Abstract
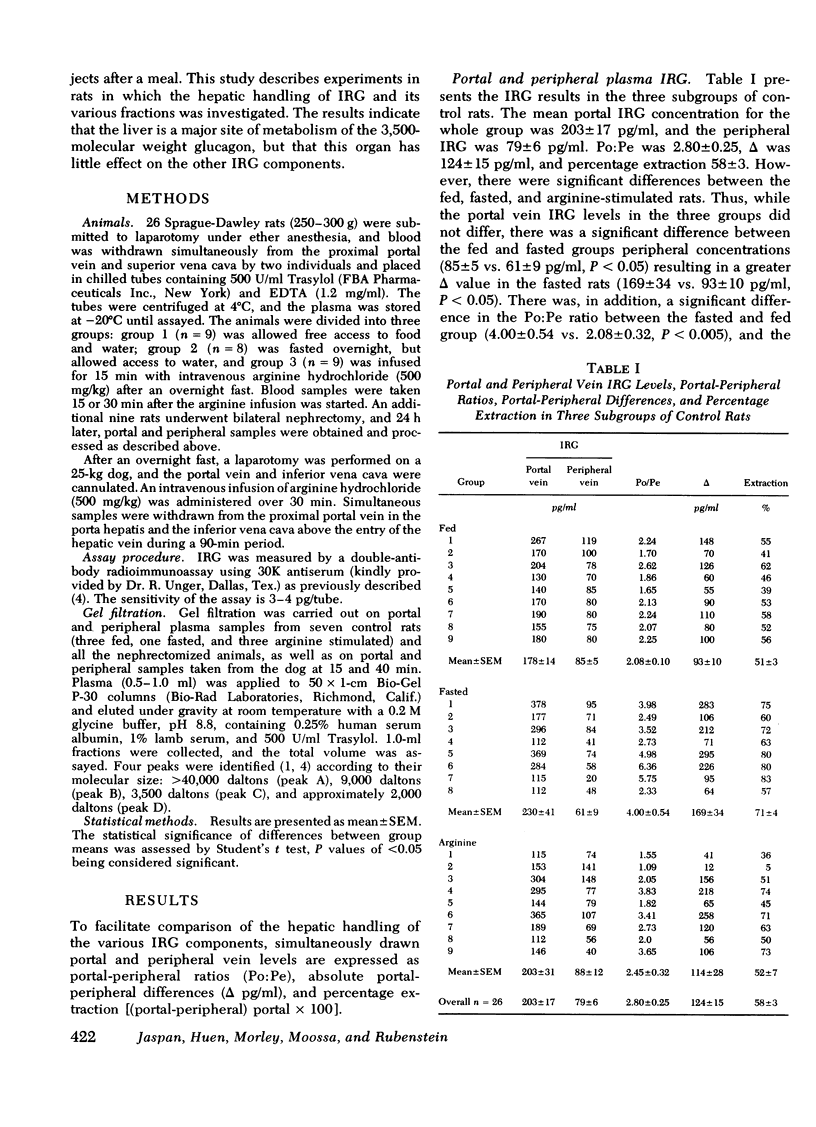
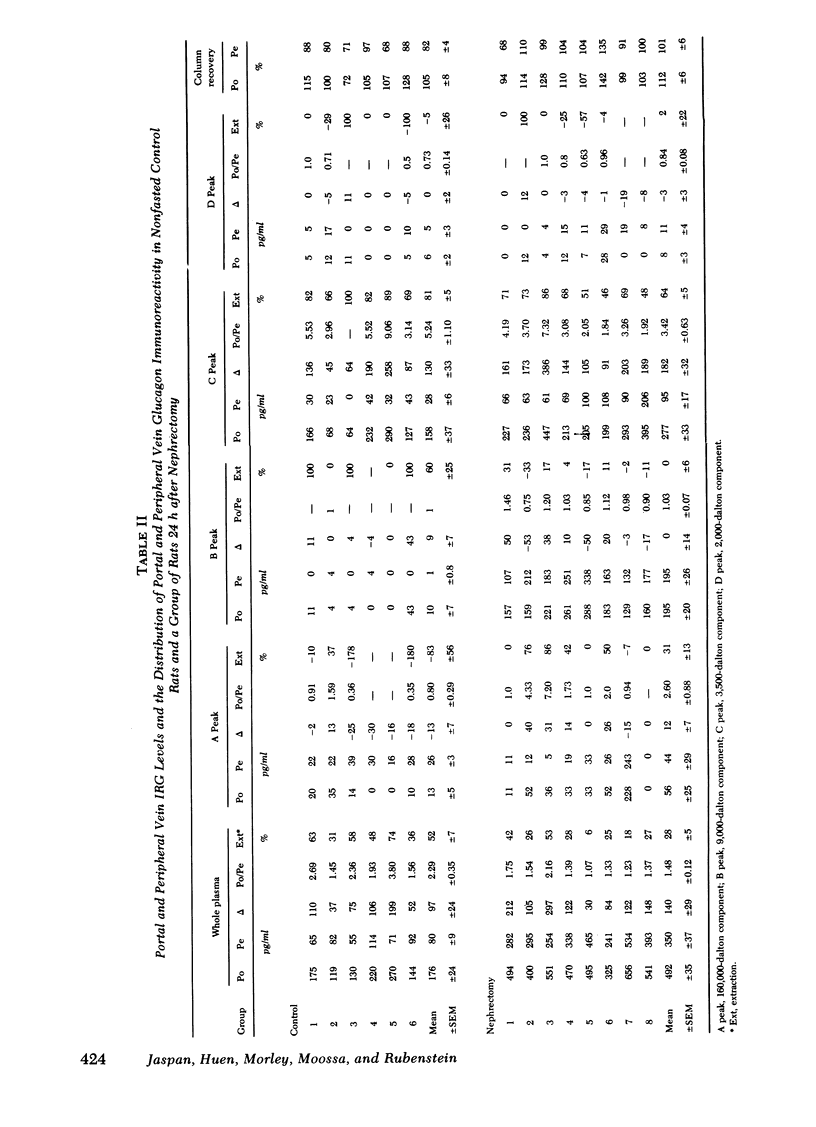
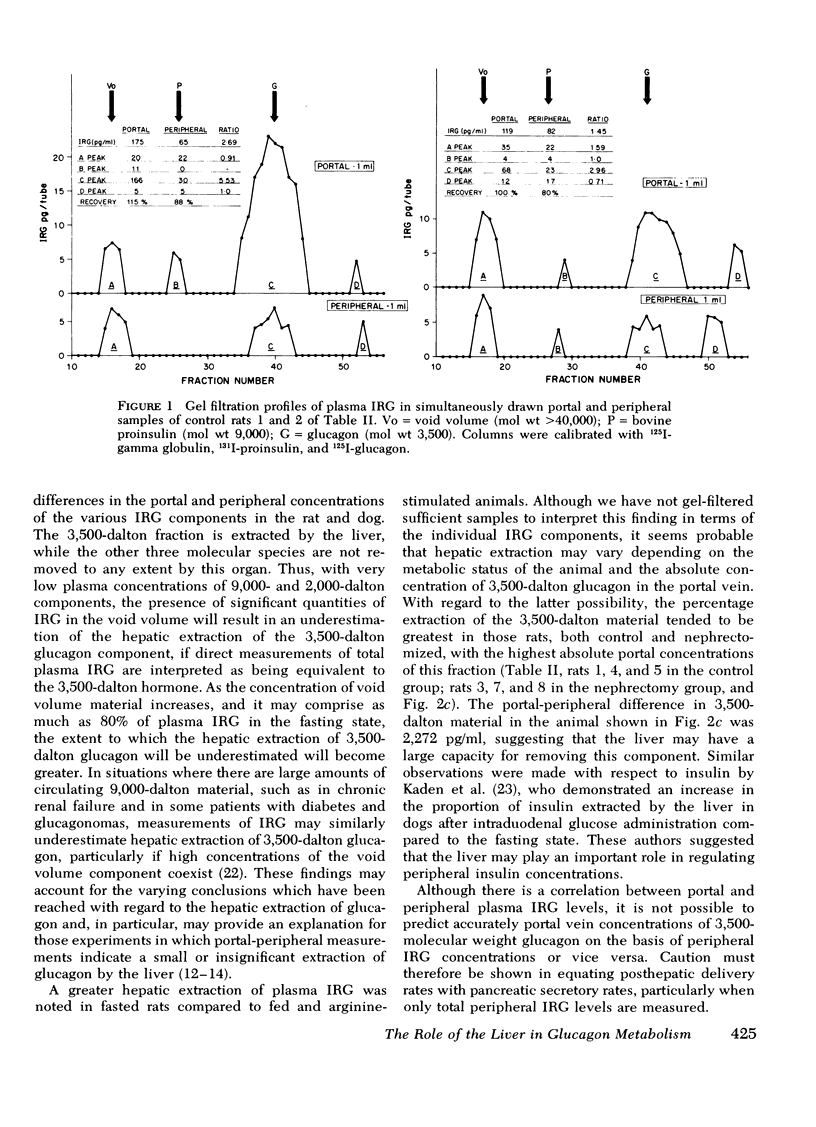
Total plasma immunoreactive pancreatic glucagon (IRG) was measured in samples taken simultaneously from the proximal portal vein and superior vena cava of 26 healthy rats. The portal-peripheral ratio of IRG was 2.80±0.25, the portal-peripheral difference (Δ) 124±15 pg/ml, and percentage extraction 58±3. Gel filtration of paired portal and peripheral vein samples showed that reduction in the 3,500-dalton IRG component (glucagon) in peripheral samples accounted for almost all the differences, there being minimal and inconsistent changes in the high molecular weight (>40,000) fraction. The portal-peripheral ratio of the 3,500-dalton glucagon was 5.24±1.10, the portal-peripheral difference 130±33 pg/ml, and the percentage extraction 81±5.
