Abstract
Background
Profound alterations in immune responses associated with uremia and exacerbated by dialysis increase the risk of active tuberculosis (TB). Evidence of the long-term risk and outcome of active TB after acute kidney injury (AKI) is limited.
Methods
This population-based-cohort study used claim records retrieved from the Taiwan National Health Insurance database. We retrieved records of all hospitalized patients, more than 18 years, who underwent dialysis for acute kidney injury (AKI) during 1999–2008 and validated using the NSARF data. Time-dependent Cox proportional hazards model to adjust for the ongoing effect of end-stage renal disease (ESRD) was conducted to predict long-term de novo active TB after discharge from index hospitalization.
Results
Out of 2,909 AKI dialysis patients surviving 90 days after index discharge, 686 did not require dialysis after hospital discharge. The control group included 11,636 hospital patients without AKI, dialysis, or history of TB. The relative risk of active TB in AKI dialysis patients, relative to the general population, after a mean follow-up period of 3.6 years was 7.71. Patients who did (hazard ratio [HR], 3.84; p<0.001) and did not (HR, 6.39; p<0.001) recover from AKI requiring dialysis had significantly higher incidence of TB than patients without AKI. The external validated data also showed nonrecovery subgroup (HR = 4.37; p = 0.049) had high risk of developing active TB compared with non-AKI. Additionally, active TB was associated with long-term all-cause mortality after AKI requiring dialysis (HR, 1.34; p = 0.032).
Conclusions
AKI requiring dialysis seems to independently increase the long-term risk of active TB, even among those who weaned from dialysis at discharge. These results raise concerns that the increasing global burden of AKI will in turn increase the incidence of active TB.
Introduction
Tuberculosis (TB) accounts for a significant proportion of all deaths caused by infectious diseases. Controlling TB is a major public health issue, especially in developing countries. The relative risk (RR) of developing active TB is 10–25times for patients with chronic kidney disease (CKD) or those on hemodialysis, and 37times for renal transplant recipients than general population [1], and their TB mortality rate is higher [2], [3]. The incidence of dialysis –requiring AKI in the United States is now higher than the incidence of end-stage renal disease (ESRD), averaging a 10% increased per year [4] and is associated with increased use of resources during and after hospitalization [5]. Depending on how AKI is defined, 7–18% of all hospitalized patients suffer from AKI [6], and as many as 5% of intensive care patients have AKI severe enough to require dialysis [7]. More hospitalized patients with AKI are discharged after temporary AKI [8], perhaps because of advances in critical care medicine and dialysis technologies.
Profound alterations in immune responses associated with uremia and exacerbated by dialysis increase the risk of active TB [9]. Some individuals, especially those with impaired T cell function, develop active tuberculosis, either as primary progression or as a reactivation [10]. Kidney disease is associated with acquired immunodeficiency due to functional abnormalities of neutrophils, reduced T cell and B cell function, and compromised monocyte and natural killer cell function [11]. Increased proinflammatory cytokines, leukocyte trafficking directly mediate pulmonary injury after AKI [12]. Evidence of the long-term risk and outcome of active TB after advanced AKI is limited.
The current study used a population-based and record-based case-control design to evaluate the association between AKI requiring dialysis and long-term risk of active TB using the National Health Insurance Research Database of Taiwan.
Methods
Study sample
The Taiwan National Health Insurance (NHI) database is a nationwide insurance program covering outpatient visits, hospital admissions, prescriptions, intervention procedures, and disease profiles for over 99% of the population in Taiwan (23.12 million in 2009) [13], [14], [15]. It is one of the largest and most comprehensive databases in the world and has been used extensively in various studies of prescription use, diagnoses, and hospitalizations.
In cooperation with the Bureau of NHI, the NHRI of Taiwan used a systematic, random sampling method to build a representative database of 1,000,000 NHI enrollees [13], [14], [16] . There were no statistically significant differences in age, sex, or health-care costs between the sample group and all the enrollees, as reported by the NHRI. This data set spans from January 1, 1999, through December 2008 and includes all claims data for these 1,000,000 individuals. It offers a good opportunity to explore outcomes of patients with AKI requiring dialysis.
Ethics Statement
Because the identification numbers of all subjects in the NHRI databases were encrypted to protect the privacy of the individuals, this study was exempted from full review by the Institution Review Board of the National Taiwan University Hospital and informed consents were waived.
