Abstract
Background
Studies have reported inconsistent findings regarding the association between obstructive sleep apnea (OSA) and future risks of cardiovascular and all-cause mortality. We conducted a meta-analysis to investigate whether OSA is an independent predictor for future cardiovascular and all-cause mortality using prospective observational studies.
Methods
Electronic literature databases (Medline and Embase) were searched for prospective observational studies published prior to December 2012. Only observational studies that assessed baseline OSA and future risk of cardiovascular and all-cause mortality were selected. Pooled hazard risk (HR) and corresponding 95% confidence intervals (CI) were calculated for categorical risk estimates. Subgroup analyses were based on the severity of OSA.
Results
Six studies with 11932 patients were identified and analyzed, with 239 reporting cardiovascular mortality, and 1397 all-cause mortality. Pooled HR of all-cause mortality was 1.19 (95% CI, 1.00 to 1.41) for moderate OSA and 1.90 (95% CI, 1.29 to 2.81) for severe OSA. Pooled HR of cardiovascular mortality was 1.40 (95% CI, 0.77 to 2.53) for moderate OSA and 2.65 (95% CI, 1.82 to 3.85) for severe OSA. There were no differences in cardiovascular mortality in continuous positive airway pressure (CPAP) treatment compared with healthy subjects (HR 0.82; 95% CI, 0.50 to 1.33).
Conclusions
Severe OSA is a strong independent predictor for future cardiovascular and all-cause mortality. CPAP treatment was associated with decrease cardiovascular mortality.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of complete or partial obstructions of the upper airway during sleep. Prevalence of OSA with an apnea-hypopnea index (AHI) exceeding 10–15 is 7–10% in the general adult population [1], and approximately 2–4% of the adult population between the ages of 30 and 60 years occurs excessive daytime somnolence [2]. Untreated OSA is associated with significant cardiovascular morbidity and mortality, debilitating daytime symptoms and increased risk of work and motor vehicle accidents.
OSA is highly prevalent in patients with hypertension, coronary artery disease, stroke, and atrial fibrillation [3], [4]. OSA has been reported to be associated with increased cardiovascular mortality [5], [6], [7], [8], [9] and all-cause mortality [6], [9], [10], [11], [12], [13], [14], and in particular with coexistence of OSA and cardiovascular disease [15], [16], [17], [18], [19]. However, many of these reports did not examine the contributing role of confounding factors [14], nor the relationship with the severity of OSA [7], [13]; conflicting results whether this association is independent of obesity and co-morbidities remain [6], [9], [12], [14].
To the best of our knowledge, no meta-analyses of such studies have been conducted on the association between OSA and future risk of cardiovascular and all-cause mortality. Given these reasons, a meta-analysis may help clarify this issue. The objective of the current meta-analysis was to quantitatively evaluate findings from prospective observational studies on OSA and future risk of cardiovascular and all-cause mortality, and determine whether OSA is an independent predictor of cardiovascular and all-cause mortality.
Methods
Search Strategy
We conducted a PubMed database and Embase search (up to December 2012) for studies assessing the association between OSA and future risk of cardiovascular and all-cause mortality. Papers could be published in English and/or Chinese. Potentially relevant studies included the word ‘mortality’, ‘death’ plus at least one of the following terms: sleep apnea, obstructive apnea, sleep-disordered breathing, obstructive sleep apnea, obstructive sleep hypopnea, sleep hypopnea syndrome, and upper airway obstruction. In addition, we also manually searched the reference lists to detect additional eligible studies.
Study Selection
Studies satisfying the following criteria were included in the prospective observational meta-analysis: 1) adults who had been diagnosed with OSA, of any severity, confirmed by using a standardized polysomnography; and 2) providing adjusted hazard risk (HR) and the 95% confidence interval (CI) dealing with the risk of cardiovascular and all-cause mortality with varying degrees of OSA severity patients compared with without OSA. In addition, of the included studies, we also compared the patients with continuous positive airway pressure (CPAP) treatment OSA with untreated subjects. CPAP treatment was defined the start of treatment and the average cumulative adherence was 4 or more hours per day. Untreated CPAP was defined as no treatment prescribed or the patient declined to utilize treatment or could not tolerate the device or was persistently noncompliant (average use <4 hours/day).Studies were excluded if 1) the study design was a case-control study or retrospective design; 2) unadjusted HR was reported; and 3) not reporting results for moderate and/or severe OSA.