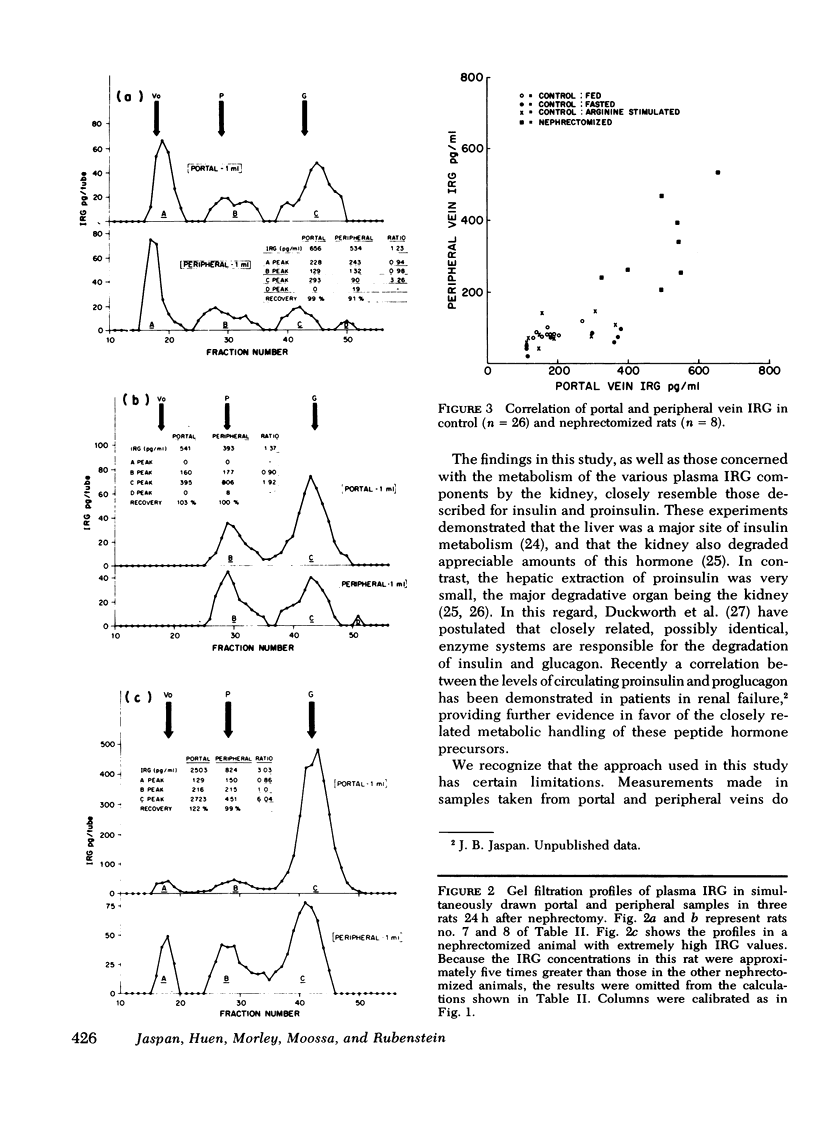
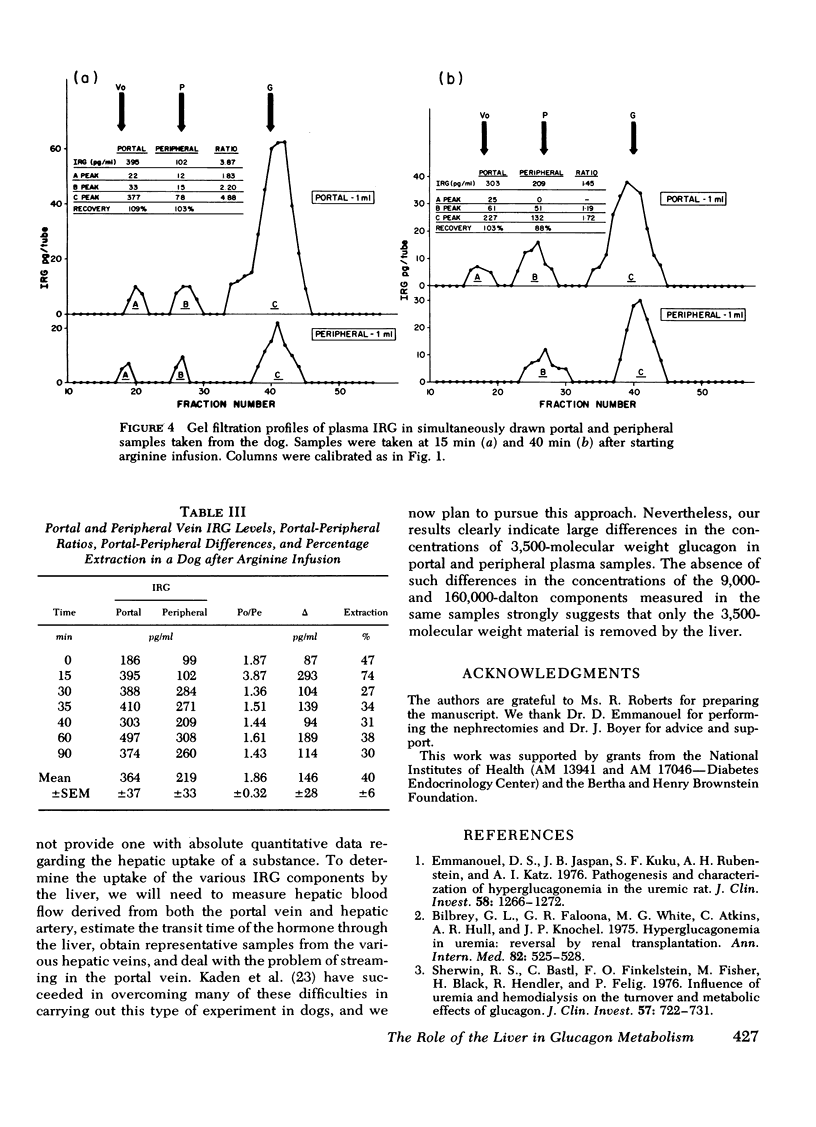
To study the transhepatic differences in the 9,000-dalton “proglucagon-like” material, the experiment was repeated in nine rats 24 h after bilateral nephrectomy, a procedure which increases plasma levels of this fraction. The portal-peripheral ratio for plasma IRG in these rats was 1.48±0.12, the portal-peripheral difference 140±29 pg/ml, and percentage extraction 28±5. Gel filtration revealed no consistent differences between portal and peripheral concentrations of the 9,000- and >40,000-dalton components, which comprised 40 and 13%, respectively, of the mean IRG level of 492±35 pg/ml. In contrast, there were marked differences between portal and peripheral levels of the 3,500-dalton component the ratio being 3.42±0.63, the portal-peripheral difference 182±32 pg/ml, and percentage extraction 64±5. Similar studies in a healthy dog, in which species there are significant circulating levels of the 9,000-dalton IRG component, confirmed the selective hepatic extraction of the 3,500-dalton fraction.