Population-based surveillance methods
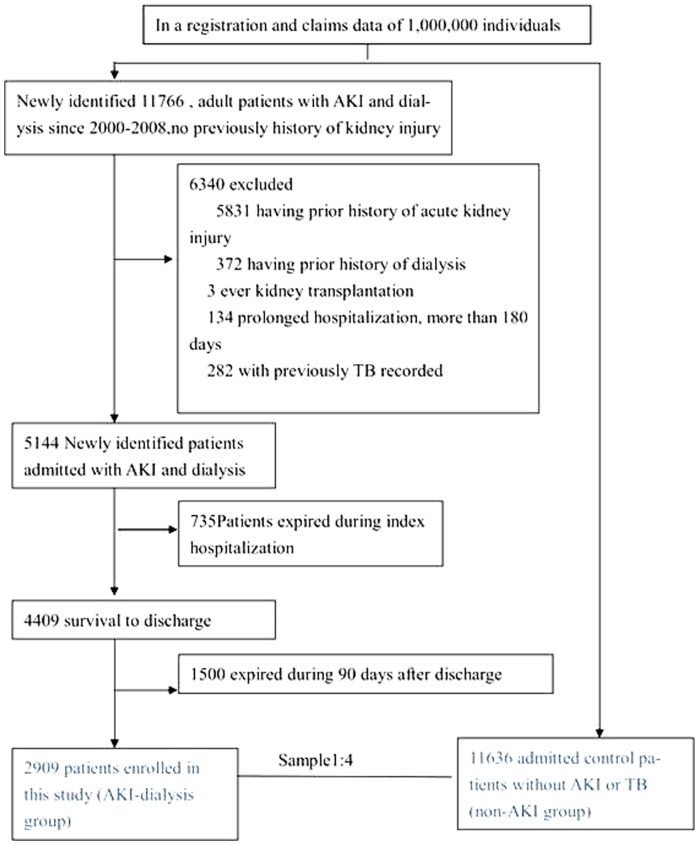
All patients, aged >18 years, and who had a first-time admission diagnosis of AKI requiring dialysis were identified. Those who survived for >90 days after discharge without re-hospitalization (AKI–dialysis group) were selected for further analysis. Patients were analyzed from January 2000 through December 2008 to confirm that there was no diagnosis of AKI, dialysis, or TB within 1 year prior to index admission. Comorbidities were defined according to the International Classification of Disease, 9th revision, Clinical Modification (ICD-9-CM) and procedure codes (including Taiwan Classification of Procedures). We excluded patients requiring prolonged hospitalization and those who underwent kidney transplantation. Figure 1 depicts the NHRI procedure for building the AKI database and selecting patients. A control cohort, 11636 patients without AKI, dialysis, or TB before and during non-AKI hospitalization (non-AKI group), was randomly selected. Four controls were selected for each AKI–dialysis case. However, we could not identify different causes of AKI from this retrospective database.
Figure 1. Flow diagram of the study sample.
(Abbreviations; AKI, acute kidney injury; CKD, chronic kidney disease; TB, tuberculosis; ESRD, end-stage renal disease; ICU, intensive care unit).
Patients in the AKI–dialysis group were further divided into 2 sub-groups, based on whether they continued dialysis (recovery and nonrecovery sub-groups). As in our previously report [17], we used a selection period of 90 days [18] to reduce the possibility of immortal time bias [19], because all patients after dialysis for more than 90 days in Taiwan will apply to the NHI for catastrophic illness registration cards.
Research variables
Charlson comorbidity index scores were based on pre-existing conditions identified from a patient's medical records [20].
Demographic and clinical characteristics of study subjects and hospitals at index hospital admission were examined, including age, sex, year of admission, and prevalence of selected comorbidities. Data representing the hospitalization period included diagnosis codes; the categories of major operations; resource use, including hemodialysis; mechanical ventilation (MV); intensive care unit (ICU) admission; and outcomes. To determine pre-existing comorbidities, we used a relatively strict criterion: at least 1 admission or at least 3 outpatient visits for treatment of a certain disease during the year prior to the index admission.
Definition of outcomes
The government of Taiwan monitors active TB, and anti-TB drugs are controlled by a central government health institute. Doctors must report every new case to the Taiwan center of disease control (CDC) within 1 week after the commencement of TB treatment. We defined active TB as an ICD-9 code in the range 010–018 on at least 3 ambulatory visits or admission, and insurance claims for at least 2 TB drugs (isoniazid, rifampicin, rifamycin, rifabutin, pyrazinamide, ethambutol, streptomycin, kanamycin, amikacin, moxifloxacin, levofloxacin, ciprofloxacin, protionamide, and cycloserine) for more than 90 days, because it takes 2 months to obtain a TB culture report in clinical practice [21], [22]. ESRD patients were defined as those who received a health card for dialysis after 90 days of dialysis.
External Validation
The main outcome of developing long-term active TB and selection criteria for identifying patients with AKI-dialysis were validated by analysis of another prospectively collected data of the National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) [23], [24]. The critical care database was constructed prospectively for outcome assurance between January 2002 and January 2008 in one medical center (National Taiwan University Hospital in Taipei, Taiwan) and its three branch hospitals in different cities [25], [26], [27], [28], [29] .The event of TB was linked to the data from Taiwan Anti-Mycobacteria Investigation (TAMI) group, a prospective collecting data recording TB events and outcomes in Taiwan [30].
