Abstract
Circulating 25-hydroxyvitamin D (25(OH)D) has been associated with cardiovascular disease (CVD) risk in observational studies. Also, SNPs to explain variation in 25(OH)D have been identified by genome-wide association studies. Detection of direct associations between SNPs that significantly affect 25(OH)D and CVD risk would indicate a causal role of vitamin D, as reverse causation could be excluded and confounding could be better controlled. Thus, a combined analysis of candidate SNPs in relation to circulating 25(OH)D and CVD risk was carried out. A case-cohort study within the EPIC-Germany study was conducted comprising a randomly drawn subcohort of 2,132 subjects (57.9% women, mean age: 50.6 years) and incident cases of myocardial infarction (n=559) and stroke (n=471) that occurred during a mean follow-up duration of 7.6 years. 25(OH)D concentrations were measured by LC-MS/MS in baseline plasma samples. Additionally, eight candidate SNPs were assayed. Associations between 25(OH)D, SNPs and the risks of myocardial infarction and stroke were assessed by multivariable regression analyses. Mean 25(OH)D level was 47.2 nmol/L in the subcohort. Four SNPs were associated with 25(OH)D (p<0.05). In subjects with 25(OH)D levels <25 nmol/L, the risks of CVD as composite endpoint (Hazard Ratio: 1.53, 95% confidence interval: 1.12–2.09), myocardial infarction, and stroke were significantly increased compared to subjects with levels ≥50 nmol/L, while no significant linear associations were observed. A SNP score was not related to the risks of total CVD (Hazard Ratio: 1.0, 95% confidence interval: 0.71–1.42), myocardial infarction, or stroke. The same was true concerning single SNPs. Given the lack of association between SNPs and the risks of stroke and myocardial infarction, the present findings do not point to a major causal role of vitamin D in the development of these diseases. However, a detection of modest associations between genetic markers and CVD risk in larger consortia cannot be ruled out.
Introduction
Beyond its role in bone health, vitamin D has been proposed to lower the risks of a variety of non-skeletal health outcomes including cardiovascular diseases (CVD) [1,2]. Its active form, 1,25(OH)2vitamin D, is a secosteroid hormone whose synthesis starts by a UV–B induced conversion of 7-dehydrocholesterol to cholecalciferol (vitamin D3) in the human skin [3]. In addition, dietary cholecalciferol from animal food sources as well as dietary ergocalciferol (vitamin D2) from mushrooms contribute to vitamin D supply, even though to a lesser extent than cholecalciferol from cutaneous production [2,4]. In the circulation, vitamin D2 and D3 are transported to the liver bound to the vitamin D binding protein and converted to 25-hydroxyvitamin D (25(OH)D) by the vitamin D 25-hydroxylase. 25(OH)D, the accepted clinical indicator of vitamin D status, is activated to 1,25(OH)2vitamin D by the vitamin D 1-alpha-hydroxylase in the kidneys and, in a paracrine fashion, in several extra-renal tissues. Biological actions of activated vitamin D in the target organs are mediated through the vitamin D receptor (VDR) [3].
The detection of the VDR and the 1-alpha-hydroxylase in cells throughout the cardiovascular system has motivated multiple studies on the role of vitamin D in the etiology of CVD [5,6]. Several pathways linking vitamin D status with atherogenesis and CVD risk have been identified in experimental studies. Vitamin D seems to down-regulate the renin-angiotensin aldosterone system (RAAS), inflammation, coagulation, proliferation of vascular smooth muscle cells and cardiomyocytes, vascular calcification, and parathyroid hormone levels [5-8]. At the same time, vitamin D may improve insulin secretion and insulin sensitivity, as well as endothelial function [6,8].
CVD risk may be lowered by these mechanisms and pooled results from observational studies actually also suggest that higher circulating 25(OH)D levels are associated with decreased risks of stroke [9] and myocardial infarction [10]. However, it has been challenged that vitamin D is causally linked with the development of CVD [11]. Instead, low circulating 25(OH)D levels might be a surrogate for a poor overall health status, rather reflecting the inability to get outdoors because of multimorbidity, low exercise resistance, or increased BMI [12].
Although large randomized controlled trials (RCT) to elucidate the effects of vitamin D supplement use on CVD risk are under way, results are not to be expected before 2017 [13]. Also, concerns have already been raised about compliance, statistical power, latitude of the study centers, target populations, or the applied vitamin D dosages in these trials [13-15]. As a complementary approach to RCTs, observational studies with combined analyses of circulating 25(OH)D and its genetic determinants in relation to CVD endpoints may help to assess, whether the link between vitamin D status and CVD risk is causal [14,16]. Several single nucleotide polymorphisms (SNPs) coding for the vitamin D binding protein and enzymes involved in vitamin D synthesis and metabolism have been found to be associated with vitamin D status in genome wide association studies (GWAS) [17,18]. Concurrent associations of such SNPs with both vitamin D status and CVD risk in observational studies would indicate a causal link between vitamin D status and CVD risk, as genetic variation is assigned randomly during meiosis and reverse causation could be ruled out [16,19].
To date, only one study has assessed the association between vitamin D related SNPs, vitamin D status and the risk of myocardial infarction (MI) [20], while no comparable study on stroke risk has been carried out. Therefore, we investigated the associations between genetic vitamin D status determinants, circulating 25(OH)D, and the risks of MI and stroke in the German branch of the European Prospective Investigation into Cancer and Nutrition (EPIC).
Methods
Population and study design
The European Investigation into Cancer and Nutrition (EPIC) is an ongoing prospective study of chronic diseases in 10 European countries. In Germany, it comprises two cohorts at centers in Heidelberg and Potsdam [21]. Between 1994 and 1998, 25 440 participants (13 612 women and 11 928 men) were recruited in Heidelberg and 27 548 participants (16 644 women and 10 904 men) in Potsdam. Subjects were mostly aged between 35 and 65 years at both centers. Baseline examinations included detailed self-administered questionnaires and interviews on health status, socio-economic status and lifestyle, anthropometric measurements, and a dietary assessment by food frequency questionnaire [21]. A blood sample from about 95% of the participants was drawn, processed and stored at -196° Celsius in liquid nitrogen [21]. Participants are being followed-up by active and passive procedures [22].
