Abstract
We conducted a meta-analysis to identify new loci for testicular germ cell tumor (TGCT) susceptibility. In the discovery phase, 931 affected individuals and 1,975 controls from three genome wide association studies (GWAS) were analyzed. Replication was conducted in six independent sample sets totaling 3,211 affected individuals and 7,591 controls. In the combined analysis, TGCT risk was significantly associated with markers at four novel loci: 4q22.2 in HPGDS (per allele odds ratio (OR) 1.19, 95%CI 1.12–1.26, P = 1.11×10−8); 7p22.3 in MAD1L1 (OR 1.21, 95%CI 1.14–1.29, P = 5.59×10−9); 16q22.3 in RFWD3 (OR 1.26, 95%CI 1.18–1.34, P = 5.15×10−12); and 17q22 (rs9905704; OR 1.27, 95%CI 1.18–1.33; P = 4.32×10−13, and rs7221274; OR 1.20, 95%CI 1.12–1.28 P = 4.04×10−9), a locus which includes TEX14, RAD51C and PPM1E. The new TGCT susceptibility loci contain biologically plausible genes encoding proteins important for male germ cell development, chromosomal segregation and DNA damage response.
In the United States, testicular germ cell tumors (TGCT) are the most common cancers in young men, with a peak incidence among those aged 25 to 34 years. The incidence of TGCT has more than doubled among white men in the United States over the past 30 years; similar increases in incidence rates have been observed in other populations of European ancestry1–3. Of note, the incidence of TGCT varies widely between populations and is much higher in individuals of European compared to African ancestry2. Established risk factors for TGCT include family history, cryptorchidism, adult height and prior TGCT history; several recent studies also have implicated marijuana use4–7. First degree relatives of affected men have been shown consistently to have an increased TGCT risk (5- to 19-fold for brothers and 2- to 4-fold for fathers)8–11, the highest for any cancer. Further, the estimated heritability of TGCT is third among all cancers, with genetic effects estimated to account for 25% of TGCT susceptibility12. These observations, coupled with twin studies13–15, support a strong genetic component contributing to TGCT susceptibility.
Despite the greatly increased relative risk of TGCT in family members, candidate gene and linkage approaches yielded little progress in identifying specific genetic risk factors. Initially, two independent genome wide association studies (GWAS) identified allele variation within KITLG on 12q22 as the strongest genetic risk factor for TGCT, with a per allele odds ratio (OR) greater than 316,17. Variants on 5p15.33 (TERT- two independent loci), 5q31.3 (SPRY4), 6p21.3 (BAK1), 9p24.3 (DMRT1- two independent loci), and 12p13.1 (ATF7IP) also have been associated with TGCT risk16–21. The per allele ORs for the identified TGCT susceptibility alleles are in large part higher than those identified for other cancers, which may be due, in part, to the homogeneity of the disease, as all TGCT are thought to arise from the primordial germ cell22,23. Multiple additional loci are expected to contribute to susceptibility as has been shown for cancers of lower heritability24. Combining multiple GWAS represents a step to increase power to detect additional genetic risk factors failing to reach genome-wide significance in individual studies.
We performed a meta-analysis of the most promising 340 SNPs (after excluding previously reported loci) observed in the adjusted pooled analysis of the combined NCI scan (STEED, US Servicemen’s Testicular Tumor Environmental and Endocrine Determinants Study; and FTCS, NCI Familial Testicular Cancer Study) with the previously reported University of Pennsylvania (UPENN) TGCT scan (Online Methods). Allelic ORs for known loci are shown in Supplementary Table 1 for the combined NCI scan. Forty SNPs from nine loci had P values below 10−4, of which 12 localized to the MAD1L1 gene locus (7p22.2) (details of correlation between NCI and UPENN study for top 40 SNPs in Supplementary Table 2). The most significant SNP marker from each of nine loci, plus eight additional markers were selected for replication (n=17). An in silico analysis of these 17 SNPs was performed in the GWAS data from the University of Southern California (USC) and the UK Testicular Cancer Collaboration (UKTCC)18, followed by genotyping in four additional TGCT case-control studies from: Fred Hutchinson Cancer Center (Adult Testicular Lifestyle and Blood Specimen [ATLAS] study), University of Pennsylvania (Testicular Cancer in Philadelphia Area Counties [TestPAC] study), Oslo University Hospital-Radium Hospital, Norway (OUHRH), and MD Anderson Cancer Center (MDA). Details of each study are included in the Supplementary Note. The combined analysis included 4,142 TGCT cases and 9,566 controls (Supplementary Table 3). In the combined meta-analysis, we observed four new loci significantly associated with TGCT (P value < 5×10−8) (Table 1; Supplementary Table 4).
