Abstract
Platelet-activating factor (PAF) is liberated from antigen-stimulated, IgE-sensitized rabbit basophils and induces aggregation of platelets and secretion of their content of vasoactive amines. Experiments were performed to determine the relationship between these two platelet responses to this stimulus.
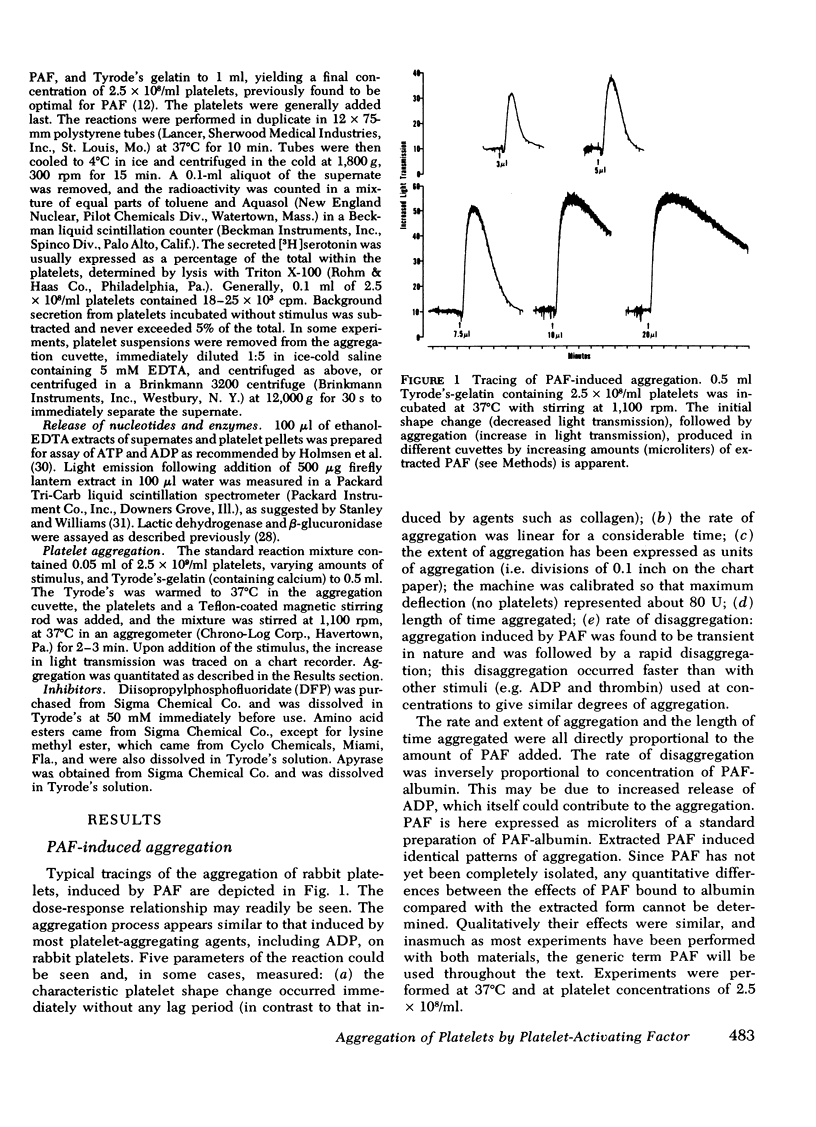
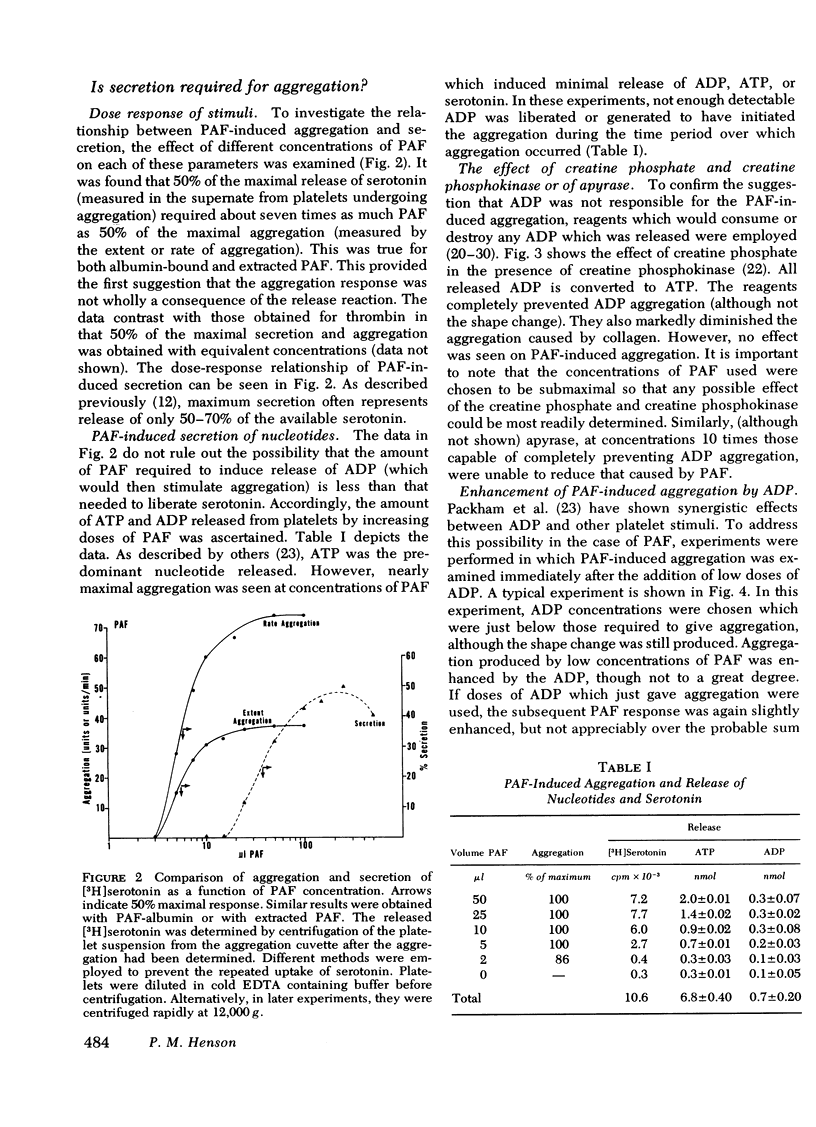
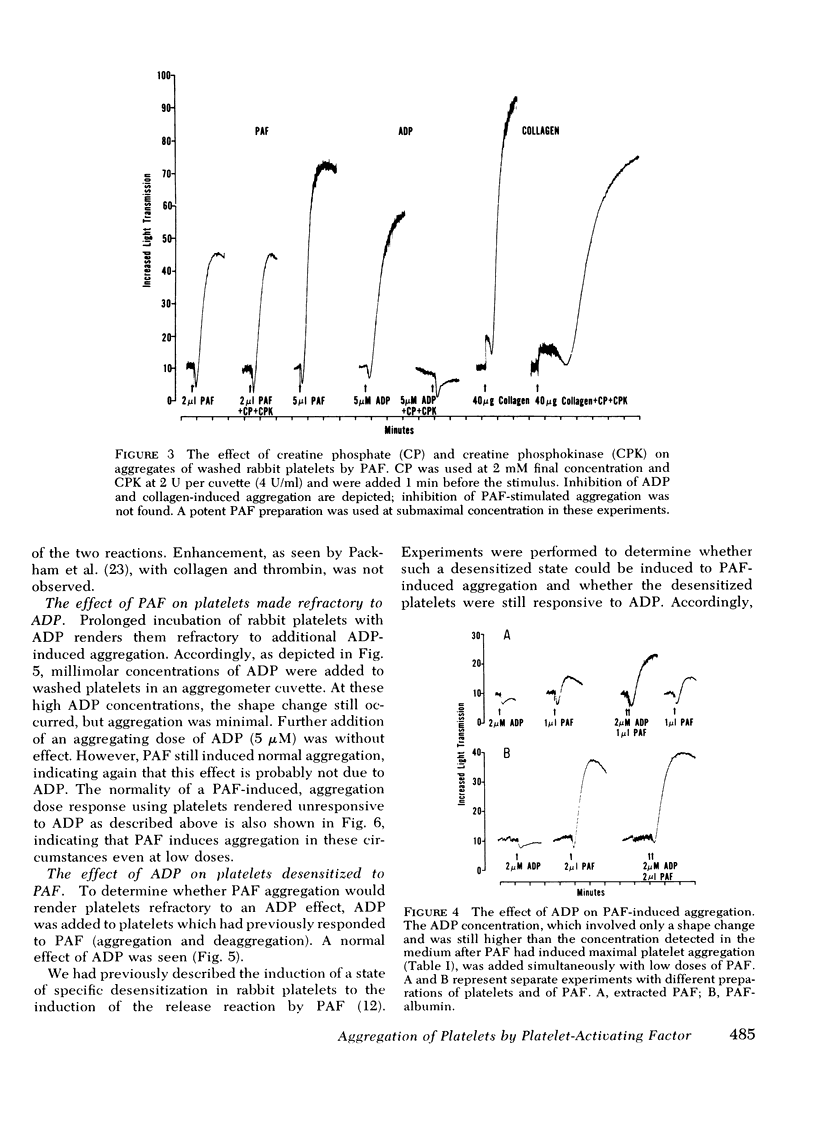
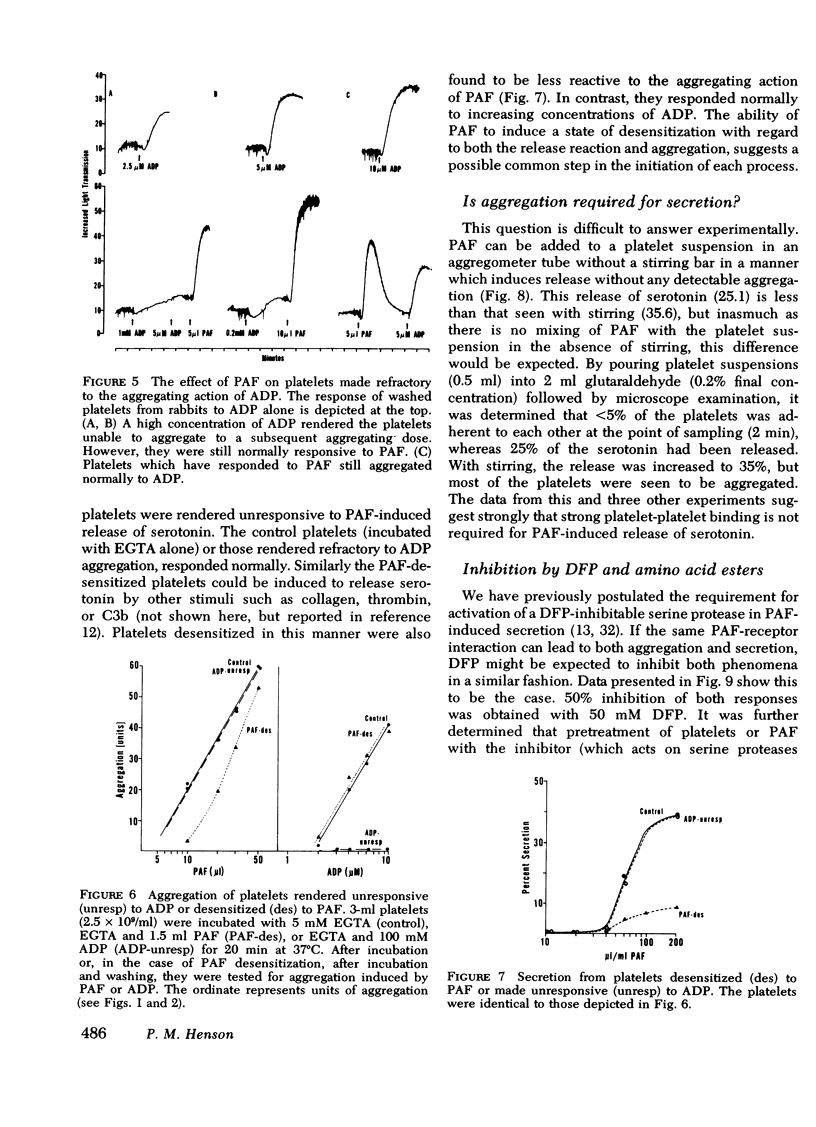
(a) Aggregation was initiated by appreciably lower concentrations of PAF than were required to produce even minimal release of constituents. PAF-induced aggregation was also resistant to agents which destroy ADP, such as creatine phosphate/creatine phosphokinase or apyrase, and occurred with platelets made unresponsive (refractory) to ADP. It was concluded that PAF can induce aggregation by a mechanism which is distinguishable from the release reaction and from the aggregating effect of ADP.