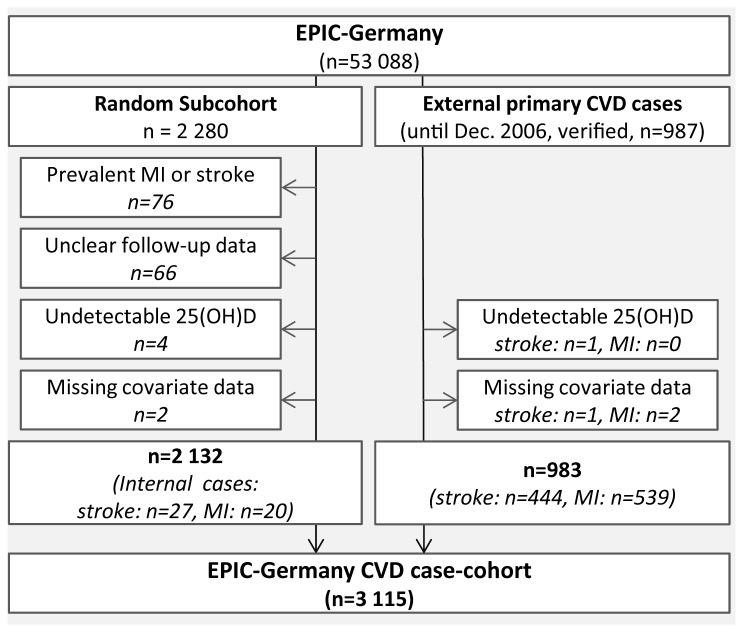
A case-cohort design was chosen for the analyses on 25(OH)D in relation to CVD risk [23,24]. Random subcohorts were drawn in Heidelberg and Potsdam according to the protocol of the Europe-wide EPIC InterAct study on diabetes type 2 [25]. As opposed to the InterAct selection criteria, prevalent cases of diabetes mellitus type 2 were kept in the present study. After the exclusion of participants with prevalent MI or stroke (n=76), without complete follow-up information (n=66), missing 25(OH)D values (n=4), and missing covariate data (n=2), the common subcohort consisted of 2 132 participants (Figure 1). In addition, incident cases of primary MI and stroke from complete follow-up rounds up to the end of December 2006 were included into the case-cohort study. MIs and strokes were ascertained by study physicians who carried out a detailed and systematic medical verification of self-reports and death certificates by clinical records in cooperation with treating physicians and hospitals. Outcomes were classified as fatal in case of death within the first 28 days after diagnosis. The following conditions were coded according to the International Statistical Classification of Diseases, 10th revision, 2011 (ICD-10): MI (ICD-10 I21), ischemic stroke (ICD-10 I63), hemorrhagic stroke (ICD-10 I60 and I61) and unspecified stroke (ICD-10 I64). After exclusion of incident cases with undetectable 25(OH)D levels (nMI=1) or missing covariate data (nMI=1, nstroke=2), 559 verified cases of MI and 471 verified cases of stroke that had occurred during average follow-up periods of 7.5 (±2.3) and 7.7 (±2.1) years, respectively, were included into the CVD case-cohort study (Figure 1). Out of these, 20 MI cases and 27 stroke cases were part of the random subcohort. 90 (16.1%) MI cases and 50 (10.6%) stroke cases were fatal. 363 (77.1%) stroke cases were classified as ischemic, 88 (18.7%) as hemorrhagic, and 20 (4.2%) cases as unspecified.
Figure 1. Flow chart indicating exclusions due to prevalent CVD and missing values.
Ethics statement
The study was approved by ethics committees at both study sites (Potsdam: Ethics Committee of the Medical Association of the State of Brandenburg; Heidelberg: Ethics Committee of the Heidelberg University Medical School) and all participants gave written informed consent.
Laboratory methods
Vitamin D status was determined in the laboratory of the Institute of Agricultural and Nutritional Sciences at the University of Halle-Wittenberg, Germany. 25(OH)D2 and 25(OH)D3 were assayed in plasma samples from baseline that had been stored at -196° Celsius for about 15 years on average. A MassChrom® reagent kit (Chromsystems, Munich, Germany) was used on an LC/MS-MS system (API 2000™, Applied Biosystems, Darmstadt, Germany) to measure both metabolites. Samples of incident cases and subcohort members were assigned to 78 analytical batches. Each batch contained at least 30 samples from both study centers and included both case and control samples. Laboratory staff was blinded to the case status of the analyzed samples. The within-batch coefficients of variation for 25(OH)D2 and 25(OH)D3 were 4.9% at 43.5 nmol/L and 4.2% at 96.8 nmol/L (n=3), and 5.6% at 41.8 nmol/L and 3.5% at 95.8 nmol/L (n=3), respectively. Between-batch coefficients of variation for 25(OH)D2 and 25(OH)D3 and were 5.8% at 43.5 nmol/L and 5.6% at 96.8 nmol/L, as well as 7.8% at 41.8 nmol/L and 6.6% at 95.8 nmol/L. The use of quality control samples from the Vitamin D External Quality Assessment Scheme (www.deqas.org) revealed that 80% of the results fell within ±15% of the ALTM (All Laboratory Trimmed Mean), as compared to ±25% being the goal. The lower limit of quantification (LLOQ) was 8.25 nmol/L for 25(OH)D2 and 11.5 nmol/L for 25(OH)D3, the lower limit of detection (LLOD) was 2.75 nmol/l for 25(OH)D2 and 4.5 nmol/L for 25(OH)D3. For measured values above the LOD, but below the LLOQ, values equal to half of the LLOQ were assigned for statistical analyses. Overall, 25 out of 3 115 detected 25(OH)D3 values, and 127 out of 174 detected 25(OH)D2 values were below the LLOQ.
Candidate SNPs were selected based on the results of two recent GWAS [17,18] and a systematic review [26]. Information regarding linkage disequilibrium (LD) and minor-allele frequencies (MAF) of candidate SNPs was obtained using HapMap data (database release 27, CEU and TSI populations). SNPs characterized by a MAF <0.15 or a high LD with other candidates (R2≥0.8) were not considered. Overall 8 SNPs from 6 regions were selected: rs1155563 and rs2282679 in the GC (group specific component) locus that codes for the vitamin D binding protein; rs3829251 and rs12785878 in the DHCR7/NADSYN1 locus that codes for the 7-dehydrocholesterol reductase involved in converting 7-dehyrocholestrol, the substrate for cholecalciferol, to cholesterol; rs10741657 in the CYP2R1 locus (25-hydroxylase); rs10877012 in the CYP27B1 locus (1-alpha-hydroxylase); rs6013897 in the CYP24A1 locus (24-hydroxylase that deactivates 1,25(OH)2vitamin D); rs6599638 in the C10orf88 locus in the vicinity of the gene coding for the acyl-Coenzyme A dehydrogenase, an enzyme involved in cholesterol synthesis;
Buffy-coat samples retrieved from liquid nitrogen storage were used for DNA extraction. Genotyping was carried out at KBioscience (Hoddesdon, Herts, UK) using the KASP assay, a homogeneous fluorescent genotyping system based on competitive allele-specific PCR. For quality control purposes non-template controls (NTC) were included on each plate. Genotyping call rates exceeded 96% for each SNP.
Statistical evaluation
It was decided to use total 25(OH)D values for the statistical analyses, because 25(OH)D2 was detected in 5.6% of the samples only. To standardize 25(OH)D levels for season of blood draw, residuals obtained from locally weighted polynomial regression of 25(OH)D on calendar week of blood draw were added to the overall case-cohort mean value [27,28].
Hazard ratios (HR) of MI and stroke were calculated with Cox proportional-hazards models modified by the Prentice method to account for the case-cohort design of the study [23,24]. 25(OH)D levels were grouped into quartiles setting the highest quartile as the reference. In addition, we used clinical categories of 25(OH)D levels as defined by the Robert Koch Institute (severe to moderate deficiency: <25 nmol/L, mild deficiency: 25-49.9 nmol/L, sufficiency: ≥50 nmol/L) as predictors in our models [29]. Age was used as the underlying timescale. The baseline hazard function was allowed to differ by age (1 year integers) and center. First, a crude model was built adjusting for sex and BMI (continuous). Further potential confounders identified by literature search, i.e. waist circumference (cm, continuous), smoking (‘never’, ‘former’, ‘1-20 cigarettes per day’, ‘>20 cigarettes per day’), physical activity according to the Cambridge Physical Activity Index [30] (‘active’, ‘moderately active’, ‘moderately inactive’, ‘inactive’), and alcohol consumption (g/d, continuous), as well as educational attainment as proxy for the subjects’ socioeconomic status (‘low’: primary school or no school graduation; ‘medium’: secondary, technical or professional school graduation; ‘high’: longer education including university degree) were additionally included in the second model. Linear trends were tested treating quartile medians as quantitative exposure scores. Besides the analyses with season-standardized 25(OH)D values, regression models were recalculated using quartiles of raw 25(OH)D values with adjustment for month of blood draw or season-specific 25(OH)D quartiles.