Table 1.
Meta-analysis identified novel loci associated with testicular germ cell tumor
| SNP1 | Nearby genes | Study2 | Cases | Controls | EAF3 | Allelic OR (95% CI) | P value | P for heterogeneity |
|---|---|---|---|---|---|---|---|---|
| rs17021463 | HPGDS | NCI | 582 | 1055 | 0.475 | 1.33 (1.14–1.55) | 2.12 × 10−4 | |
| G|T | 4q22.2 | UPENN | 349 | 919 | 0.433 | 1.28 (1.07–1.53) | 6.67 × 10−3 | |
| Discovery | 931 | 1974 | 1.31 (1.16–1.47) | 6.09 × 10−6 | 0.750 | |||
|
|
||||||||
| UKTCC | 979 | 4947 | 0.420 | 1.19 (1.08–1.31) | 5.66 × 10−4 | |||
| USC | 358 | 258 | 0.428 | 1.09 (0.82–1.46) | 0.464 | |||
| OUHRH | 798 | 377 | 0.399 | 1.16 (0.98–1.39) | 0.092 | |||
| TestPAC | 267 | 575 | 0.417 | 1.14 (0.92–1.41) | 0.225 | |||
| ATLAS | 297 | 664 | 0.420 | 1.11 (0.91–1.35) | 0.292 | |||
| MDA | 236 | 351 | 0.412 | 1.01 (0.79–1.27) | 0.960 | |||
| Replication | 2935 | 7172 | 1.15 (1.07–1.23) | 7.01 × 10−5 | 0.862 | |||
|
|
||||||||
| Combined | 3866 | 9146 | 1.19 (1.12–1.26) | 1.11 × 10−8 | 0.583 | |||
|
| ||||||||
| rs12699477 | MAD1L1 | NCI | 582 | 1056 | 0.412 | 1.31 (1.13–1.52) | 4.64 × 10−4 | |
| T|C | 7p22.3 | UPENN | 349 | 919 | 0.407 | 1.37 (1.15–1.63) | 4.25 × 10−4 | |
| Discovery | 931 | 1975 | 1.34 (1.19–1.50) | 7.16 × 10−7 | 0.704 | |||
|
|
||||||||
| UKTCC | 979 | 4947 | 0.380 | 1.17 (1.06–1.30) | 1.34 × 10−3 | |||
| USC | 358 | 258 | 0.352 | 1.32 (0.99–1.74) | 0.024 | |||
| TestPAC | 266 | 573 | 0.375 | 1.09 (0.88–1.35) | 0.440 | |||
| ATLAS | 298 | 671 | 0.373 | 1.08 (0.89–1.32) | 0.480 | |||
| Replication | 1901 | 6449 | 1.16 (1.07–1.25) | 2.41 × 10−4 | 0.645 | |||
|
|
||||||||
| Combined | 2832 | 8424 | 1.21 (1.14–1.29) | 5.59 × 10−9 | 0.318 | |||
|
| ||||||||
| rs4888262 | RFWD3 | NCI | 582 | 1052 | 0.552 | 1.36 (1.17–1.59) | 9.12 × 10−5 | |
| T|C | 16q22.3 | UPENN | 349 | 919 | 0.498 | 1.39 (1.16–1.67) | 3.12 × 10−4 | |
| Discovery | 931 | 1971 | 1.37 (1.22–1.54) | 1.39 × 10−7 | 0.858 | |||
|
|
||||||||
| UKTCC | 957 | 4940 | 0.458 | 1.22 (1.10–1.34) | 1.02 × 10−4 | |||
| USC | 358 | 258 | 0.440 | 1.41 (1.09–1.83) | 5.14 × 10−3 | |||
| TestPAC | 260 | 575 | 0.465 | 1.12 (0.91–1.38) | 0.2876 | |||
| ATLAS | 295 | 649 | 0.495 | 1.16 (0.95–1.41) | 0.1328 | |||
| Replication | 1870 | 6422 | 1.21 (1.12–1.31) | 1.62 × 10−6 | 0.568 | |||
|
|
||||||||
| Combined | 2801 | 8393 | 1.26 (1.18–1.34) | 5.15 × 10−12 | 0.397 | |||
|
| ||||||||
| rs9905704 | TEX14 | NCI | 582 | 1054 | 0.719 | 1.37 (1.16–1.61) | 1.88 × 10−4 | |
