [see comments] N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Discovery of vascular permeability factor (VPF) Exp Cell Res. 2006;312:522–526. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enria D, Padula P, Segura EL, Pini N, Edelstein A, Posse CR, Weissenbacher MC. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina. 1996;56:709–711. [PubMed] [Google Scholar]
- Galeno H, Mora J, Villagra E, Fernandez J, Hernandez J, Mertz GJ, Ramirez E. First human isolate of Hantavirus (Andes virus) in the Americas. Emerg Infect Dis. 2002;8:657–661. doi: 10.3201/eid0807.010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. Elevated VEGF Levels in Pulmonary Edema Fluid and PBMCs from Patients with Acute Hantavirus Pulmonary Syndrome. Adv Virol. 2012a;2012:674360. doi: 10.1155/2012/674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic Hantaviruses Direct the Adherence of Quiescent Platelets to Infected Endothelial Cells. Journal of Virology. 2010;84:4832–4839. doi: 10.1128/JVI.02405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Andes virus infection of lymphatic endothelial cells causes giant cell and enhanced permeability responses that are rapamycin and vascular endothelial growth factor C sensitive. J Virol. 2012b;86:8765–8772. doi: 10.1128/JVI.00817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol. 2008;82:5797–5806. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci U S A. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc Natl Acad Sci U S A. 2002;99:13837–13842. doi: 10.1073/pnas.192298899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J Virol. 2010;84:7405–7411. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. VEGFR2 and Src Kinase Inhibitors Suppress ANDV Induced Endothelial Cell Permeability. J Virol. 2011;85:2296–2303. doi: 10.1128/JVI.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin GW, Simpson SQ, Crowell RE, James DS, Koster FT, Mertz GJ, Levy H. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med. 1996;24:252–258. doi: 10.1097/00003246-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;171:83–87. doi: 10.1164/rccm.200406-707OC. [DOI] [PubMed] [Google Scholar]
- Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, Hayallah AM, Thomas KR, Famulok M, Zhang K, Ginsberg MH, Li DY. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson CB, Hooper J, Mertz G. Treatment of hantavirus pulmonary syndrome. Antiviral Res. 2008;78:162–169. doi: 10.1016/j.antiviral.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Samant GV, Pramanik K, Loscombe PW, Pendrak ML, Roberts DD, Ramchandran R. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol. 2008;9:61. doi: 10.1186/1471-2121-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvree K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Breant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Kosmidou I, Karmpaliotis D, Kirtane AJ, Barron HV, Gibson CM. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis. 2008;25:259–264. doi: 10.1007/s11239-007-0062-4. [DOI] [PubMed] [Google Scholar]
- Koster F, Foucar K, Hjelle B, Scott A, Chong YY, Larson R, McCabe M. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;116:665–672. doi: 10.1309/CNWF-DC72-QYMR-M8DA. [DOI] [PubMed] [Google Scholar]
- Koster F, Mackow E. Pathogenesis of the Hantavirus Pulmonary Syndrome. Future Virology. 2012;7:41–51. [Google Scholar]
- Levis S, Rowe J, Morzunov S, Enria D, St Jeor S. New Hantaviruses causing hantavirus pulmonary syndrome in central America. Lancet. 1997;349:998–999. doi: 10.1016/s0140-6736(05)62895-4. [DOI] [PubMed] [Google Scholar]
- London NR, Li DY. Robo4-dependent Slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Curr Opin Hematol. 2011;18:186–190. doi: 10.1097/MOH.0b013e328345a4b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London NR, Smith MC, Li DY. Emerging mechanisms of vascular stabilization. J Thromb Haemost. 2009;7(Suppl 1):57–60. doi: 10.1111/j.1538-7836.2009.03421.x. [DOI] [PubMed] [Google Scholar]
- Lopez N, Padula P, Rossi C, Lazaro ME, Franze-Fernandez MT. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology. 1996;220:223–226. doi: 10.1006/viro.1996.0305. [DOI] [PubMed] [Google Scholar]
- Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102:1030–1041. doi: 10.1160/TH09-09-0640. [DOI] [PubMed] [Google Scholar]
- Macneil A, Nichol ST, Spiropoulou CF. Hantavirus pulmonary syndrome. Virus Res. 2011;162:138–147. doi: 10.1016/j.virusres.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Marlow R, Binnewies M, Sorensen LK, Monica SD, Strickland P, Forsberg EC, Li DY, Hinck L. Vascular Robo4 restricts proangiogenic VEGF signaling in breast. Proc Natl Acad Sci U S A. 2010;107:10520–10525. doi: 10.1073/pnas.1001896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte KB, Feddersen RM, Foucar K, Zaki SR, Koster FT, Madar D, Merlin TL, McFeeley PJ, Umland ET, Zumwalt RE. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Human Pathology. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Padula PJ, Edelstein A, Miguel SD, Lopez NM, Rossi CM, Rabinovich RD. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology. 1998;241:323–330. doi: 10.1006/viro.1997.8976. [DOI] [PubMed] [Google Scholar]
- Pepini T, Gorbunova EE, Gavrilovskaya I, Mackow JE, Mackow ER. Andes Virus Regulation of Cellular MicroRNAs Contributes to Hantavirus Induced Endothelial Cell Permeability. J Virol. 2010;84:11929–11936. doi: 10.1128/JVI.01658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc Natl Acad Sci U S A. 2005;102:1163–1168. doi: 10.1073/pnas.0406743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL, Bicknell R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J Virol. 2010;84:11227–11234. doi: 10.1128/JVI.01405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol. 2007;293:C1309–1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]
- Zaki S, Greer P, Coffield L, Goldsmith C, Nolte K, Foucar K, Feddersen R, Zumwalt R, Miller G, Rollin P, Ksiazek T, Nichol S, Peters C. Hantavirus Pulmonary Syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]