Outcomes Measures and Data Extraction
Outcome measures included cardiovascular mortality (defined as death from stroke, heart failure, myocardial infarction or arrhythmia), and all-cause mortality. Death at the end of follow-up was obtained from the medical records, or from official death certificates.
AHI or the respiratory disturbance index (RDI) is the most commonly used to assess the severity of the OSA. According to the International Classification of Sleep Disorders, OSA is defined as AHI >15/h in an asymptomatic patient or AHI >5/h in a patient with excessive daytime sleepiness or combining symptoms and an RDI ≥5 or by an RDI ≥15 without symptoms [20]. A widely-used cutpoint at 5, 15 and 30 identified mild, moderate, and severe OSA, respectively.
Two reviewers (Xiahui Ge and Xuejun Guo) independently extracted the data from each trial. The HR and 95% CI were extracted. We used the fully adjusted HR for all of the included studies. We also extracted the following items from everyone study: author; year of publication; the location of study; methods used in diagnosing OSA; CPAP treatment; duration of follow-up; the sample size, gender, and the mean age or age range of participants; mortality events; and statistical adjustments for confounding factors. Where discrepancies were identified, reviewers resolved these by discussion.
Quality Assessment
Quality assessment was performed with consideration for the following aspects followed the Meta-analysis of Observational Studies in Epidemiology guidelines [21]: 1) clear inclusion and exclusion criteria; 2) documentation of the loss to follow-up rate; 3) clear definition of outcome and outcome assessment; 4) sufficient duration of follow-up; 5) appropriate statistical analysis; and 6) important confounded and prognostic factors identified. All items had the following answer options: yes/no/too little information to answer the question.
Statistical Analyses
The HR was used as the common measure estimate across studies. Data analyses used multivariate-adjusted HR and 95% CI. Before pooling the data, adjusted HR from each study was converted to their logHR to stabilize the variances and to normalize the distributions.
Homogeneity of HR across studies was assessed using the Cochrane Q statistic (p<0.10 was considered indicative of statistically significant heterogeneity) and I2 statistic (values of less than 40% as “heterogeneity might not be important” and of more than 75% as “considerable heterogeneity, based on the suggestion of the Cochrane Handbook for Systematic Review of Interventions) [22]. As there was substantial heterogeneity in the types of OSA diagnosis and follow-up duration between the different studies, a random effects model (Mantel-Haenszel heterogeneity) was used to calculate the pooled HR.
Potential publication bias was also assessed by both the Begg’s rank correlation test [23] and Egger linear regression test at p<0.10 [24]. Finally, sensitivity analysis was used to investigate the influence of a single study on the overall risk estimate, and was carried out by sequentially omitting one study at each turn with the metaninf algorithm in STATA. All analyses were performed using STATA version 12.0 statistical software (Stata Corp LP, College Station). P<0.05 was considered as statistically significant.