| G|T | 17q22 | UPENN | 349 | 919 | 0.663 | 1.30 (1.08–1.56) | 5.46 × 10−3 | |
| Discovery | 931 | 1973 | 1.33 (1.19–1.52) | 3.44 × 10−6 | 0.674 | |||
|
|
||||||||
| UKTCC | 979 | 4947 | 0.680 | 1.28 (1.16–1.43) | 3.65 × 10−6 | |||
| USC | 358 | 258 | 0.649 | 1.35 (1.08–1.72) | 0.017 | |||
| OUHRH | 802 | 382 | 0.695 | 1.23 (1.02–1.49) | 0.028 | |||
| TestPAC | 259 | 575 | 0.669 | 1.14 (0.91–1.43) | 0.253 | |||
| ATLAS | 300 | 666 | 0.669 | 1.09 (0.88–1.33) | 0.403 | |||
| MDA | 234 | 351 | 0.672 | 1.15 (0.89–1.47) | 0.273 | |||
| Replication | 2932 | 7179 | 1.23 (1.15–1.32) | 1.37 × 10−8 | 0.628 | |||
|
|
||||||||
| Combined | 3863 | 9152 | 1.27 (1.18–1.33) | 4.32 × 10−13 | 0.668 | |||
| rs7221274 | PPM1E | NCI | 582 | 1056 | 0.660 | 1.39 (1.19–1.61) | 3.56 × 10−5 | |
| G|A | 17q22 | UPENN | 349 | 919 | 0.598 | 1.19 (1.00–1.43) | 0.053 | |
| Discovery | 931 | 1975 | 1.30 (1.16–1.47) | 7.79 × 10−6 | 0.197 | |||
|
|
||||||||
| UKTCC | 979 | 4947 | 0.620 | 1.23 (1.12–1.37) | 3.08 × 10−5 | |||
| USC | 358 | 258 | 0.558 | 1.37 (0.87–2.13) | 9.43 × 10−3 | |||
| OUHRH | 802 | 380 | 0.630 | 1.22 (1.02–1.47) | 0.029 | |||
| TestPAC | 243 | 538 | 0.612 | 1.01 (0.81–1.27) | 0.890 | |||
| ATLAS | 301 | 671 | 0.604 | 1.00 (0.83–1.22) | 0.989 | |||
| MDA | 215 | 351 | 0.615 | 1.05 (0.83–1.33) | 0.682 | |||
| Replication | 2898 | 7145 | 1.16 (1.09–1.25) | 3.27 × 10−5 | 0.228 | |||
|
|
||||||||
| Combined | 3829 | 9120 | 1.20 (1.12–1.28) | 4.04 × 10−9 | 0.130 | |||
SNP genotype depicted as reference allele|effect allele
NCI depicts combined analysis results of the two GWAS scans STEED and FTCS performed at NCI
Discovery depicts initial meta-analysis of NCI and UPENN and Replication depicts meta-analysis of the rest
EAF depicts effect allele frequency in control
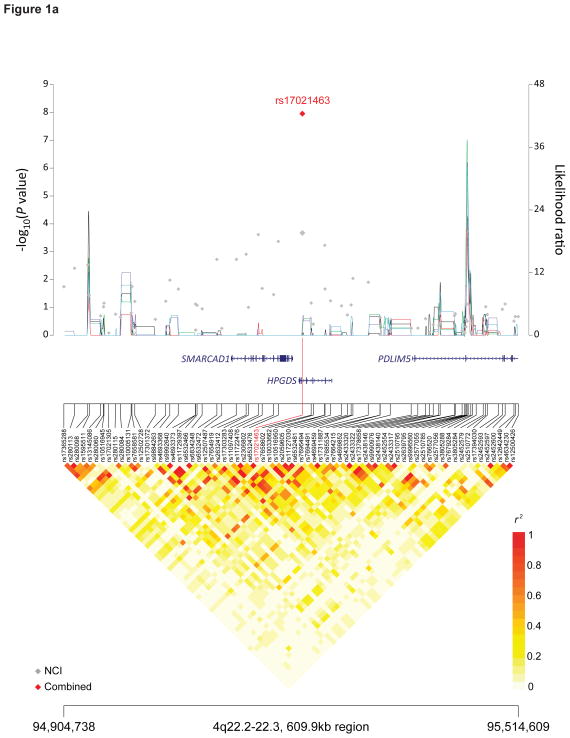
The most significant 4q22.2 SNP marker, rs17021463, is located within the intron of the hematopoietic prostaglandin D synthase gene, HPGDS (P = 1.11×10−8, OR 1.19, 95%CI 1.12–1.26) (Figure 1A, Table 1). In mice, hpgds is expressed in the early embryonic male gonad and appears to regulate nuclear localization of the sox9 protein25. Disruption of hpgds leads to modification of the phenotype of apcMin/+ mice26. Seventy-one surrogate markers highly correlate with HPGDS rs17021463 (r2 ≥ 0.8, 1000 Genomes CEU data, Supplementary Table 5). Notably, rs35744894 (r2 = 0.87) changes a DMRT2 binding motif (Supplementary Table 6); variation in DMRT1 has been associated with TGCT risk19.