To check for non-linearity, Cox models with first and second degree fractional polynomials of 25(OH)D levels were applied allowing for seven transformations of continuous 25(OH)D values (by x-2, x-1, x-0.5, x0.5, x2, x3, and log(x)) [31]. 25(OH)D values below the lower limit of quantification and in the highest percentile of the distribution were omitted from fractional polynomial analyses. The fractional polynomial with the best fit according to deviance testing was selected [31]. Log likelihood ratio tests were conducted to detect interactions between 25(OH)D levels with adjustment variables. Sensitivity analyses were carried out excluding the cases that occurred within the first two years after baseline.
Correlation coefficients and Lewontin’s D' were calculated as measures for the Linkage disequilibrium (LD) between SNP pairs. Chi-squared tests were conducted to assess if genotypes were in Hardy-Weinberg equilibrium (HWE). Generalized Linear Models were used to assess the association between SNPs and 25(OH)D in the subcohort adjusting for age, sex and study center. P values were multiplied by 8 to account for multiple testing. Partial R2 values were calculated as estimates for the effect sizes of the SNPs. HRs of MI and stroke across alleles were calculated by Cox proportional-hazards models adjusted for sex and center using age as the underlying timescale. Co-dominant, dominant, log-additive, and recessive models were used for analyses on single SNPs. Further, risk allele scores were calculated based on SNPs that were significantly associated with 25(OH)D levels. First, a simple score was created summing the number of alleles associated with lower 25(OH)D levels. Alternatively, risk alleles were weighted by beta coefficients obtained from the regression of SNPs on 25(OH)D levels using an additive model. Statistical tests were two-sided and p-values of <0.05 were considered statistically significant. SAS 9.2 (SAS Institute, NC, USA) was used for all analyses.
Results
Characteristics of the study participants
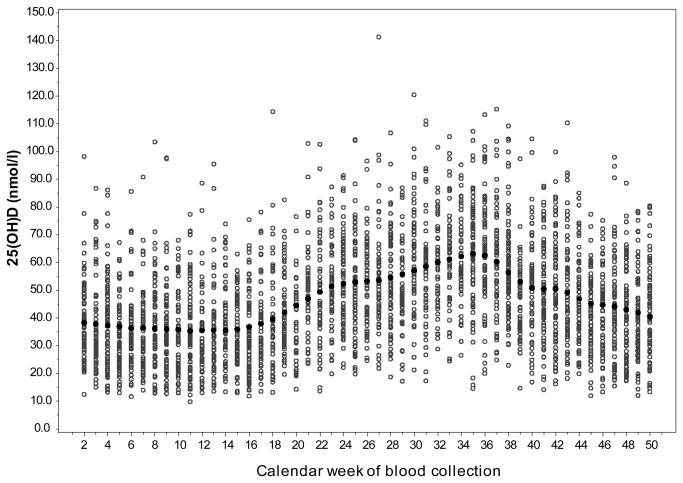
The seasonal variation in 25(OH)D levels in the subcohort is shown in Figure 2. Mean season-adjusted 25(OH)D levels were higher among non-cases in the subcohort (47.3 nmol/L, ±18.3) and in the total subcohort (47.2 nmol/L, ±18.3) when compared to the mean levels in MI cases (44.6 nmol/L, ±18.9) and stroke cases (44.6 nmol/L, ±18.3). Further baseline characteristics of the subcohort and the incident cases are presented in Table 1. Incident MI and stroke cases were older and more likely to be men compared to the subcohort members. They had higher risk profiles concerning classical CVD risk factors including overweight and obesity, smoking, physical activity, hypertension, and diabetes. Also, CVD cases had higher waist circumference values and lower formal education levels. Finally, average alcohol intake levels in stroke cases were higher than in subcohort members.
Figure 2. Seasonal variation in plasma 25(OH)D levels.
The light circles represent individual 25(OH)D plasma concentrations, the black circles represent weekly average 25(OH)D plasma concentrations after smoothing by locally weighted polynomial regression.
Table 1. Characteristics of the subcohort presented by quartiles of season-standardized 25(OH)D.
| Subcohort* (quartiles of season-standardized plasma 25(OH)D) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | All | MI cases | stroke cases | |
| N | 533 | 533 | 533 | 533 | 2132 | 559 | 471 |
| Women | 62.5 | 58.5 | 55.4 | 55.2 | 57.9 | 22.4 | 41.1 |
| Age at baseline (years) | 50.7 (±8.5) | 51.2 (±8.4) | 50.6 (±8.7) | 49.9 (±8.5) | 50.6 (±8.6) | 55.7 (±6.7) | 56.3 (±7.3) |
| 25(OH)D (nmol/L)† | 27.3 (±6.3) | 40.4 (±2.9) | 50.8 (±3.3) | 70.3 (±16.9) | 47.2 (±18.3) | 44.6 (±18.9) | 44.6 (±18.3) |
| Season of blood draw | |||||||
| Jan–Mar | 25.9 | 29.8 | 26.1 | 21.0 | 25.7 | 23.2 | 28.5 |
| Apr-Jun | 21.6 | 26.8 | 23.5 | 24.6 | 24.1 | 21.5 | 22.6 |
| Jul–Sept | 26.8 | 22.2 | 27.0 | 27.8 | 25.9 | 28.3 | 24.9 |
| Oct–Sept | 25.7 | 21.2 | 23.4 | 26.6 | 24.3 | 27.0 | 24.0 |
| BMI categories | |||||||
| Normal weight | 43.0 | 37.3 | 46.3 | 52.7 | 44.8 | 22.2 | 32.7 |
| Overweight | 34.5 | 42.4 | 40.2 | 38.5 | 38.9 | 51.3 | 47.0 |
| Obese | 22.5 | 20.3 | 13.5 | 8.8 | 16.3 | 26.5 | 21.3 |
| Waist circumference (cm) | |||||||
| Women | 82.9 (±13.1) | 82.6 (±12.2) | 79.2 (±9.9) | 77.1 (±9.1) | 80.6 (±11.5) | 86.5 (±12.5) | 84.5 (±12.0) |
| Men | 96.5 (±11.2) | 96.9 (±10.7) | 94.8 (±9.9) | 93.1 (±8.8) | 95.2 (±10.2) | 98.9 (±9.7) | 97.5 (±10.8) |
| Alcohol intake (g/day) | 14.8 (±22.6) | 14.6 (±16.8) | 15.3 (±17.1) | 19.0 (±21.0) | 15.9 (±19.6) | 16.8 (±19.7) | 20.1 (±26.4) |
| Smoking status | |||||||
| Never | 47.8 | 46.9 | 46.9 | 45.4 | 46.8 | 29.2 | 37.3 |
| Former | 25.2 | 31.9 | 35.7 | 38.1 | 32.7 | 30.6 | 34.2 |
| Current | 27.0 | 21.2 | 17.4 | 16.5 | 20.5 | 40.2 | 28.5 |
| Physical activity index | |||||||
| Inactive | 21.4 | 20.3 | 13.7 | 12.8 | 17.0 | 22.9 | 25.5 |
| Moderately Inactive | 38.3 | 35.5 | 38.6 | 36.2 | 37.2 | 36.9 | 35.7 |
| Moderately Active | 25.7 | 25.5 | 25.0 | 28.3 | 26.1 | 23.6 | 20.2 |
| Active | 14.6 | 18.7 | 22.7 | 22.7 | 19.7 | 16.6 | 18.6 |
| Education level | |||||||
| Low | 24.0 | 21.8 | 21.6 | 20.8 | 22.1 | 34.0 | 33.5 |
| Medium | 44.5 | 44.1 | 40.9 | 44.7 | 43.5 | 36.3 | 39.3 |
| High | 31.5 | 34.1 | 37.5 | 34.5 | 34.4 | 29.7 | 27.2 |
| Prevalent hypertension‡ | 32.8 | 35.5 | 32.1 | 27.4 | 31.9 | 48.4 | 52.1 |
| Prevalent diabetes‡ | 5.6 | 4.9 | 3.0 | 2.4 | 4.0 | 14.5 | 13.9 |
Includes 20 incident cases of MI and 27 incident cases of stroke;
Season-standardized;
Self-reported;
Presented values are percentages. Means (standard deviations) are shown for age, 25(OH)D, waist circumference, and alcohol intake;
In the subcohort, there were more women in the lowest 25(OH)D quartile compared to the overall percentage of women. Age at baseline was similar across 25(OH)D quartiles. The proportions of overweight and obese subjects, current smokers, physically inactive subjects, and subjects with prevalent hypertension or diabetes were lower at higher 25(OH)D levels, while average alcohol intakes increased across 25(OH)D quartiles. There was no clear relation between education levels and 25(OH)D levels. Mean plasma 25(OH)D levels were similar among participants from Heidelberg (47.4 nmol/L, ±19.6) and Potsdam (47.0 nmol/L, ±17.2) (p=0.63).