Data analysis
Continuous variables were reported as mean ± standard deviations; discrete variables were presented as counts or percentages. All data were analyzed using R software, version 2.14.1 (Free Software Foundation, Inc., Boston, MA, USA.). Two-sided p values<0.05 were considered statistically significant. Analyses with Cox proportional hazards regression model and propensity score model were conducted separately within each stratum to evaluate the risk of the outcomes after adjustments for all variables. For outcomes measurements, an individual was censored at active TB identification or at the end of the measurement period.
Due to the strong correlation between ESRD and TB, we used a Cox proportional hazards model with time-dependent covariates to evaluate the impact of ESRD on the risk of active TB [31], assuming that changes in ESRD status could appear at the middle time point. The time from index hospital discharge to TB event was analyzed by fitting Cox proportional hazards model. Basic model-fitting techniques for (1) variable selection, (2) goodness-of-fit assessment, and (3) regression diagnostics (including residual analysis, influence analysis, and check of multi-collinearity) were used in regression analyses to ensure good quality of results.
In specificity testing, we constructed a propensity score using a non-parsimonious multi-variable logistic regression model in an attempt to make an unbiased estimate of the confounders predicting dialysis during index admission, a binary dependent variable, under a set of covariates (Table S1 in File S1). The predicted probability derived from the logistic equation was used as the propensity score for each individual. To restore the interval validity of subsequent group comparisons and reduce the influence of observations on the overall results [32], we also truncated the samples by discarding cases in the region of non-overlap that appears with weight-trimmed propensity scores estimated by logistic regression [33], [34]. The degree of trimming was chosen to minimize mean squared error.
Results
Demographic characteristics of patients
We identified 2,909 patients (men, 49.9%, mean age = 61.9±14.9 years), more than 18 years of age, who had a first-time diagnosis of AKI requiring dialysis and who survived for >90 days after index discharge (AKI: dialysis group). Each AKI–dialysis patient was matched to 4 hospital patients without AKI or dialysis, for a control group of 1,1636 patients (non-AKI group: men, 45.7%; mean age = 46.2±18.4 years) ( Table 1 ). Among the AKI–dialysis group, 686 recovered and no longer required dialysis after hospital discharge (recovery subgroup) ( Figure 1 ). The average age of the overall cohort was 49.3±18.8 years, and the Charlson score before admission was 0.95±1.63. The prevalence of preadmission baseline and index hospital comorbidities was higher in the AKI group than in the non-AKI group, except for tumor metastasis ( Table 1 ). Compared to the nonrecovery sub-group, the recovery sub-group had lower Charlson score; higher prevalences of myocardial infarction, dementia, and tumor with metastasis; and lower prevalence of chronic kidney disease.
Table 1. Characteristics of enrolled patients.
| AKI dialysis group (n = 2909) | |||||||
| Items | Non-AKI group (n = 11636) | AKI-dialysis group (n = 2909) | p | Non-recovery (n = 2223) | Recovery (n = 686) | p ¶ | p § |