We conclude that the various IRG fractions are metabolized differently by the liver, and that portal-peripheral ratios based on direct assay of plasma IRG will vary depending on the percentage glucagon immunoreactivity in each fraction; the greater the combined contribution of fractions other than the 3,500-dalton component to total plasma IRG, the lower will be the ratio. Because of the heterogeneity of circulating IRG and significant differences in the metabolism of its various components, gel filtration of plasma samples is necessary for precise quantitation of the hepatic uptake of each particular fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilbrey G. L., Faloona G. R., White M. G., Atkins C., Hull A. R., Knochel J. P. Hyperglucagonemia in uremia: reversal by renal transplantation. Ann Intern Med. 1975 Apr;82(4):525–528. doi: 10.7326/0003-4819-82-4-525. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Faloona G. R., White M. G., Knochel J. P. Hyperglucagonemia of renal failure. J Clin Invest. 1974 Mar;53(3):841–847. doi: 10.1172/JCI107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C., Andrews S. S. Portal and peripheral vein immunoreactive glucagon concentrations after arginine or glucose infusions. Diabetes. 1974 Mar;23(3):199–202. doi: 10.2337/diab.23.3.199. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Solomon S. S., Vance J. E., Porter H. P., Williams R. H. Glucagon clearance by the isolated perfused rat liver. Proc Soc Exp Biol Med. 1968 Jun;128(2):620–623. doi: 10.3181/00379727-128-33081. [DOI] [PubMed] [Google Scholar]
- Dencker H., Hedner P., Holst J., Tranberg K. G. Pancreatic glucagon response to an ordinary meal. Scand J Gastroenterol. 1975;10(5):471–474. [PubMed] [Google Scholar]
- Emmanouel D. S., Jaspan J. B., Kuku S. F., Rubenstein A. H., Katz A. I., Huen A. H. Pathogenesis and characterization of hyperglucagonemia in the uremic rat. J Clin Invest. 1976 Nov;58(5):1266–1272. doi: 10.1172/JCI108581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Gusberg R., Hendler R., Gump F. E., Kinney J. M., Mulrow P. J. Concentrations of glucagon and the insulin:glucagon ratio in the portal and peripheral circulation. Proc Soc Exp Biol Med. 1974 Oct;147(1):88–90. doi: 10.3181/00379727-147-38286. [DOI] [PubMed] [Google Scholar]
- Fisher M., Sherwin R. S., Hendler R., Felig P. Kinetics of glucagon in man: effects of starvation. Proc Natl Acad Sci U S A. 1976 May;73(5):1735–1739. doi: 10.1073/pnas.73.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDNER M. G., JAUREGUI R. H., WEISENFELD S. Disappearance of HGF fron insulin after liver perfusion. Am J Physiol. 1954 Oct;179(1):25–28. doi: 10.1152/ajplegacy.1954.179.1.25. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Kuku S. F., Locker J. D., Huen A., Emmanouel D. S., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon in man. Metabolism. 1976 Nov;25(11 Suppl 1):1397–1401. doi: 10.1016/s0026-0495(76)80150-3. [DOI] [PubMed] [Google Scholar]
- Kaden M., Harding P., Field J. B. Effect of intraduodenal glucose administration on hepatic extraction of insulin in the anesthetized dog. J Clin Invest. 1973 Aug;52(8):2016–2028. doi: 10.1172/JCI107386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuku S. F., Jaspan J. B., Emmanouel D. S., Zeidler A., Katz A. I., Rubenstein A. H. Heterogeneity of plasma glucagon. Circulating components in normal subjects and patients with chronic renal failure. J Clin Invest. 1976 Sep;58(3):742–750. doi: 10.1172/JCI108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S. Plasma glucagon after kidney exclusion: experiments in somatostatin-infused and in eviscerated dogs. Metabolism. 1976 Jul;25(7):761–768. doi: 10.1016/0026-0495(76)90147-5. [DOI] [PubMed] [Google Scholar]
- Marco J., Diego J., Villanueva M. L., Diaz-Fierros M., Valverde I., Segovia J. M. Elevated plasma glucagon levels in cirrhosis of the liver. N Engl J Med. 1973 Nov 22;289(21):1107–1111. doi: 10.1056/NEJM197311222892103. [DOI] [PubMed] [Google Scholar]
- Morley C. G., Kuku S., Rubenstein A. H., Boyer J. L. Serum hormone levels following partial hepatectomy in the rat. Biochem Biophys Res Commun. 1975 Nov 17;67(2):653–661. doi: 10.1016/0006-291x(75)90862-1. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Pottenger L. A., Mako M., Getz G. S., Steiner D. F. The metabolism of proinsulin and insulin by the liver. J Clin Invest. 1972 Apr;51(4):912–921. doi: 10.1172/JCI106886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R., Joshi P., Hendler R., Felig P., Conn H. O. Hyperglucagonemia in Laennec's cirrhosis. The role of portal-systemic shunting. N Engl J Med. 1974 Jan 31;290(5):239–242. doi: 10.1056/NEJM197401312900502. [DOI] [PubMed] [Google Scholar]
- Valverde I., Dobbs R., Unger R. H. Heterogeneity of plasma glucagon immunoreactivity in normal, depancreatized, and alloxan-diabetic dogs. Metabolism. 1975 Sep;24(9):1021–1028. doi: 10.1016/0026-0495(75)90095-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. H., HAY J. S., TJADEN M. B. Degradation of insulin-I 131 and glucagon-I 131 and factors influencing it. Ann N Y Acad Sci. 1959 Mar 30;74(3):513–529. doi: 10.1111/j.1749-6632.1959.tb39576.x. [DOI] [PubMed] [Google Scholar]