Associations between plasma 25(OH)D and incident myocardial infarction and stroke
Prospective associations between plasma 25(OH)D levels and the risks of MI and stroke are shown in Table 2. In the basic model adjusted for sex and BMI, 25(OH)D was significantly inversely associated with the risk of MI (HR between quartile 1 and quartile 4 [95% confidence interval]: 1.43 [1.07-1.92], plinear trend<0.01). After additional adjustment for waist circumference, alcohol intake, physical activity, smoking, and education levels, the association attenuated and was no longer significant (HR [95% CI]: 1.17 [0.86-1.58], plinear trend=0.19). Concerning incident stroke, the basic model revealed a significant association with 25(OH)D levels (HR [95% CI]: 1.37 [1.02-1.84], plinear trend=0.05). This association also attenuated by multiple adjustment (HR [95% CI]: 1.25 [0.92-1.70], plinear trend=0.19). Similarly, 25(OH)D levels were significantly inversely related to CVD risk in the basic model (HR [95% CI]: 1.41 [1.11-1.79], plinear trend<0.01), but not in the multivariable model (HR [95% CI]: 1.19 [0.93-1.52], plinear trend=0.12) when using MI and stroke as a composite endpoint (Table 2).
Table 2. Hazard ratios for cardiovascular diseases according to quartiles of 25(OH)D.
| 25(OH)D quartile |
Cases
|
Model 1
|
Model 2
|
|||||
|---|---|---|---|---|---|---|---|---|
| (Median nmol/L)* | N | (%) | HR | 95% CI | P trend† | HR | 95% CI | P trend† |
| Myocardial Infarction | ||||||||
| Q4 (66.5) | 118 | (21.1) | 1.0 | (Referent) | 1.0 | (Referent) | ||
| Q3 (50.5) | 117 | (20.9) | 0.95 | (0.70-1.28) | 0.85 | (0.62-1.17) | ||
| Q2 (40.4) | 158 | (28.3) | 1.24 | (0.93-1.66) | 1.07 | (0.78-1.45) | ||
| Q1 (28.9) | 166 | (29.7) | 1.43 | (1.07-1.92) | <0.01 | 1.17 | (0.86-1.58) | 0.19 |
| Stroke | ||||||||
| Q4 (66.6) | 111 | (23.6) | 1.0 | (Referent) | 1.0 | (Referent) | ||
| Q3 (50.5) | 101 | (21.4) | 0.86 | (0.63-1.17) | 0.83 | (0.60-1.13) | ||
| Q2 (40.4) | 102 | (21.7) | 0.83 | (0.61-1.12) | 0.83 | (0.61-1.14) | ||
| Q1 (28.9) | 157 | (33.3) | 1.37 | (1.02-1.84) | 0.05 | 1.25 | (0.92-1.70) | 0.19 |
| CVD as composite endpoint | ||||||||
| Q4 (66.6) | 229 | (22.2) | 1.0 | (Referent) | 1.0 | (Referent) | ||
| Q3 (50.5) | 218 | (21.2) | 0.89 | (0.70-1.14) | 0.84 | (0.65-1.09) | ||
| Q2 (40.4) | 260 | (25.2) | 1.06 | (0.83-1.35) | 0.96 | (0.75-1.24) | ||
| Q1 (28.9) | 323 | (31.4) | 1.41 | (1.11-1.79) | <0.01 | 1.19 | (0.93-1.52) | 0.12 |
Hazard Ratios calculated by Cox regression analyses using Prentice weights to account for the case-cohort design;
Model 1 adjusted for BMI and sex, stratified by center and age at baseline;
Model 2 additionally adjusted for waist circumference, alcohol intake, education level, physical activity, and smoking
Quartile medians from the subcohort;
P for trend calculated modeling quartile medians as continuous variable;
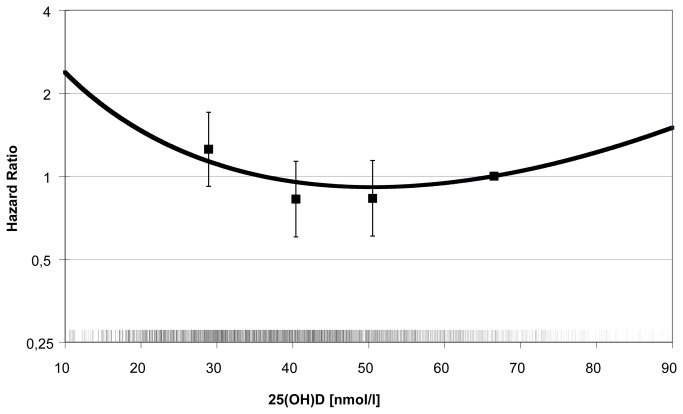
With regard to MI and total CVD, there was no indication for a nonlinear relation with 25(OH)D using fractional polynomials. By contrast, there was a significant (p<0.01) nonlinear association between 25(OH)D and incident stroke characterized by a j-shaped curve after transforming 25(OH)D levels to a second degree polynomial (Figure 3). A categorization by clinical cut-points revealed an increased risk of stroke in participants with deficient 25(OH)D levels (<25 nmol/L) as compared to participants with levels above 50 nmol/L (HR [95% CI]: 1.54 [1.05-2.27], p=0.03, Table 3). The same was true concerning MI risk (HR [95% CI]: 1.56 [1.08-2.25], p=0.02) and CVD risk (HR [95% CI]: 1.53 [1.12-2.09], p<0.01). The risks of both MI and stroke in subjects with 25(OH)D levels between 25 and 49.9 nmol/L did not differ significantly from those of subjects with levels of ≥50 nmol/L.
Figure 3. Nonlinear association between plasma 25(OH)D and incident stroke.
The curve represents the following second degree fractional polynomial function of 25(OH)D that is significantly (p<0.01) associated with incident stroke after multivariable adjustment: HR stroke = exp[((x)1 ×(-0.19457) + (x)1 ×(0.03954)×log(x)) – ((66.5)1 ×(-0.19457) + (66.5)1 ×(0. 03954)×log(x))];
Additionally, hazard ratios of stroke by 25(OH)D quartiles from the fully adjusted model are presented. The reference for the polynomial function was set at 66.5 nmol/L, the median value in the highest 25(OH)D quartile. The short vertical ticks above the horizontal axis represent the distribution of season-standardized 25(OH)D values in incident stroke cases and the subcohort.