| Male | 5319(45.7%) | 1452(49.9%) | <0.001 | 1062(47.8%) | 390(56.9%) | <0.001 | <0.001 |
| Age (years) | 46.2±18.4 | 61.9±14.9 | <0.001 | 61.4±14.4 | 63.6±16.4 | <0.001 | <0.001 |
| Comorbidity | <0.001 | ||||||
| Charlson score | 0.43±1.03 | 3.01±1.92 | <0.001 | 3.2±1.82 | 2.39±2.1 | <0.001 | <0.001 |
| Myocardial infarction | 49(0.4%) | 55(1.9%) | <0.001 | 34(1.5%) | 21(3.1%) | 0.015 | <0.001 |
| Congestive heart failure | 135(1.2%) | 414(14.2%) | <0.001 | 312(14%) | 102(14.9%) | 0.574 | <0.001 |
| Peripheral vascular disease | 45(0.4%) | 40(1.4%) | <0.001 | 32(1.4%) | 8(1.2%) | 0.709 | 0.009 |
| Cerebrovascular disease | 264(2.3%) | 254(8.7%) | <0.001 | 193(8.7%) | 61(8.9%) | 0.877 | <0.001 |
| Dementia | 58(0.5%) | 61(2.1%) | <0.001 | 34(1.5%) | 27(3.9%) | <0.001 | <0.001 |
| COPD | 512(4.4%) | 254(8.7%) | <0.001 | 192(8.6%) | 62(9%) | 0.757 | <0.001 |
| Rheumatologic disease | 61(0.5%) | 38(1.3%) | 0.001 | 28(1.3%) | 10(1.5%) | 0.701 | 0.006 |
| Peptic Ulcer | 671(5.8%) | 448(15.4%) | <0.001 | 340(15.3%) | 108(15.7%) | 0.763 | <0.001 |
| Hemiplegia | 32(0.3%) | 36(1.2%) | <0.001 | 27(1.2%) | 9(1.3%) | 0.844 | <0.001 |
| Chronic Kidney disease | 236(2%) | 1754(60.3%) | <0.001 | 1563(70.3%) | 208(30.3%) | <0.001 | <0.001 |
| Solid tumor | 328(2.8%) | 150(5.2%) | <0.001 | 107(4.8%) | 43(6.3%) | 0.139 | <0.001 |
| Tumor with metastasis | 90(0.8%) | 31(1.1%) | 0.137 | 16(0.7%) | 15(2.2%) | 0.002 | 0.001 |
| Diabetes Mellitus | 841(7.2%) | 1347(46.3%) | <0.001 | 1056(47.5%) | 291(42.4%) | 0.020 | <0.001 |
| Moderate or Severe liver disease | 376(3.2%) | 169(5.8%) | <0.001 | 127(5.7%) | 42(6.1%) | 0.709 | <0.001 |
| Index hospital co-morbidities | |||||||
| Cardiovascular | 41(0.4%) | 81(2.8%) | <0.001 | 28(1.3%) | 53(7.7%) | <0.001 | <0.001 |
| Respiratory | 71(0.6%) | 275(9.5%) | <0.001 | 131(5.9%) | 144(21%) | <0.001 | <0.001 |
| Hepatic | 65(0.6%) | 35(1.2%) | 0.009 | 20(0.9%) | 15(2.2%) | 0.014 | <0.001 |
| Neurologic | 10(0.1%) | 48(1.7%) | <0.001 | 36(1.6%) | 12(1.7%) | 0.864 | <0.001 |
| Hematologic | 39(0.3%) | 29(1%) | <0.001 | 25(1.1%) | 4(0.6%) | 0.274 | 0.301 |
| Metabolic | 3(0%) | 96(3.3%) | <0.001 | 65(2.9%) | 31(4.5%) | 0.050 | <0.001 |
| Operative categories | |||||||
| Cardiothoracic | 51(0.4%) | 49(1.7%) | <0.001 | 19(0.9%) | 30(4.4%) | <0.001 | <0.001 |
| Upper GI | 44(0.4%) | 11(0.4%) | 0.999 | 3(0.1%) | 8(1.2%) | 0.001 | 0.008 |
| Lower GI | 83(0.7%) | 20(0.7%) | 0.999 | 10(0.4%) | 10(1.5%) | 0.013 | 0.038 |
| Hepatobiliary | 181(1.6%) | 13(0.4%) | <0.001 | 4(0.2%) | 9(1.3%) | 0.001 | 0.750 |
| Mechanical ventilation | 255(2.2%) | 508(17.5%) | <0.001 | 253(11.4%) | 255(37.2%) | <0.001 | <0.001 |
| ICU admission during index hospitalization | 539(4.6%) | 920(31.6%) | <0.001 | 526(23.7%) | 394(57.4%) | <0.001 | <0.001 |
| Long term outcomes | |||||||
| ESRD | 21(0.2%) | 1626(55.9%) | <0.001 | 1500(67.5%) | 126(18.4%) | <0.001 | <0.001 |
| TB | 53(0.5%) | 66(2.3%) | <0.001 | 53(2.4%) | 13(1.9%) | 0.557 | <0.001 |
recovery subgroup vs. nonrecovery subgroup.
recovery subgroup vs. non-AKI group.
Abbreviations: AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; ESRD, end stage renal disease; GI, Gastrointestinal; ICU, intensive care unit; TB, tuberculosis.
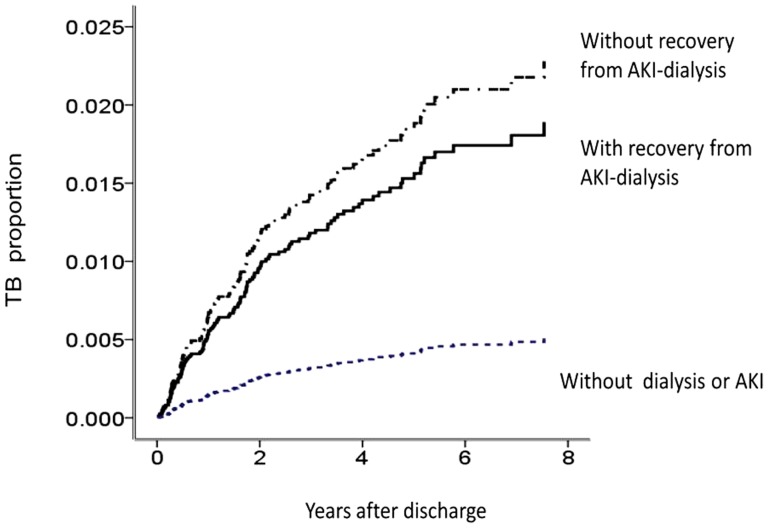
Long-term active TB (Fig. 2)
Figure 2. Cox proportional hazards model for long-term active TB events among the non-AKI group, recovery AKI subgroup, and nonrecovery AKI sub-group (AKI, acute kidney injury).