Results
Literature Search
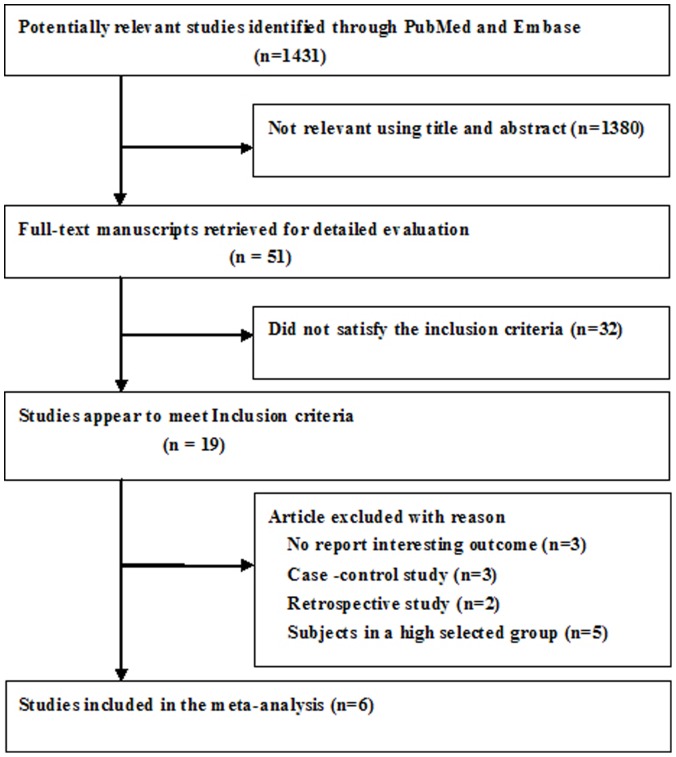
Following the application of the search strategy, a total of 1431 potentially relevant citations were identified in our initial literature search. After screening the abstracts or titles, 1380 studies were excluded, mainly because they were reviews, case-control studies, or not relevant to our analysis. After reviewing the full texts, six studies [5], [6], [8], [9], [10], [11] met the inclusion criteria. A flow chart showing the study selection is presented in Figure 1.
Figure 1. Flow chart of study selection process for meta-analysis.
Baseline Characteristics and Quality Assessment
The characteristics of the included studies are listed in Table 1. Qualities of the included studies are listed in Table S1.
Table 1. Summary of clinical studies included in meta-analysis.
| Study/year | Country | Design | Subjects (%men) | OSA assessment/Patient Selection | Age/Mean (SD) | BMI/median/mean(SD) | Follow-up(Year) | Outcome assessment | Outcome/Events number/HR or OR (95% CI) | Adjustment for Covariates |
| Marin et al 2005 [5] | Spain | Prospective study | 1651(100) | AHI ≥30 (235)AHI 5 to <30 (403)AHI <5 (264)Snorers (377)AHI>30 treated(372) | 50·3 (8·1)49·9 (7·2)49·6 (8·1)49·9 (9·1)49·9 (8·5) | 27·5(4.·4)30·3 (4·2)29·8 (4·4)26·1 (3·6)30·7 (4·4) | 10·1 | National Death Index Death certificate | CVD death (75)1·15 (0·34 to 2·69) S2·87 (1·17 to 7·51) m+M1.05 (0.39 to 2.21) CPAP | Age, diagnostic group, CVD, DB, hypertension, lipid disorders, smoking status, alcohol use, SBP, DBP, blood glucose, TC, triglycerides, and use of antihypertensive, lipid-lowering, and antidiabetic drugs. |
| Young et al 2008 [6] | USA | Prospective study | 1522 (55) | AHI ≥30(63)AHI 15 to <30(82)AHI 5 to <15(220)AHI <5(1157) | 50 (9)50 (8)50 (8)47 (8) | 37.233.331.527.6 | 18 | Social Security Death Index, Wisconsin State Bureau of Health Information and Policy, Vital Records Section | CVD death (23)5.20 (1.4 to 19.2) S1.5 (0.3 to 7.3) M1.3 (0.4 to 4.1) mAll-cause(80)3.8 (1.6 to 9.0) S1.7(0.7 to 4.1) M1.4 (0.7 to 2.6) m | Age, sex, BMI, hypertension (or use of antihypertensive medication), CHD, CVD, self-reported diagnosis of DB, heart failure, MI, cardiac surgery, and stroke. |