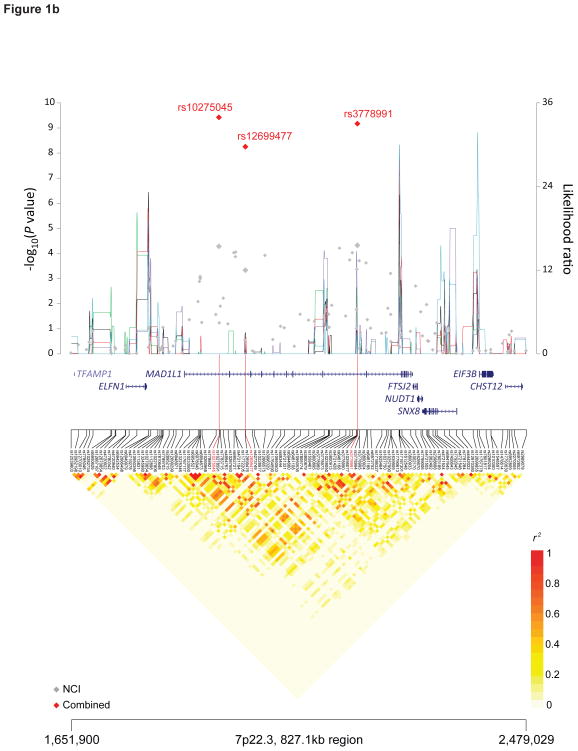
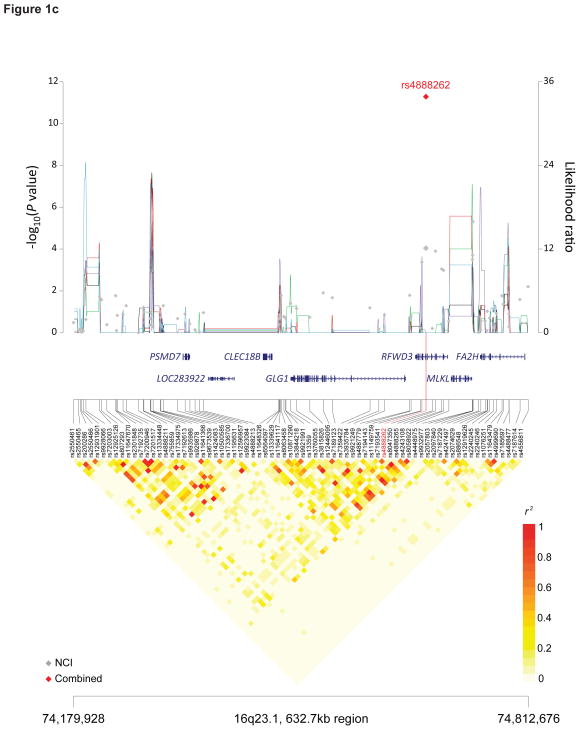
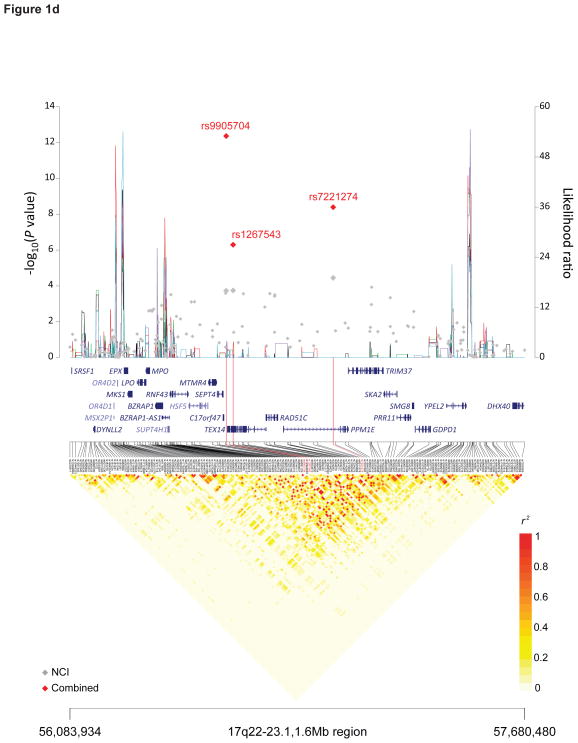
Figure 1. Recombination plot and linkage disequilibrium structure for the four new TGCT susceptibility regions at 4q22.2, 7p22.3, 16q22.3 and 17q22 (a–d).