Table 3. Hazard ratios for cardiovascular diseases according to clinical vitamin D status categories.
| Clinical category | 25(OH)D (nmol/L) | N cases/subco. |
Model 1
|
Model2
|
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Myocardial Infarction | ||||||||
| Sufficiency | ≥50 | 183/802 | 1.0 | (Referent) | ||||
| Mild deficiency | 25-49.9 | 315/1162 | 1.24 | (0.99-1.55) | 0.06 | 1.10 | (0.87-1.39) | 0.43 |
| Moderate to severe deficiency | <25 | 61/168 | 1.81 | (1.25-2.64) | <0.01 | 1.56 | (1.08-2.25) | 0.02 |
| Stroke | ||||||||
| Sufficiency | ≥50 | 171/802 | 1.0 | (Referent) | ||||
| Mild deficiency | 25-49.9 | 240/1162 | 0.99 | (0.79-1.24) | 0.93 | 0.95 | (0.75-1.19) | 0.65 |
| Moderate to severe deficiency | <25 | 60/168 | 1.81 | (1.25-2.62) | <0.01 | 1.54 | (1.05-2.27) | 0.03 |
| CVD (composite) | ||||||||
| Sufficiency | ≥50 | 354/802 | 1.0 | (Referent) | 1.0 | (Referent) | ||
| Mild deficiency | 25-49.9 | 555/1162 | 1.11 | (0.93-1.33) | 0.25 | 1.01 | (0.84-1.22) | 0.90 |
| Moderate to severe deficiency | <25 | 121/168 | 1.80 | (1.33-2.45) | <0.01 | 1.53 | (1.12-2.09) | <0.01 |
Hazard ratios calculated by Cox regression analyses using Prentice weights to account for the case-cohort design;
Model 1 adjusted for BMI and sex, stratified by center and age at baseline;
Model 2 additionally adjusted for waist circumference, alcohol intake, education level, physical activity, and smoking
Additional adjustment for self-reported prevalent hypertension, dyslipidemia, diabetes, drug use (anti-hypertensive drugs, NSAIDS, glucocorticosteroids, bisphosphonates, anti-epileptics, oral contraceptives, or hormone replacement therapy), and general use of vitamin and mineral supplements did not affect the reported associations substantially (data not shown). There was no evidence for effect modification of the association between 25(OH)D and disease risks by other risk variables. Also, subgroup analyses for nonfatal MI, fatal MI, ischemic stroke, and hemorrhagic stroke did not reveal significant associations. Finally, in sensitivity analyses excluding cases that had occurred within the first two years after baseline, 25(OH)D levels were not related to MI or stroke risk (data not shown). Analyses showed similar results when using raw 25(OH)D levels adjusting for month of blood draw or season-specific quartiles (data not shown).
Associations between SNPs and plasma 25(OH)D
Four SNPs (rs1155563 and rs2282679 in the GC locus, as well as rs12785878 and rs3829251 in the DHCR7/NADSYN1 locus) were significantly associated with season-adjusted 25(OH)D levels in the subcohort (Table 4). The variance in 25(OH)D explained by these four SNPs ranged from 0.7% (rs3829251) to 3.0% (rs2282679). As the two SNP pairs in the GC locus (rs1155563 and rs2282679) and the DHCR7/NADSYN1 locus (rs12785878 and rs3829251) were in high LD (r2>0.8), only rs2282679 and rs12785878 were used to build SNP scores. A simple SNP score based on the sum of risk alleles from both SNPs explained 4.4% of the variance in plasma 25(OH)D levels, while a SNP score based on risk alleles weighted by beta coefficients from linear regression models explained 4.2% adjusting for age, sex and center (data not shown). SNP associations with 25(OH)D levels were similar when analyzing data of the entire case-cohort or when using raw 25(OH)D levels adjusting for month of blood draw instead of season-standardized levels (data not shown).
Table 4. Associations between candidate SNPs, season-standardized 25(OH)D levels, and the risks cardiovascular diseases.
|
|
25(OH)D levels by alleles
|
Risk of myocardial Infarction†
|
Risk of stroke†
|
Risk of CVD (composite)†
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP, locus* | Allele |
N subcohort
|
Mean 25(OH)D | P** | R2 | N cases | HR | 95% CI | P | N cases | HR | 95% CI | P | N cases | HR | 95% CI | P | |
| rs1155563, | T/T | 1059 |
49.7 | 281 | Ref. | 243 | Ref. | 524 | Ref. | |||||||||
| GC | T/C | 832 |
45.1 | 224 | 1.07 | (0.86-1.33) | 185 | 0.99 | (0.79-1.24) | 409 | 1.05 | (0.88-1.25) | ||||||
| C/C | 164 |
42.4 | <0.01 | 2.1 | 36 | 0.80 | (0.53-1.21) | 0.73 | 32 | 0.90 | (0.59-1.37) | 0.69 | 68 | 0.84 | (0.60-1.18) | 0.72 | ||
| rs2282679, | A/A | 1058 | 50.0 | 288 | Ref. | 249 | Ref. | 537 | Ref. | |||||||||
| GC | A/C | 827 | 45.0 | 216 | 0.97 | (0.77-1.21) | 183 | 0.91 | (0.73-1.14) | 399 | 0.94 | (0.79-1.13) | ||||||
| C/C | 166 | 40.3 | <0.01 | 3.0 | 38 | 0.85 | (0.57-1.27) | 0.48 | 31 | 0.85 | (0.56-1.31) | 0.32 | 69 | 0.85 | (0.61-1.19) | 0.31 | ||
| rs3829251, | G/G | 1415 | 48.2 | 380 | Ref. | 314 | Ref. | 694 | Ref. | |||||||||
| DHCR7/ | G/A | 594 | 45.1 | 150 | 1.04 | (0.83-1.31) | 140 | 1.15 | (0.91-1.45) | 290 | 1.09 | (0.90-1.31) | ||||||
| NADSYN1 | A/A | 60 | 44.0 | <0.01 | 0.7 | 15 | 1.17 | (0.63-2.17) | 0.61 | 11 | 1.0 | (0.50-1.98) | 0.34 | 26 | 1.09 | (0.65-1.83) | 0.38 | |
| rs12785878, | T/T | 1081 | 49.0 | 290 | Ref. | 241 | Ref. | 531 | Ref. | |||||||||
| DHCR7/ | T/G | 806 | 46.2 | 210 | 1.04 | (0.84-1.29) | 181 | 1.05 | (0.84-1.31) | 391 | 1.06 | (0.89-1.27) | ||||||
| NADSYN1 | G/G | 172 | 40.3 | <0.01 | 1.7 | 42 | 1.12 | (0.76-1.65) | 0.55 | 42 | 1.23 | (0.83-1.81) | 0.35 | 84 | 1.20 | (0.87-1.65) | 0.38 | |
| rs6599638, | G/G | 555 | 46.3 | 135 | Ref. | 127 | Ref. | 262 | Ref. | |||||||||
| C10orff88 | G/A | 1020 | 47.6 | 268 | 1.10 | (0.86-1.41) | 231 | 1.02 | (0.79-1.31) | 499 | 1.07 | (0.87-1.31) | ||||||
| A/A | 488 | 47.1 | 1.0 | 0.0 | 141 | 1.14 | (0.85-1.52) | 0.36 | 106 | 0.92 | (0.68-1.23) | 0.56 | 247 | 1.05 | (0.83-1.33) | 0.70 | ||
| rs10741657, | G/G | 768 | 46.2 | 208 | Ref. | 183 | Ref. | 391 | Ref. | |||||||||
| CYP2R1 | G/A | 966 | 47.0 | 251 | 1.07 | (0.86-1.34) | 212 | 1.03 | (0.82-1.30) | 463 | 1.06 | (0.88-1.28) | ||||||
| A/A | 317 | 49.2 | 0.10 | 0.3 | 87 | 0.94 | (0.69-1.28) | 0.87 | 66 | 0.81 | (0.60-1.24) | 0.33 | 153 | 0.90 | (0.69-1.16) | 0.62 | ||
| rs10877012, | G/G | 919 | 48.1 | 243 | Ref. | 213 | Ref. | 456 | Ref. | |||||||||
| CYP27B1 | T/G | 910 | 46.7 | 251 | 1.10 | (0.89-1.37) | 203 | 1.00 | (0.80-1.25) | 454 | 1.11 | (0.89-1.40) | ||||||
| T/T | 238 | 45.3 | 0.16 | 0.3 | 49 | 0.79 | (0.55-1.13) | 0.59 | 47 | 0.86 | (0.59-1.23) | 0.53 | 96 | 1.07 | (0.86-1.35) | 0.38 | ||
| rs6013897, | T/T | 1315 | 47.7 | 337 | Ref. | 276 | Ref. | 613 | Ref. | |||||||||
| CYP24A1 | T/A | 680 | 46.3 | 185 | 1.07 | (0.86-1.34) | 164 | 1.16 | (0.92-1.45) | 349 | 1.09 | (0.90-1.31) | ||||||