| Punjabi et al2009 [11] | USA | Prospective cohort study | 6294(46.7) | AHI ≥30(341)AHI 15 to <30(727)AHI 5 to <15(1797)AHI <5(3429) | 64.6 (10.7)65.1 (10.5)64.8 (10.6)61.3 (11.1) | 32.1 (6.1)30.7 (5.8)29.5 (5.3)27.0 (4.5) | 8.2 | Multiple concurrent approaches | All-cause(1047)1.46(1.14 to 1.86) S1.17 (0.97 to 1.42) M0.93 (0.80 to 1.08) m | Age, sex, race, smoking status, BMI, SBP, DSP, hypertension, diabetes, and CVD. |
| Marshall et al 2008 [10] | Australia | Prospective study | 380 (73.2) | RDI ≥15(18)RDI 5 to <15(77)RDI <5(285) | 55.1 (8.2)54.3 (7.2)52.6 (7.5) | 34.3 (7.3)27.9 (4.1)26.2 (3.7) | 13.4 | National Death Index Death certificates | All-cause(33)6.24(2.01 to 19.39) M+S0.47 (0.17 to 1.29) m | Age, gender, BMI, mean arterial pressure, TC, HDL, DB, and medically diagnosed angina |
| Martinez-Garciaet al 2012 [9] | Spain | Prospective study | 939 (36) | AHI ≥30(173)AHI 15 to <30(108)AHI <15(155)OSA treated (n = 503) | 71.9(4.5)71.7 (5.2)70.9 (4.4)70.1 (4.2) | 34.8 (6)33.6 (4.4)32.4 (5.1)35.1 (5.9) | 5.8 | Death certificate Medical records | CVD death (100)2.25 (1.41 to 3.61) S1.38 (0.73 to 2.64) M0.93 (0.46 to 1.89) CPAPAll-cause(190)1.99 (1.42 to 2.81) S1.17 (0.75 to 1.81) M | OSA group, age, gender, type of sleep study, sleep clinic, BMI, DB, smoke habit, ESS, cardiovascular events, dyslipidemia, and hypertension |
| Campos-Rodriguez et al 2012 [8] | Spain | Prospective cohort study | 1116 (0) | AHI >30 (95)AHI of 10–29 (167)AHI <10 (278)AHI>30 treated(421)AHI10–29 treated(155) | 64.2 (11.4)58.2 (12.0)52.1 (12.5)59.1 (11.1)58.3 (9.8) | 37.9 (7.3)35.1 (5.9)33.1 (6.2)38.7 (7.5)37.7 (7.3) | 3 | Death certificate Medical record | CVD death (41)3.50 (1.23 to 9.98) S1.60 (0.52 to 4.90) m+M0.55(0.17 to 1.74) CPAP(>30)0.19(0.02 to 1.67) CPAP (10–29) | Age, BMI, hypertension, DB, study group, and previous cardiovascular events. |
Abbreviations: BMI, body mass index; AHI, apnea-hypopnea index; RDI, respiratory disturbance index; HR, hazard risk; OR, odds ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; MI, myocardial infarction; DB, diabetes mellitus; CVD, cardiovascular disease; CRP, C-Reactive protein; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; AF, atrial fibrillation; CHD, coronary heart disease; ESS, Epworth Sleepiness Scale; m, mild OAS;M, moderate OSA;S, severe OSA; SSDI, Social Security Death Index; CPAP, continuous positive airway pressure.