Regional plots of association results, recombination hotspots and linkage disequilibrium for the (a) 4q22.2–22.3:94,904,738–95,514,609, (b) 7p22.3:1,651,900–2,479,029, (c) 16q23.1:74,179,928–74,812,676 and (d) 17q22–23.1:56,083,934–57,680,480 TGCT susceptibility loci. (a–d) Combined meta-analysis results are shown as red diamonds with rs numbers labeled, and the NCI scan in gray. For each plot, −log10P values (y axis, left) of the SNPs are shown according to their chromosomal positions (x axis). Linkage disequilibrium structure based on NCI controls (n=1,188) was visualized by snp.plotter software. The line graph shows likelihood ratio statistics (y axis, right) for recombination hotspot by SequenceLDhot software and five different colors represent 5 tests of 100 controls from NCI without resampling. Physical locations of each region are based on NCBI Build 37 of the human genome.
Fifty-three of 71 surrogate markers that were highly correlated with HPGDS rs17021463 (r2 ≥ 0.8, 1000 Genomes CEU data, Supplementary Table 5) across a 200kb window mapped within or near an adjacent gene, SMARCAD1 (SWI/SNF-related, matrix-associated actin-dependent regulator of chromatin, subfamily a, containing DEAD/H box 1). SMARCAD1 is a chromatin remodeler, which restores silenced heterochromatin domains in dividing cells and participates in DNA damage response27,28. Homozygous mutant mice display developmental defects, including impaired fertility29. Surrogate markers included one nonsynonymous substitution, rs7439869, at codon 301 (r2 = 0.93, 1000 Genomes CEU, T>C, Val>Ala). Although it is predicted to be tolerated by PolyPhen230, it changes an OCT4 (POUF5F1) and SOX4 binding motif (Supplementary Table 6). OCT4 is a transcription factor, which regulates pluripotency in a number of cell types, including primordial germ cells, and is expressed in TGCT31–36.
We identified a locus on 7p22.3, harboring mitotic arrest deficient-like 1 (MAD1L1) gene, which encodes MAD1. The most significant SNP without study heterogeneity, rs12699477, localized in intron 17 (P = 5.59 × 10−9, OR 1.21, 95%CI (1.14–1.29)) (Figure 1B). Of note, the risk allele (C) at rs12699477 is more prevalent in populations of European (29%) than those of African ancestry (8%) in 1000 Genomes37. MAD1 is a spindle assembly checkpoint protein that delays the onset of anaphase in the mitotic cell cycle until all sister chromatids achieve proper alignment and microtubule attachment, thereby preventing aneuploidy and maintaining genomic stability38.
Among the 35 SNPs that are highly correlated with MAD1L1 rs12699477 (r2 ≥ 0.7, 1000 Genomes CEU data, Supplementary Table 5), rs1801368 is a missense mutation at codon 558 (G>A, Arg>His) that resides in the MAD1L1 second leucine zipper domain. Arg558His has been reported to be associated with lung cancer risk39 and may lead to reduced binding of MAD2 to MAD1, resulting in decreased proficiency in enforcing mitotic arrest40. We observed additional statistically significant associations with TGCT for neighboring SNPs in the MAD1L1 region, including rs10275045 (P=3.78×10−10, OR 1.20, 95%CI (1.13–1.27)) and rs3778991 (P=6.73×10−10, OR 1.21, 95%CI (1.14–1.28)). However, both displayed significant study heterogeneity (Supplementary Table 4). The r2 between our strongest signal at rs12699477 and these markers is 0.66 and 0.50, respectively, in the STEED controls. A conditional analysis resulted in a marked attenuation of the signal, supporting a single TGCT susceptibility locus across MAD1L1 on 7q22.3 (Supplementary Table 7).