| A/A | 67 | 46.5 | 0.99 | 0.1 | 27 | 1.50 | (0.90-2.52) | 0.18 | 22 | 1.54 | (0.90-2.63) | 0.07 | 49 | 1.48 | (0.96-2.30) | 0.07 | ||
| SNP Score‡ | Risk alleles | |||||||||||||||||
| 0 | 546 | 52.4 | 159 | Ref. | 127 | Ref. | 286 | Ref. | ||||||||||
| 1 | 865 | 47.3 | 210 | 0.88 | (0.69-1.13) | 190 | 1.00 | (0.77-1.29) | 400 | 0.94 | (0.76-1.13) | |||||||
| 2 | 462 | 43.9 | 123 | 0.93 | (0.70-1.25) | 113 | 1.05 | (0.78-1.42) | 236 | 1.01 | (0.79-1.29) | |||||||
| ≥3 | 161 | 38.9 | <0.01 | 4.4 | 41 | 1.07 | (0.71-1.62) | 1.0 | 30 | 0.90 | (0.57-1.42) | 0.95 | 71 | 1.00 | (0.71-1.42) | 0.90 | ||
Percentages of missing values for single SNPs: rs1155563: 3.5%, rs2282679:3.4%, rs3829251:2.8%, rs6013897: 2.9%, rs6599638:3.0%, rs10741657:3.4%, rs10877012:3.0%, and rs12785878:3.3%;
P for linear trend calculated by ANCOVA within Generalized Linear Models modeling the number of risk alleles as continuous score, adjusted for age, sex and study center; Initial p values were multiplied by 8 to account for the number of tested SNPs;
Calculated by Cox regression analyses stratified by age and center, and adjusted for sex; P for linear trend calculated modeling the number of risk alleles as continuous score; P values from Cox regressions were not corrected for multiple testing because all associations were non-significant;
Consisting of rs2282679 and rs12785878; rs1155563 and rs2282679 in the GC locus, as well as rs3829251 and rs12785878 in the DHCR7/NADSYN1 loci were in strong linkage disequilibrium (R2>0.8);
No deviation from Hardy-Weinberg-Equilibrium was detected for any of the SNPs
Associations between SNPs and incident cardiovascular diseases
Associations between genetic 25(OH)D determinants and incident MI and stroke are shown in Table 4. None of the SNPs was significantly related to the risks of MI or stroke in the co-dominant model, irrespective of the variance in 25(OH)D explained. Further, the selected SNPs were not significantly associated with incident CVD, i.e. stroke and MI as a composite endpoint. There were no significant linear trends for associations between the SNPs and incident MI, stroke, or CVD when using SNPs as continuous variables in additive models, even without correcting p-values for multiple testing. With respect to the SNP score, Cox regression revealed no significant association with the risk of MI (HR of subjects with ≥3 risk alleles compared to subjects with none [95% CI]: 1.07 [0.71-1.62], plinear trend=1.0). The same was true regarding incident stroke (HR [95% CI]: 0.90 [0.57-1.42], plinear trend=0.95). Finally, there was no significant association between the SNP score and incident total CVD (HR [95% CI]: 1.0 [0.71-1.42], plinear trend=0.90). Modeling circulating 25(OH)D as continuous score by steps of 13.5 nmol/L, i.e. the difference in 25(OH)D between extreme categories of our SNP score, yielded no significant associations concerning incident MI (HR [95% CI]: 0.92 [0.84-1.01], plinear trend=0.07) and stroke (HR [95% CI]: 0.95 [0.86-1.04], plinear trend=0.26), while a non-significant trend for an inverse association was observed concerning overall CVD (HR [95% CI]: 0.93 [0.87-1.002], plinear trend=0.06) in fully adjusted Cox models.
Regression models on SNPs and CVD endpoints including prevalent cases of MI and stroke that had been excluded from analyses on circulating 25(OH)D and incident CVD did not reveal results that differed substantially from those presented in Table 4 (data not shown). Dominant or recessive models showed no significant associations for any of the SNPs (data not shown). Lastly, allele scores including additional SNPs or alleles weighted by beta coefficients were not related to incident MI, stroke, or CVD (data not shown).
Discussion
In the present study, we found no significant linear associations between 25(OH)D levels and the risks of stroke and MI, although we did observe increased risks of both diseases at 25(OH)D levels below 25 nmol/L. Four out of eight candidate SNPs were significantly associated with 25(OH)D levels. However, neither single SNPs nor allele scores were related to the risks of MI or stroke. Thus, our results provide no support for a major causal role of vitamin D in the development of MI and stroke.
With regard to the lack of linear associations between vitamin D status and the risks of MI and stroke in our study two aspects are worth noting. First, plasma 25(OH)D levels in our population were relatively low compared to other populations worldwide [32]. Only few subjects in our study had 25(OH)D values above 75 nmol/L (5.8% in the subcohort) that are considered optimal regarding CVD prevention by some researchers [33]. Second, the majority of incident cases in our study were nonfatal. Notably, a large prospective study from Denmark has recently shown that 25(OH)D levels were associated with incident fatal MI, but not nonfatal MI [10]. The authors discussed that their finding supported the notion that 25(OH)D may rather be a marker of overall health than a causal factor in CVD etiology. In the Health Professionals Study, the association with 25(OH)D was also stronger for fatal CHD than for nonfatal MI [34], and a similar tendency was observed in the MIDSPAN study [35]. Hence, the hypothesis that 25(OH)D may be related to fatal MI rather than nonfatal MI deserves further study, even though the Intermountain study and the Cardiovascular Health Study 25(OH)D did show associations between 25(OH)D and the risks of incident nonfatal MI [36,37]. Nonetheless, the observation that inverse associations detected in our raw models attenuated after adjusting for classical CVD risk factors may imply that low 25(OH)D is a marker of an adverse CVD risk profile as well.
Interestingly, associations between 25(OH)D and incident MI have been assessed only recently in the MONICA/KORA-Augsburg study that resembled our study regarding the evaluation concept and the population [38]. In agreement with our results, 25(OH)D levels were not related to incident MI in men, whereas a significant inverse association that remained stable after rigorous adjustment was observed in women. No such association restricted to female participants was evident in the EPIC-Germany CVD case-cohort study. However, it should be noted that the numbers of incident cases in women were rather low in both studies and that a possible gender difference concerning 25(OH)D and the risk of MI therefore requires further investigation.