During a mean follow-up period of 3.6 years, a significantly greater proportion of AKI patients developed end-stage renal disease (ESRD), compared to the non-AKI group (55.9% vs. 0.2%, p<0.001) ( Table 1 ). AKI patients had a higher long-term risk of active TB (2.3% vs. 0.5%, p<0.001).
The incidence of TB was 590, 650, and 83 per 100,000 person-years in the recovery sub-group, nonrecovery sub-group, and non-AKI group, respectively. The risk of TB infection in AKI–dialysis patients relative to the general population was 7.71. The hazard ratio for developing active TB, relative to non-AKI patients, was 6.39 (95% CI, 3.57–11.45; p<0.001) for the nonrecovery sub-group and 3.84 (95% CI, 2.07–7.10; p<0.001) for the recovery sub-group. Other risk factors predicting the development of active TB were age >45 years (HR, 3.64; 95% CI, 2.07–6.39; p<0.001), male sex (HR, 1.94; 95% CI, 1.33–2.83; p<0.001), and chronic obstructive pulmonary disease (HR, 1.87; 95% CI, 1.10–3.19; p = 0.021). This model had good validity (C-index = 0.79). However, ESRD after AKI, the time-dependent explanatory variable, was not associated with development of active TB according to our Cox proportional hazards model (HR, 1.19; p = 0.58). Additionally, in the AKI–dialysis group, active TB was associated with increased all-cause mortality (HR, 1.34; 95% CI, 1.03–1.74; p = 0.032).
Sensitivity analysis was consistent with our main finding that the AKI–dialysis group had a higher incidence of active TB than the non-AKI group ( Fig. 3 ). This was also true for patients with or without diabetes (all, p<0.005). AKI-recovery groups had higher ratio of TB than non-AKI group in spite of subsequent CKD.
Figure 3. The hazard ratios (HRs) and 95% CIs for long-term tuberculosis, adjusted for the AKI–dialysis and non-AKI groups.
*adjusted for age and sex. Abbreviations; COPD, chronic obstructive pulmonary disease; ICU.
External Validation using NSARF data
We validated our main findings using prospective critical care data from the multi-centers , NSARF, to guide us in conducting more broad-based research on AKI from nationally representative sources. Among 234 AKI-dialysis patients survival to discharge, 180 (76.9%) recovered from dialysis within 90 days after discharge from index admission. In the NSARF cohort, 8788 patients without AKI and survival to 90 days after hospital discharge were enrolled as controls. There were 70 (0.8%), 5 (2.8%) and 2 (3.7%) in non-AKI, recovery and non-recovery groups respectively had active TB after a mean follow-up of 3.3 years. (1.01–5.13 years). Consistent with our previous findings, the results obtained using the Cox proportional hazard model showed that recovery group (HR = 3.03; 95% CI, 1.20 −7.67; p = 0.019) and nonrecovery subgroup (HR = 4.37; 95% CI, 1.00 −19.07; p = 0.049) had high risk of developing long-term active tuberculosis compared with non-AKI.
Specificity test using propensity matching
The risk factors predicting dialysis during index hospitalization were components of propensity scores and are listed in Table S1 in File S1. The propensity score for predicting dialysis during index hospitalization in all study groups was highly discriminative (estimated area under the receiver operating characteristic curve [eAUC–ROC] = 0.975), and it fit the observed binary data well (adjusted generalized R 2 = 0.819; Hosmer–Lemeshow goodness-of-fit [GOF] test, p<0.001).
To restore the interval validity of subsequent group comparisons and reduce the influence of observations on the overall results, we weight-trimmed 888 patients by estimated propensity scores. After careful matching, there were 526 patients in the recovery sub-group, 1,935 in the nonrecovery sub-group, and 11.196 in the non-AKI group.
Consistent with our previous finding, the AKI–dialysis group had a higher long-term incidence of active TB than the non-AKI group, after adjustment by weight-trimmed propensity scores (HR, 4.77; 95% CI, 2.09–10.89; p<0.001) (C-index = 0.79) (Table S2 and Figure S1 in File S1).
We also weight-trimmed between the recovery sub-group and the non-AKI group, according to each patient's propensity score. This propensity model had good validity (eAUC–ROC = 0.984; adjusted generalized R 2 = 0.79; GOF test, p<0.001). After adjustment according to weight-trimmed propensity scores, patients in the nonrecovery sub-group had a greater long-term risk of active TB (HR, 1.25; 95% CI, 1.06–1.48; p = 0.008) (Figure S1 in File S1) than the non-AKI group.