(b) Secretion can probably occur independently of aggregation because incubation without agitation resulted in secretion without detectable aggregation.
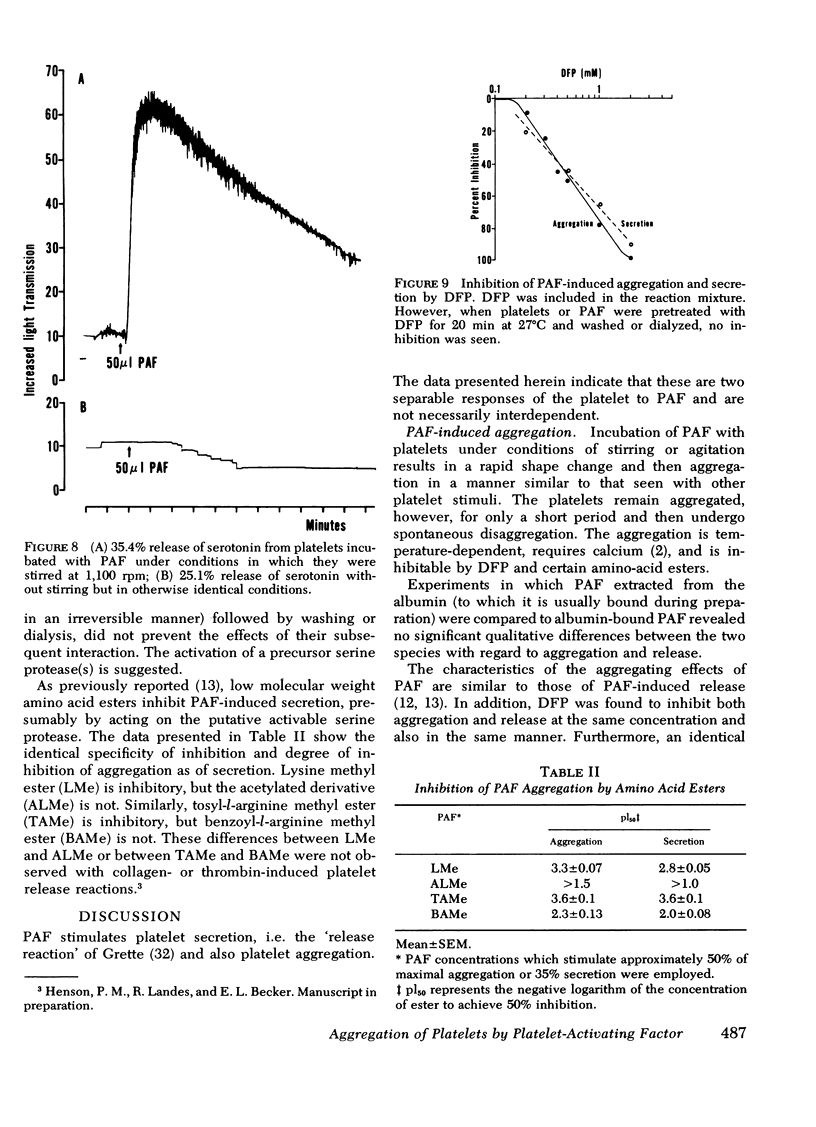
(c) Despite the suggestion that the two platelet responses can be independent, they may be induced by the same stimulus. This was indicated by experiments in which platelets specifically desensitized to PAF-induced secretion were also found to be unresponsive to PAF-stimulated aggregation. Moreover, identical levels of inhibition and identical inhibition profiles were obtained for both responses with serine esterase inhibitor, diisopropylphosphofluoridate, and with amino acid esters. It was concluded that the same stimulus-specific activable protease (esterase) was most likely involved in PAF-induced aggregation and secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Barbaro J. F., Zvaifler N. J. Antigen induced histamine release from platelets of rabbits producing homologous PCA antibody. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1245–1247. doi: 10.3181/00379727-122-31371. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974 Jun 7;249(457):581–582. doi: 10.1038/249581a0. [DOI] [PubMed] [Google Scholar]
- GRETTE K. Studies on the mechanism of thrombin-catalyzed hemostatic reactions in blood platelets. Acta Physiol Scand Suppl. 1962;195:1–93. [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- Halonen M., Pinckard R. N., Meng A. L. Characterization of IgE-induced systemic anaphylaxis in the rabbit, lack of correlation between the intravascular release of histamine and anaphylactic sensitivity. J Immunol. 1973 Aug;111(2):331–340. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Activation and desensitization of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. I. Characteristics of the secretory response. J Exp Med. 1976 Apr 1;143(4):937–952. doi: 10.1084/jem.143.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Gould D., Becker E. L. Activation of stimulus-specific serine esterases (proteases) in the initiation of platelet secretion. I. Demonstration with organophosphorus inhibitors. J Exp Med. 1976 Dec 1;144(6):1657–1673. doi: 10.1084/jem.144.6.