All-Cause Mortality
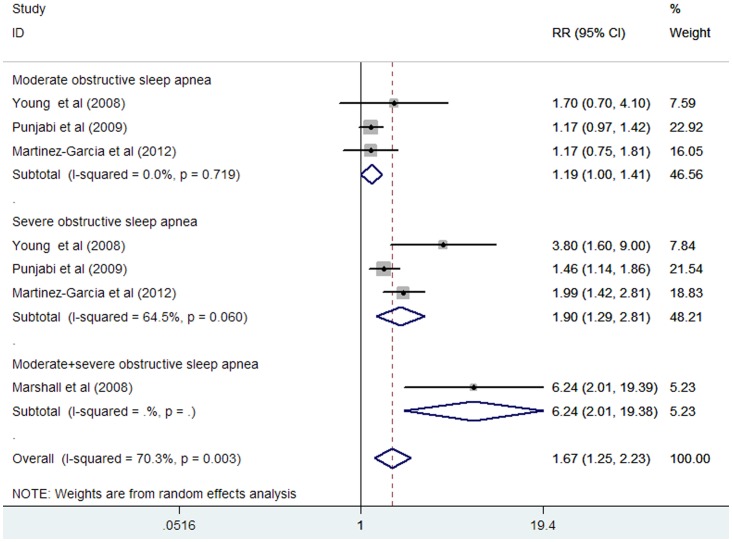
Three studies [6], [8], [11] reported on all-cause mortality for moderate and severe OSA, separately. One study reported the outcome of all-cause mortality for moderate to severe OSA [10]. The total number of participants included in this meta-analysis was 9165, with 1397 all-cause mortality. As shown in Figure 2, moderate to severe OSA was associated with an increase in all-cause mortality in a random effect model compared without OSA subjects (HR 1.67; 95% CI, 1.25 to 2.23; P = 0.001). Substantial heterogeneity was observed (I2 = 70.3%; P = 0.003). Evidence of publication bias for studies reporting adjusted HR of all-cause mortality for moderate to severe OSA was noted by the Begg’s rank correlation test (P = 0.051) and Egger’s linear regression test (P = 0.036).
Figure 2. HR and 95% CI from the included studies of moderate and severe OSA with all-cause mortality comparing OSA to the control.
Subgroup analyses showed that moderate OSA was not associated with an increase in all-cause mortality compared without OSA subject (HR 1.19; 95% CI, 1.00 to 1.41;P = 0.051). Substantial heterogeneity was not observed (I2 = 0%; p = 0.867). Severe OSA was associated with an increase in all-cause mortality compared without OSA subjects (HR 1.90; 95% CI, 1.29 to 2.81; P = 0.001). Substantial heterogeneity was observed (I2 = 64.5%; P = 0.060).
Cardiovascular Mortality
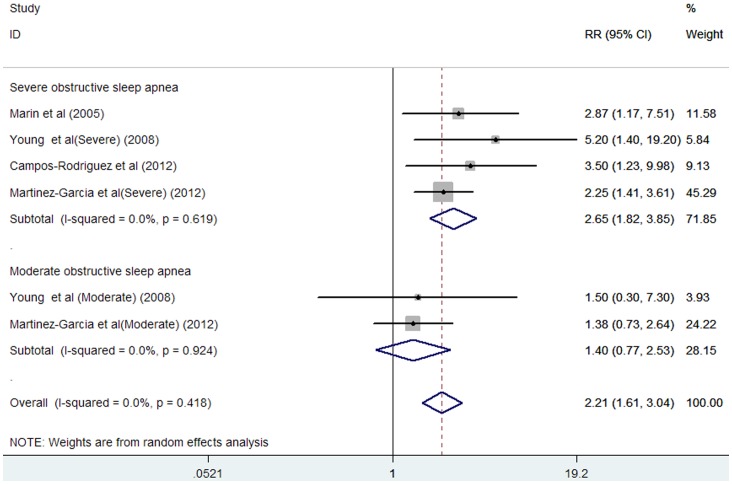
Four studies [5], [6], [8], [9] reported on cardiovascular mortality for moderate to severe OSA. The total number of participants included in this meta-analysis was 5228, with 239 reporting cardiovascular mortality. As shown in Figure 3, moderate to severe OSA was associated with an increase in cardiovascular mortality in a fixed-effect model compared without OSA subjects (HR 2.21; 95% CI, 1.61 to 3.04; P = 0.000). Substantial heterogeneity was not observed (I2 = 0%; P = 0.418). There was no evidence of publication bias for studies reporting adjusted HR of cardiovascular mortality, as suggested by the Begg’s rank correlation test (P = 0.452) and Egger’s linear regression test (P = 0.448).
Figure 3. HR and 95% CI from the included studies of moderate and severe OSA with cardiovascular mortality comparing OSA to the control.