Discussion
Despite recovery from AKI requiring dialysis, the most severe form of AKI, patients still had a greater long-term risk of active TB than those without AKI or dialysis during hospitalization. In addition, AKI requiring dialysis appears to be a major risk factor (7.71-increased relative risk) for active TB, independent of possible confounders. These findings are noteworthy from the perspective of a clinician providing long-term care to an individual with AKI requiring dialysis. Our study is the first to elucidate nationwide epidemiological characteristics of de novo AKI requiring dialysis in hospitalized patients and to compare the long-term risk of active TB among those continuing and not continuing dialysis and those without AKI or dialysis. The result is true in our native critical cohort; that is, patients survival from dialysis -requiring AKI, even recovery from dialysis, had higher risk of developing active TB than patients without AKI or dialysis.
Taiwan has an intermediate burden of TB. The annual incidence of TB in Taiwanese dialysis patients was 490 per 100,000 in 1997, which was 6.9 times greater than that of the general population [35]. The incidence of TB was 590 per 100,000 person-years in survivors with temporary dialysis. An epidemiological study in Taiwan found that the incidence of infection was 62–75 per 100,000 person-years during 2002–2008 [36]. The incidence in our matched patients without AKI or dialysis, 83 per 100,000 person-years, was slightly greater than the incidence in the general population. The discrepancy could be related to the greater rate of comorbidities in our study subjects, all of whom had been hospitalized.
The kidneys are uniquely positioned to serve as immunomodulatory organs [37]. There is evidence to suggest that the deleterious effects of AKI on lung function could, at least in part, be due to loss of the normal balance of immune, inflammatory, and mediators that occurs with injury of the tubular epithelium [38]. It is also notable that leukocytes passing through the kidney are exposed to a uniquely hostile environment. The kidney could well modulate leukocyte trafficking in a number of important organs,—such as the lungs,—via both adhesion molecule expression and physical characteristics of neutrophils, such as cytoplasmic “stiffness” [37], [39]. Consequently, it is reasonable to suppose that the immune system of AKI patients (mainly T lymphocytes and antigen-presenting cells) undergo disturbances that increase susceptibility to TB infection.
In a large cohort study of the risk of active TB, the hazard ratio for patients with chronic obstructive pulmonary disease (COPD) was 3 times greater than that of the general population [40]. As in patients with advanced CKD [21], COPD is a risk factor for active TB after hospital discharge. It is biologically plausible that COPD increases the risk of TB and other pulmonary infections, because hypersecretion of mucus and ciliary dysfunction can lead to impaired clearance of pathogens [41]. In agreement with a previous study, our results showed that diabetes did not affect the long-term risk of active TB in patients with advanced CKD [21]. AKI survivors with complete recovery of renal function remain at elevated risk of developing de-novo CKD, which may influence long-term survival; however, recovery of kidney function after AKI is associated with better long-term survival and renal function.26 While the time-dependent ESRD episode after AKI was neither related to long-term TB disease, the strong impacts of AKI-dialysis attenuated other's impacts on incident TB, ex. DM, ESRD.
TB patients with greater frailty were expected to have higher mortality and higher adjusted HRs for death, compared with non-TB patients, especially patients who recovered from AKI requiring dialysis. This phenomenon underlines the importance of evaluating the inherent frailty of research study subjects when comparing outcomes in patients with and without TB. Nonetheless, in all scenarios, patients who recovered from AKI still fared more poorly than patients without AKI or dialysis. Further studies are needed to disentangle the underlying factors contributing to this finding.
Limitations
Major strengths of our study are the large sample and the nature of the cohort, which was based on the general population. AKI–dialysis cases in this study were presumably more severe by reason of the stringent definition that was set to avoid miss-classification of patients. Because of the retrospective nature of the claims data, the standard procedure for diagnosing TB infection, for example, acid-fast staining and culturing, was not identified. Additionally, some data had to be inferred indirectly from administrative records, including smoking status and nutritional status, because they were not available from the National Health Research Institute (NHRI). Nonetheless, we do the external data validation using the well designed, prospective collecting, native critical data, and the results were consisted with the finding from nationwide data. Some future perspectives in AKI research are highlighted with respect to novel therapeutic strategies in the prevention and control of TB.
Conclusion
Our findings suggest that the occurrence of TB could be associated with AKI episode. New AKI therapeutic and caring strategies are needed for improved outcomes in AKI patients, targeting not only renal dysfunction alone, but also long-term frailty, which is expected to be associated with greater risk of active TB. Accordingly, the high incidence of TB after recovery from AKI requiring dialysis will correspond to high mortality. Our results raise concerns that the increasing global burden of AKI will include increased incidence of active TB.