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of release of constituents from rabbit platelets by antigen-antibody complexes and complement. I. Lytic and nonlytic reactions. J Immunol. 1970 Aug;105(2):476–489. [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Activation of platelets by platelet-activating factor (PAF) derived from IgE-sensitized basophils. II. The role of serine proteases, cyclic nucleotides, and contractile elements in PAF-induced secretion. J Exp Med. 1976 Apr 1;143(4):953–968. doi: 10.1084/jem.143.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Storm E., Day H. J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972 Apr;46(2):489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Goetzl E. J., Wasserman S. I., Valone F. H., Rubin R. H., Austen K. F. The release of four mediators of immediate hypersensitivity from human leukemic basophils. J Immunol. 1975 Jan;114(1 Pt 1):87–92. [PubMed] [Google Scholar]
- Malmsten C., Hamberg M., Svensson J., Samuelsson B. Physiological role of an endoperoxide in human platelets: hemostatic defect due to platelet cyclo-oxygenase deficiency. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1446–1450. doi: 10.1073/pnas.72.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Chang P. L., Mustard J. F. Platelet aggregation and release: effects of low concentrations of thrombin or collagen. Am J Physiol. 1973 Jul;225(1):38–47. doi: 10.1152/ajplegacy.1973.225.1.38. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Osler A. G. Destruction of rabbit platelets in the allergic response of sensitized leukocytes. I. Demonstration of a fluid phase intermediate. J Immunol. 1971 May;106(5):1244–1251. [PubMed] [Google Scholar]
- Siraganian R. P., Osler A. G. Destruction of rabbit platelets in the allergic response of sensitized leukocytes. II. Evidence for basophil involvement. J Immunol. 1971 May;106(5):1252–1259. [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of an intermediate in prostaglandin biosynthesis and its association with the platelet release reaction. J Clin Invest. 1974 May;53(5):1468–1472. doi: 10.1172/JCI107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]