Subgroup analyses showed that moderate OSA was associated with an increase in cardiovascular mortality compared to those without OSA (HR 1.40; 95% CI, 0.77 to 2.53; P = 0.273). Substantial heterogeneity was not observed (I2 = 0%; P = 0.924).Severe OSA was associated with an increase in all-cause mortality compared to subjects without OSA (HR 2.65; 95% CI, 1.82 to 3.85; P = 0.000). Substantial heterogeneity was not observed (I2 = 0%; P = 0.619).
Effects of CPAP Treatment on Cardiovascular Mortality
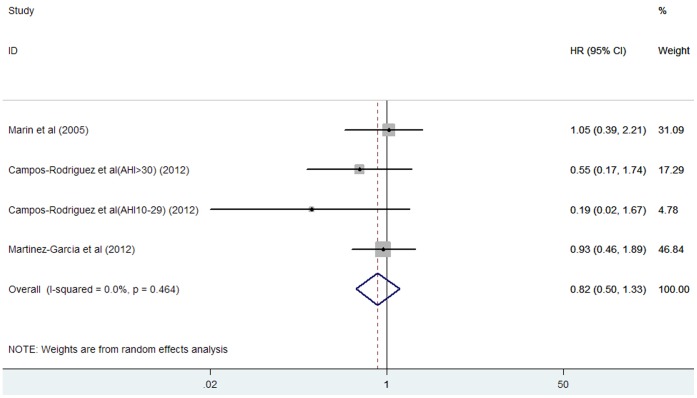
Three studies [5], [8], [9] reported on cardiovascular mortality for CPAP treatment. The total number of participants included in this meta-analysis was 1451, with 62 reporting cardiovascular mortality. As shown in Figure 4, there were no differences in cardiovascular mortality in CPAP treatment group compared with no OSA subjects (HR 0.82; 95% CI, 0.50 to 1.33; P = 0.414). Substantial heterogeneity was not observed (I2 = 0%; P = 0.464). There was no evidence of publication bias for the studies reporting adjusted HR of CPAP treatment on cardiovascular mortality, as suggested by the Begg’s rank correlation test (P = 0.734) and Egger’s linear regression test (P = 0.335).
Figure 4. HR and 95% CI from the included studies of continuous positive airway pressure treatment with cardiovascular mortality comparing OSA to the control.
Sensitivity Analyses
Sensitivity analyses were performed based on all-cause and cardiovascular mortality. In the metainf analysis, there was little influence in the quantitative pooled measure of HR or 95% CI when omission of anyone studies as shown in Table S2 (all-cause mortality) and S3 (cardiovascular mortality).
Discussion
The findings of the current meta-analysis provided evidence that severe OSA is a strong independent predictor for future cardiovascular and all-cause mortality. Subjects with severe OSA increased 67% risk of all-cause mortality and 265% risk of cardiovascular mortality. In addition, CPAP treatment was associated with decrease cardiovascular mortality.
OSA affects both sexes, but a two to threefold higher prevalence in men than women [1], [25]; the difference might be related to the differences in pharyngeal collapsibility and central respiratory drive [26]. In the current included studies, women with severe OSA increased risk of cardiovascular mortality (HR 3.50) [8]; in contrast, men with severe OSA were at a lower risk (HR 2.87) [5].As for all-cause mortality, OSA was associated with increased mortality in male, but not in female subjects [11]. However, due to the limited number of studies, we were unable to conduct subgroup analyses on gender. Therefore, whether men or women with severe OSA had more risk of mortality is still unclear.
The association between age and OSA is complex. OSA subjects had a higher mortality rate than subjects of the same age in the general population [27], [28], and appeared to have more excess mortality in patients younger than 50 years of age [28], [29].In the Sleep Heart Health Study, OSA was not associated with increased mortality in subjects over 70 years of age [11]. Another recently published study further demonstrated that OSA was not associated with mortality in persons >75 years old [14].OSA in the elderly may not be associated with the same increase in mortality than in middle-aged patients [30].In the current meta-analysis, the age of subjects in the included studies varied in these studies, and it might influence the findings of the meta-analysis. Hence, the generalizability of these findings is limited, as for the population studied varied with respect to age, sex, and obesity.