Supporting Information
Table S1. Demographics and comorbidities added into a non-parsimonious propensity model to predict dialysis during index hospitalization in the AKI-dialysis and non-AKI groups. Table S2. Characteristics of patients in the AKI-dialysis and non-AKI groups at index hospitalization matched by weight trimmed logistic regression estimated propensity scores. Figure S1. Cox proportional hazards model for long-term active TB events, stratified by AKI–dialysis status at index hospitalization and adjusted by weight-trimmed logistic regression estimated propensity scores.
(DOC)
Acknowledgments
The authors would like to thank the staff of the Second Core Lab of Department of Medical Research in National Taiwan University Hospital for technical assistance. We express our sincere gratitude to all participants of the NSARF.
National Taiwan University Hospital Study Group for Acute Renal Failure (NSARF) includes Wen-Je Ko, MD, PhD, Vin-Cent Wu, MD, PhD, Yu-Feng Lin, MD, Chun-Fu Lai, MD, Yih-Sharng Chen, MD, PhD, Tzong-Shinn Chu, MD, PhD, Yung-Ming Chen, MD, Wei-Jie Wang, MD, Cheng-Yi Wang, MD, Pei-Chen Wu, MD, Pi-Ru Tsai, RN, Yu-Chang Yeh, MD, Fu-Chang Hu, MS, ScD, and Kwan-Dun Wu, MD, PhD in the National Taiwan University Hospital Che-Hsiung Wu, MD. in the Buddhist Tzu Chi General Hospital, Taipei branch. Chih-Chung Shiao, MD, in the Division of Nephrology, Department of Internal Medicine, Saint Mary's Hospital Luodong. Tao-Min Huang, MD, in the Yun-Lin branch, National Taiwan University Hospital.
Taiwan Anti-Mycobacteria Investigation group includes Chun-Ta Huang, MD, Chin-Chung Shu, MD, Sheng-Yuan Ruan, MD, Yu-Tzu Tseng, MD, Chia-Lin Hsu, MD, Jann-Yuan Wang, MD, PhD, Chong-Jen Yu, MD, PhD, Li-Na Lee, MD, PhD, Pan-Chyr Yang, MD, PhD in the National Taiwan University Hospital; Chih-Hsin Lee, MD, PhD in the Buddhist Tzu Chi General Hospital, Taipei branch; Ming-Chih Yu, MD in the Wan Fang Hospital; Wei-Juin Su, MD, PhD in the Taipei Veterans General Hospital; and Hsin-Chih Lai, MD, PhD in the Chang Gung University.
Funding Statement
This study was supported by the Ta-Tung Kidney Foundation and Taiwan National Science Council (grants NSC 101-2314-B-002-132-MY3, NSC 100-2314-B-002-119, NSC 101-2314-B-002-085-MY3) and NTUH 098-001177, NTUH 100-001667. Role of the Sponsors: No role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
References
- 1. Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, et al. (2010) Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax 65: 557–570. [DOI] [PubMed] [Google Scholar]
- 2. Lundin AP, Adler AJ, Berlyne GM, Friedman EA (1979) Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med 67: 597–602. [DOI] [PubMed] [Google Scholar]
- 3. Kumar VA, Craig M, Depner TA, Yeun JY (2000) Extended daily dialysis: A new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis 36: 294–300. [DOI] [PubMed] [Google Scholar]
- 4. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, et al. (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, et al. (2006) Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 1: 43–51. [DOI] [PubMed] [Google Scholar]
- 6. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C (2006) An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917. [DOI] [PubMed] [Google Scholar]
- 7. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, et al. (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. Jama 294: 813–818. [DOI] [PubMed] [Google Scholar]
- 8. Waikar SS, Winkelmayer WC (2009) Chronic on acute renal failure: long-term implications of severe acute kidney injury. Jama 302: 1227–1229. [DOI] [PubMed] [Google Scholar]
- 9. Descamps-Latscha B (1993) The immune system in end-stage renal disease. Curr Opin Nephrol Hypertens 2: 883–891. [DOI] [PubMed] [Google Scholar]
- 10. Harding CV, Boom WH (2010) Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 8: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kay NE, Raij LR (1986) Immune abnormalities in renal failure and hemodialysis. Blood Purif 4: 120–129. [DOI] [PubMed] [Google Scholar]
- 12. Yap SC, Lee HT (2012) Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 116: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 13. Chang CH, Lin JW, Chen HC, Kuo CW, Shau WY, et al. (2011) Non-steroidal anti-inflammatory drugs and risk of lower gastrointestinal adverse events: a nationwide study in Taiwan. Gut 60: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 14. Cheng SH, Chen CC, Chang WL (2009) Hospital response to a global budget program under universal health insurance in Taiwan. Health Policy 92: 158–164. [DOI] [PubMed] [Google Scholar]
- 15. Lee CH, Lee MC, Lin HH, Shu CC, Wang JY, et al. (2012) Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One 7: e37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YH, Chen YK, Keller JJ, Lin HC (2012) A population-based case-control analysis of the association between herpes zoster and erectile dysfunction. J Infect 65: 150–156. [DOI] [PubMed] [Google Scholar]