The SNP selection in the present study appears to be in line with recent suggestions for genetic markers in Mendelian Randomization studies on vitamin D [16,20,39,40]. As opposed to us, rs4588 in the GC locus instead of rs2282679 was used in the 1958 British birth cohort study [16]. However, both SNPs were in strong LD so that similar results using rs4588 instead of rs2282679 in our study can be assumed. Likewise, rs2298850 was used instead of rs2282679 in the Tromsø Study. Yet, both SNPs were in high LD and between allele-differences in 25(OH)D were similar [20]. In the DHCR7/NADSYN1 locus, rs3794060 was used in the Tromsø Study and reported to be in perfect LD with rs12785878, the SNP that we chose [20]. In a recent GWAS based on 5 cohorts, rs2282679, rs12785878, rs3829251, and rs6013897 were identified as the best predictors of circulating 25(OH)D [39]. Interestingly, it appeared that the explanatory power with respect to circulating 25(OH)D was better when using a score out of these SNPs as compared to polygenic scores consisting of several thousand SNPs [39]. Also, the variance explained by the two strongest SNPs was similar to the variance explained by the listed four candidate SNPs [39]. These observations are in line with our finding that adding further SNPs to an allele score consisting of rs2282679 and rs12785878 did not increase the explained variance in 25(OH)D.
Although we selected the most relevant SNPs, the variance in plasma 25(OH)D explained by our SNP score did not exceed 4.4%. This modest proportion of explained variance in 25(OH)D levels may have contributed to our failure to detect significant associations between SNPs and the risks of MI and stroke. Nevertheless, the average difference in 25(OH)D between extreme categories of our SNP score was 13.5 nmol/L. Considering the increased risks of stroke and MI at 25(OH)D levels below 25 nmol/L in our study, it does not seem implausible that even a smaller fraction of genetically determined 25(OH)D may affect CVD risk. That said, the limited sample size of our study did not allow for separate analyses of associations between SNPs and CVD risk in subjects with insufficient 25(OH)D levels.
Thus far, two observational studies have reported on genetic determinants of circulating 25(OH)D in relation to CVD risk. The Tromsø Study included some 2000 incident MI cases [20], and, like our study, did not show associations between SNPs and MI risk, the HR of MI being 0.93 (95% CI: 0.82–1.06) for subjects in the highest quartile of a genotype score based on rs2298850 (GC), rs10741657 (CYP2R1), rs3794060 (DHCR7/NADSYN1), and rs6013897 (CYP24A1). Also, no association between genetic vitamin D status determinants (rs2282679, rs12785878, and rs10741657) and CVD mortality was observed in a population of 3316 subjects scheduled for coronary angiography comprising 619 later cases of fatal CVD [41]. Still, we cannot rule out the detection of modest associations between SNPs related to vitamin D status and MI or stroke in larger consortia, as a post hoc calculation revealed that we only had the power of 0.62, 0.46 and 0.42 to detect HRs of 1.3 for incident overall CVD, MI, and stroke, respectively, between extreme categories of our SNP score. Pooling data from observational studies in larger Mendelian Randomization projects may help overcome such limitations of statistical power.
Besides the sample size, a further limitation of our study was that we could assess 25(OH)D levels only at a single occasion. However, despite the seasonal variation in 25(OH)D, several large studies have shown acceptable long-term correlations between repeated 25(OH)D measurements that are comparable to those of other biomarkers of CVD [42-45]. Moreover, we did not have the opportunity to measure levels of the vitamin D binding protein or 1,25(OH)2D. Vitamin D binding protein levels would have been of particular interest, as the strongest genetic determinants of 25(OH)D in GWAS and in our study were from the GC gene. Of note, recent prospective studies on cancer suggest that circulating levels of the vitamin D binding protein may crucially affect associations between 25(OH)D and disease risks by determining the free fraction of 25(OH)D [46-49]. Finally, we could not adjust for further potential confounders, such as blood lipids, blood pressure, or CRP.
In summary, we did not detect clear linear inverse relationships between 25(OH)D and CVD risk. The attenuation of the associations between 25(OH)D and CVD risks by adjustment for classical CVD risk factors in our study support the notion of 25(OH)D being a global health status marker rather than an independent causal factor in CVD etiology. The lack of associations between genetic vitamin D status determinants and CVD risk suggests that associations between low vitamin D levels and CVD risk may be due to uncontrolled confounding or reverse causation. However, a detection of genetic effects related to vitamin D status on CVD risk remains possible, and our results on genetic determinants of 25(OH)D require a cautious interpretation due to limited statistical power. The results of large consortia applying the Mendelian Randomization concept as well as upcoming RCTs will facilitate an evidence-based appraisal of the role of vitamin D with respect to CVD risk.
Funding Statement
The present work was part of the joint project "Vitamin D and Cardiovascular Health" funded by the German Federal Ministry of Education and Ressearch (BMBF, http://www.bmbf.de/en/index.php), grant number 0315668. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB et al. (2012) The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 33: 456-492. doi:10.1210/er.2012-1000. PubMed: 22596255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281. doi:10.1056/NEJMra070553. PubMed: 17634462. [DOI] [PubMed] [Google Scholar]
- 3. Norman AW, Bouillon R (2010) Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 235: 1034-1045. doi:10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- 4. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204-210. PubMed: 12499343. [DOI] [PubMed] [Google Scholar]
- 5. Van der Schueren BJ, Verstuyf A, Mathieu C (2012) Straight from D-Heart: vitamin D status and cardiovascular disease. Curr Opin Lipidol 23: 17-23. doi:10.1097/MOL.0b013e32834d7357. PubMed: 22123672. [DOI] [PubMed] [Google Scholar]
- 6. Motiwala SR, Wang TJ (2011) Vitamin D and cardiovascular disease. Curr Opin Nephrol Hypertens 20: 345-353. doi:10.1097/MNH.0b013e3283474985. PubMed: 21519252. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt N, Brandsch C, Kühne H, Thiele A, Hirche F et al. (2012) Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLOS ONE 7: e35316. doi:10.1371/journal.pone.0035316. PubMed: 22536373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandenburg VM, Vervloet MG, Marx N (2012) The role of vitamin D in cardiovascular disease: from present evidence to future perspectives. Atherosclerosis 225: 253-263. doi:10.1016/j.atherosclerosis.2012.08.005. PubMed: 22921424. [DOI] [PubMed] [Google Scholar]
- 9. Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM (2012) 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke 43: 1470-1477. doi:10.1161/STROKEAHA.111.636910. PubMed: 22442173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG (2012) 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol 32: 2794-2802. doi:10.1161/ATVBAHA.112.248039. PubMed: 22936341. [DOI] [PubMed] [Google Scholar]
- 11. Institute of Medicine (US) Committee to Review Dietary Reference. Intakes for Vitamin D and Calcium (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington: The National Academies Press; . 9780309163941 9780309163941 [PubMed] [Google Scholar]
- 12. McGreevy C, Williams D (2011) New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med 155: 820-826. doi:10.7326/0003-4819-155-12-201112200-00004. PubMed: 22184689. [DOI] [PubMed] [Google Scholar]
- 13. Kupferschmidt K (2012) Uncertain verdict as vitamin D goes on trial. Science 337: 1476-1478. doi:10.1126/science.337.6101.1476. PubMed: 22997323. [DOI] [PubMed] [Google Scholar]