A few studies that did not meet the inclusion criteria for the meta-analysis also found severe OSA was associated with future cardiovascular and all-cause mortality. In a prospective study which recruited 147 hypertensive patients, the adjusted HR for mortality with severe OSA was 9.20 (1.177 to 72.00) [15]. In a total of 1022 consecutive patients, severe OSA was associated with the development of stroke or death (HR 1.97; 95% CI, 1.12 to 3.48) [16].In a retrospective cohort study of 281 consecutive OSA patients with a history of myocardial injury or with known existing ischemic heart disease, the adjusted HR for mortality with severe OSA was 1.72 (95% CI, 1.01 to 2.91) [17]. A recently published well-designed meta-analyses suggested that OSA was associated with an increased rate of stroke and cardiovascular mortality [31].However, these studies are mostly based on clinical samples rather than general population cohorts.
Exact mechanisms linking OSA with cardiovascular and all-cause mortality are not fully elucidated; however, several possible explanations are as follows: sympathetic activation, inflammation, oxidative stress, and endothelial dysfunction. All consequences of OSA are directly linked to intermittent hypoxia in the pathogenesis of cardiovascular disease in patients with OSA [32], [33]. An important issue is whether treatment of severe OSA with CPAP reduces mortality rates. CPAP is the mainstay of therapy for moderate to severe OSA. The current meta-analysis suggested that CPAP treatment was not associated with decrease cardiovascular mortality (HR 0.82; 95% CI, 0.50 to 1.33) compared with healthy subjects. A well–designed meta-analysis also demonstrated that CPAP was an effective treatment for OSA compared with conservative/usual care and placebo in populations with moderate to severe daytime sleepiness [34].
There are several potential limitations of this study. First, we only included studies published in English, and some relevant studies might be not included in the meta-analysis. Second, a major limitation was the possibility of uncontrolled confounding, and the individual studies did not adjust for potential risk factors in a consistent way. There are several known risk factors for OSA such as obesity, age, cigarette smoking, and craniofacial abnormalities. OSA often coexists with cardiovascular diseases that are often associated with a high mortality rate and that could obscure the influence of OSA on mortality. The lack of adjustment for these confounding factors might have resulted in a slight overestimation of the HR. Third, another limitation of the current study is that the severity of OSA at the initial diagnosis did not reflect the severity of the syndrome at the end. However, previous studies on variability on sleep and breathing patterns have shown that one night of recording provides a reasonably accurate estimate of OSA severity [35]. Fourth, AHI does not account for the severity of the hypoxemia induced by the apneas and hypopnea [36]. Fifth, subgroup analyses will further explain the source of heterogeneity. However, due to the limited studies included, we could not conduct further deepen our analyses of the data, such as by gender, age and obesity. In addition, the length of follow-up in studies (ranges from 3 to 18 years) is an additional limitation. It is difficult to determine beyond the duration of the follow-up studies in the meta-analysis with respect to long-term impact on cardiovascular and all-cause mortality.
In conclusion, this meta-analysis provides evidence that severe OSA is an independent predictor for future cardiovascular and all-cause mortality. CPAP treatment was associated with decrease cardiovascular mortality in OSA patients. More well-designed studies are needed to confirm how age and gender in OSA are potential predictors of cardiovascular and all-cause mortality.
Supporting Information
Quality assessment of studies included in the meta-analysis.
(DOC)
HR and 95% CI by omitting each study from the eligible studies of all-cause mortality.
(DOC)
HR and 95% CI by omitting each study from the eligible studies of cardiovascular mortality.
(DOC)
Acknowledgments
We gratefully thank Shijun Wang for his assistance in proofreading.
Funding Statement
The authors have no support or funding to report.