- 17. Lin YF, Ko WJ, Chu TS, Chen YS, Wu VC, et al. (2009) The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg 198: 325–332. [DOI] [PubMed] [Google Scholar]
- 18. Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, et al. (2006) Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 19. Levesque LE, Hanley JA, Kezouh A, Suissa S (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340: b5087. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 21. Li SY, Chen TJ, Chung KW, Tsai LW, Yang WC, et al. (2011) Mycobacterium tuberculosis infection of end-stage renal disease patients in Taiwan: a nationwide longitudinal study. Clin Microbiol Infect 17: 1646–1652. [DOI] [PubMed] [Google Scholar]
- 22. Lin HH, Ezzati M, Chang HY, Murray M (2009) Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 180: 475–480. [DOI] [PubMed] [Google Scholar]
- 23. Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, et al. (2011) Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80: 1222–1230. [DOI] [PubMed] [Google Scholar]
- 24. Wu VC, Lai CF, Shiao CC, Lin YF, Wu PC, et al. (2012) Effect of diuretic use on 30-day postdialysis mortality in critically ill patients receiving acute dialysis. PLoS One 7: e30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, et al. (2011) Preoperative Proteinuria Predicts Adverse Renal Outcomes after Coronary Artery Bypass Grafting. J Am Soc Nephrol 22: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu VC, Wang CH, Wang WJ, Lin YF, Hu FC, et al. (2009) Sustained low-efficiency dialysis versus continuous veno-venous hemofiltration for postsurgical acute renal failure. Am J Surg 199: 466–476. [DOI] [PubMed] [Google Scholar]
- 27. Wu VC, Ko WJ, Chang HW, Chen YS, Chen YW, et al. (2007) Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg 205: 266–276. [DOI] [PubMed] [Google Scholar]
- 28. Wu VC, Ko WJ, Chang HW, Chen YW, Lin YF, et al. (2008) Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med 34: 101–108. [DOI] [PubMed] [Google Scholar]
- 29. Shiao CC, Wu VC, Li WY, Lin YF, Hu FC, et al. (2009) Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care 13: R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shu CC, Lee CH, Hsu CL, Wang JT, Wang JY, et al. (2011) Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung 189: 467–474. [DOI] [PubMed] [Google Scholar]
- 31. Fisher LD, Lin DY (1999) Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 20: 145–157. [DOI] [PubMed] [Google Scholar]
- 32. MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, et al. (2006) A national evaluation of the effect of trauma-center care on mortality. N Engl J Med 354: 366–378. [DOI] [PubMed] [Google Scholar]
- 33. Lee BK, Lessler J, Stuart EA (2011) Weight trimming and propensity score weighting. PLoS One 6: e18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cole SR, Hernan MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chou KJ, Fang HC, Bai KJ, Hwang SJ, Yang WC, et al. (2001) Tuberculosis in maintenance dialysis patients. Nephron 88: 138–143. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control: Department of Health EYT (2009) Taiwan tuberculosis incidence and mortality rate, 2002–2008 (in Chinese). http://wwwcdcgovtw/public/Data/9123117163971pdf.
- 37. Kluth DC, Erwig LP, Rees AJ (2004) Multiple facets of macrophages in renal injury. Kidney Int 66: 542–557. [DOI] [PubMed] [Google Scholar]
- 38. Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, et al. (1999) Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367. [DOI] [PubMed] [Google Scholar]
- 39. Motosugi H, Graham L, Noblitt TW, Doyle NA, Quinlan WM, et al. (1996) Changes in neutrophil actin and shape during sequestration induced by complement fragments in rabbits. Am J Pathol 149: 963–973. [PMC free article] [PubMed] [Google Scholar]
- 40. Inghammar M, Ekbom A, Engstrom G, Ljungberg B, Romanus V, et al. (2010) COPD and the risk of tuberculosis–a population-based cohort study. PLoS One 5: e10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benfield T, Lange P, Vestbo J (2008) COPD stage and risk of hospitalization for infectious disease. Chest 134: 46–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and comorbidities added into a non-parsimonious propensity model to predict dialysis during index hospitalization in the AKI-dialysis and non-AKI groups. Table S2. Characteristics of patients in the AKI-dialysis and non-AKI groups at index hospitalization matched by weight trimmed logistic regression estimated propensity scores. Figure S1. Cox proportional hazards model for long-term active TB events, stratified by AKI–dialysis status at index hospitalization and adjusted by weight-trimmed logistic regression estimated propensity scores.
(DOC)