- 14. Zittermann A, Börgermann J, Gummert JF, Pilz S (2012) Future directions in vitamin D and cardiovascular research. Nutr Metab Cardiovasc Dis 22: 541-546. doi:10.1016/j.numecd.2012.02.004. PubMed: 22633567. [DOI] [PubMed] [Google Scholar]
- 15. Pilz S, Rutters F, Dekker JM (2012) Disease prevention: vitamin D trials. Science 338: 883. doi:10.1126/science.338.6109.883-b. PubMed: 23161977. [DOI] [PubMed] [Google Scholar]
- 16. Berry DJ, Vimaleswaran KS, Whittaker JC, Hingorani AD, Hyppönen E (2012) Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLOS ONE 7: e37465. doi:10.1371/journal.pone.0037465. PubMed: 22629401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML et al. (2010) Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 19: 2739-2745. doi:10.1093/hmg/ddq155. PubMed: 20418485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB et al. (2010) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376: 180-188. doi:10.1016/S0140-6736(10)60588-0. PubMed: 20541252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheehan NA, Didelez V, Burton PR, Tobin MD (2008) Mendelian randomisation and causal inference in observational epidemiology. PLOS Med 5: e177. doi:10.1371/journal.pmed.0050177. PubMed: 18752343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB et al. (2012) Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromso Study. PLOS ONE 7: e37295. doi:10.1371/journal.pone.0037295. PubMed: 22649517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boeing H, Wahrendorf J, Becker N (1999) EPIC-Germany--A source for studies into diet and risk of chronic diseases. Eur Investig Into Cancer Nutr. Ann Nutr Metab 43: 195-204. [DOI] [PubMed] [Google Scholar]
- 22. Bergmann MM, Bussas U, Boeing H (1999) Follow-up procedures in EPIC-Germany--data quality aspects. European Prospective Investigation into Cancer and Nutrition. Ann Nutr Metab 43: 225-234. doi:10.1159/000012789. PubMed: 10592371. [DOI] [PubMed] [Google Scholar]
- 23. Kulathinal S, Karvanen J, Saarela O, Kuulasmaa K (2007) Case-cohort design in practice - experiences from the MORGAM Project. Epidemiol Perspect Innov 4: 15. doi:10.1186/1742-5573-4-15. PubMed: 18053196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prentice RL (1986) A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 73: 1-11. doi:10.1093/biomet/73.1.1. [Google Scholar]
- 25. Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB et al. (2011) Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia 54: 2272-2282. doi:10.1007/s00125-011-2182-9. PubMed: 21717116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGrath JJ, Saha S, Burne TH, Eyles DW (2010) A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol 121: 471-477. doi:10.1016/j.jsbmb.2010.03.073. PubMed: 20363324. [DOI] [PubMed] [Google Scholar]
- 27. Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC et al. (2008) Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst 100: 796-804. doi:10.1093/jnci/djn152. PubMed: 18505967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borkowf CB, Albert PS, Abnet CC (2003) Using lowess to remove systematic trends over time in predictor variables prior to logistic regression with quantile categories. Stat Med 22: 1477-1493. doi:10.1002/sim.1507. PubMed: 12704611. [DOI] [PubMed] [Google Scholar]
- 29.http://wwwrki.de/SharedDocs/FAQ/vitamind3/vitamind3.html?nn=2444038#FAQId2437556 Website of the Robert Koch Institute. Available in German: Accessed 2013.
- 30. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J et al. (2003) Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 6: 407-413. PubMed: 12795830. [DOI] [PubMed] [Google Scholar]
- 31. Sauerbrei W, Meier-Hirmer C, Benner A, Royston P (2006) Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Comput Statist Data Anal 50: 3464-3485. doi:10.1016/j.csda.2005.07.015. [Google Scholar]
- 32. Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J et al. (2012) A global representation of vitamin D status in healthy populations. Arch Osteoporos 7: 155-172. doi:10.1007/s11657-012-0093-0. PubMed: 23225293. [DOI] [PubMed] [Google Scholar]
- 33. Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E et al. (2010) Benefit-risk assessment of vitamin D supplementation. Osteoporos Int 21: 1121-1132. doi:10.1007/s00198-009-1119-3. PubMed: 19957164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giovannucci E, Liu Y, Hollis BW, Rimm EB (2008) 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 168: 1174-1180. doi:10.1001/archinte.168.11.1174. PubMed: 18541825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welsh P, Doolin O, McConnachie A, Boulton E, McNeil G et al. (2012) Circulating 25OHD, dietary vitamin D, PTH, and calcium associations with incident cardiovascular disease and mortality: the MIDSPAN Family Study. J Clin Endocrinol Metab 97: 4578-4587. doi:10.1210/jc.2012-2272. PubMed: 23071162. [DOI] [PubMed] [Google Scholar]
- 36. Anderson JL, May HT, Horne BD, Bair TL, Hall NL et al. (2010) Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 106: 963-968. doi:10.1016/j.amjcard.2010.05.027. PubMed: 20854958. [DOI] [PubMed] [Google Scholar]
- 37. Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ et al. (2011) Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 58: 1433-1441. doi:10.1016/j.jacc.2011.03.069. PubMed: 21939825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karakas M, Thorand B, Zierer A, Huth C, Meisinger C et al. (2013) Low Levels of Serum 25-Hydroxyvitamin D Are Associated with Increased Risk of Myocardial Infarction, Especially in Women: Results from the MONICA/KORA Augsburg Case-Cohort Study. J Clin Endocrinol Metab 98: 272-280. doi:10.1210/jc.2012-2368. PubMed: 23150690. [DOI] [PubMed] [Google Scholar]
- 39. Hiraki LT, Major JM, Chen C, Cornelis MC, Hunter DJ et al. (2013) Exploring the genetic architecture of circulating 25-hydroxyvitamin D. Genet Epidemiol 37: 92-98. doi:10.1002/gepi.21694. PubMed: 23135809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S et al. (2013) Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLOS Med 10: e1001383 PubMed: 23393431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO et al. (2013) Vitamin D and mortality: a Mendelian randomization study. Clin Chem 59: 793-797. doi:10.1373/clinchem.2012.193185. PubMed: 23319826. [DOI] [PubMed] [Google Scholar]
- 42. Kotsopoulos J, Tworoger SS, Campos H, Chung FL, Clevenger CV et al. (2010) Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev 19: 938-946. doi:10.1158/1055-9965.EPI-09-1318. PubMed: 20332276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E (2004) Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control 15: 255-265. doi:10.1023/B:CACO.0000024245.24880.8a. PubMed: 15090720. [DOI] [PubMed] [Google Scholar]
- 44. Sonderman JS, Munro HM, Blot WJ, Signorello LB (2012) Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol 176: 615-621. doi:10.1093/aje/kws141. PubMed: 22975199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y et al. (2010) Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 171: 903-908. doi:10.1093/aje/kwq005. PubMed: 20219763. [DOI] [PubMed] [Google Scholar]
- 46. Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J et al. (2012) Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case-control study. Cancer Res 72: 1190-1198. doi:10.1158/1538-7445.AM2012-1190. PubMed: 22232734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mondul AM, Weinstein SJ, Virtamo J, Albanes D (2012) Influence of vitamin D binding protein on the association between circulating vitamin D and risk of bladder cancer. Br J Cancer 107: 1589-1594. doi:10.1038/bjc.2012.417. PubMed: 22990651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J et al. (2013) Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer 132: 2940-2947. doi:10.1002/ijc.27969. PubMed: 23180681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chun RF (2012) New perspectives on the vitamin D binding protein. Cell Biochem Funct 30: 445-456. doi:10.1002/cbf.2835. PubMed: 22528806. [DOI] [PubMed] [Google Scholar]