References
- 1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, et al. (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239. [DOI] [PubMed] [Google Scholar]
- 3. Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360: 237–245. [DOI] [PubMed] [Google Scholar]
- 4. Caples SM, Gami AS, Somers VK (2005) Obstructive sleep apnea. Ann Intern Med 142: 187–197. [DOI] [PubMed] [Google Scholar]
- 5. Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 6. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, et al. (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31: 1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 7. Shah NA, Yaggi HK, Concato J, Mohsenin V (2010) Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 14: 131–136. [DOI] [PubMed] [Google Scholar]
- 8. Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, et al. (2012) Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med 156: 115–122. [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, et al. (2012) Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 186: 909–916. [DOI] [PubMed] [Google Scholar]
- 10. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, et al. (2008) Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 31: 1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 11. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, et al. (2009) Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6: e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hudgel DW, Lamerato LE, Jacobsen GR, Drake CL (2012) Assessment of multiple health risks in a single obstructive sleep apnea population. J Clin Sleep Med 8: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, et al. (2011) Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 219: 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson P, Alehagen U, Ulander M, Svanborg E, Dahlstrom U, et al. (2011) Sleep disordered breathing in community dwelling elderly: associations with cardiovascular disease, impaired systolic function, and mortality after a six-year follow-up. Sleep Med 12: 748–753. [DOI] [PubMed] [Google Scholar]
- 15. Korostovtseva LS, Sviryaev YV, Zvartau NE, Konradi AO, Kalinkin AL (2011) Prognosis and cardiovascular morbidity and mortality in prospective study of hypertensive patients with obstructive sleep apnea syndrome in St Petersburg, Russia. Med Sci Monit 17: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, et al. (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 17.Won CH, Chun HJ, Chandra SM, Sarinas PS, Chitkara RK, et al.. (2012) Severe obstructive sleep apnea increases mortality in patients with ischemic heart disease and myocardial injury. Sleep Breath. [DOI] [PubMed]
- 18. Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, et al. (2008) Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10 year follow-up. Arch Intern Med 168: 297–301. [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Parker JD, Newton GE, Floras JS, Mak S, et al. (2007) Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 49: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 20. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, et al. (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5: 263–276. [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duran J, Esnaola S, Rubio R, Iztueta A (2001) Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 163: 685–689. [DOI] [PubMed] [Google Scholar]
- 26. Valipour A (2012) Gender-related differences in the obstructive sleep apnea syndrome. Pneumologie 66: 584–588. [DOI] [PubMed] [Google Scholar]
- 27. Marti S, Sampol G, Munoz X, Torres F, Roca A, et al. (2002) Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J 20: 1511–1518. [DOI] [PubMed] [Google Scholar]
- 28. Lavie P, Lavie L, Herer P (2005) All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J 25: 514–520. [DOI] [PubMed] [Google Scholar]
- 29. Rich J, Raviv A, Raviv N, Brietzke SE (2012) All-cause mortality and obstructive sleep apnea severity revisited. Otolaryngol Head Neck Surg 147: 583–587. [DOI] [PubMed] [Google Scholar]
- 30. Launois SH, Pepin JL, Levy P (2007) Sleep apnea in the elderly: a specific entity? Sleep Med Rev 11: 87–97. [DOI] [PubMed] [Google Scholar]
- 31. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK (2012) Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 5: 720–728. [DOI] [PubMed] [Google Scholar]
- 32. Khayat R, Patt B, Hayes D Jr (2009) Obstructive sleep apnea: the new cardiovascular disease. Part I: Obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev 14: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373: 82–93. [DOI] [PubMed] [Google Scholar]
- 34. McDaid C, Griffin S, Weatherly H, Duree K, van der Burgt M, et al. (2009) Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess 13: 143–274. [DOI] [PubMed] [Google Scholar]
- 35. Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, et al. (2002) Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography–the Sleep Heart Health Study. [corrected]. Sleep 25: 843–849. [PubMed] [Google Scholar]
- 36.Kokkarinen J (2011) Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 183: 950; author reply 950. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of studies included in the meta-analysis.
(DOC)
HR and 95% CI by omitting each study from the eligible studies of all-cause mortality.
(DOC)
HR and 95% CI by omitting each study from the eligible studies of cardiovascular mortality.
(DOC)