We observed a significant TGCT association with rs4888262 on 16q22.3 (P = 5.15×10−12, OR 1.26, 95%CI 1.18–1.34), which is a synonymous SNP in codon 404 (G>A, Thr) of the ring finger WD domain 3 (RFWD3) (Table 1, Figure 1C). RFWD3 is an E3 ubiquitin ligase that positively regulates p53 stability by forming a RFWD3-MDM2-p53 complex, thereby protecting p53 from degradation by MDM2 polyubiquitination41,42. Within the LD interval are SNPs that map to two additional genes, the golgi glycoprotein 1 (GLG1) and mixed lineage kinase domain-like (MLKL); the latter of which has been recently identified as a key of mediator of TNF-induced necrosis, downstream of receptor interacting protein kinase 3 (RIP3)43,44 (Figure 1C). We note that rs3851729, which is highly correlated with rs4888262 (r2 = 0.77, 1000 Genomes CEU), maps to a highly conserved sequence in the 3′ UTR of GLG1; similarly, rs4072222 (r2 = 0.87, 1000 Genomes CEU) maps to an intron of MLKL (Supplementary Table 5). Both susceptibility variants are cis-eQTLs that influence MLKL and RFWD3 expression in monocytes45.
We identified two highly correlated SNPs (r2 = 0.74 in the STEED controls) on 17q22, rs9905704 (P = 4.32×10−13, OR 1.27, 95%CI 1.18–1.33) and rs7221274 (P = 4.04×10−9, OR 1.20, 95%CI 1.12–1.28) (Table 1, Figure 1D). In a conditional analysis, the signal at one SNP was markedly attenuated by the other, indicating a single 17q22 TGCT susceptibility locus (Supplementary Table 7). Within this LD block are at least six plausible candidate genes: RAD51C (RAD51 homolog C [S. cerevisiae]), TEX14 (testis expressed 14), PPM1E (protein phosphatase, Mg2+/Mn2+ dependent, 1E), SEPT4 (septin 4), TRIM37 (tripartite motif containing 37), and SKA2 (spindle and kinetochore associated complex subunit 2) (Figure 1D). Proteins encoded by these candidate genes, except for SKA2, have been implicated as having roles in spermatogenesis46–51. RAD51C is a DNA repair gene, in which rare mutations confer susceptibly to ovarian cancer52,53. Of male rad51cko/neo mice, approximately one-third were found to be infertile due to impaired spermatogenesis49. TEX14 is an essential component of germ cell intercellular bridges, evolutionarily conserved structures from invertebrates to humans that allows clonal development of daughter cells in syncytium; targeted disruption of Tex14 results in male sterility in mice48. TEX14 also has been implicated as an important component of kinetochores (KTs) and interacts with MAD154. PPM1E encodes a phosphatase that dephosphorylates to switch-off CaMK4 (calcium/calmodulin-dependent protein kinase IV), deficiency of which causes infertility in mice50,55. TRIM37 encodes a RING-B-box-coiled-coil protein; rare mutations in this gene cause the autosomal recessive disease mulibrey nanism (MUL; MIM 253250)56, in which adult males have testicular failure57. Three SNPs - rs8077332, rs11652713, and rs9898048 - map within TRIM37 and are in perfect LD with rs7221274 (r2 = 1, 1000 Genomes CEU, Supplementary Table 5); all are cis-eQTL affecting RAD51C expression in monocytes45. Thus, fine mapping and functional studies will be required to elucidate the biological basis of the association signal in this interval on 17q22.
In our meta-analysis of GWAS studies, we have identified four new TGCT susceptibility loci at 4q22, 7q22, 16q22.3, and 17q22. In total, 10 loci now have been conclusively associated with TGCT susceptibility. The four newly identified susceptibility alleles account for 2% of the risk to the brothers and 3% of risk to the sons of TGCT patients, increasing the cumulative total of 12 susceptibility alleles (two susceptibility alleles from TERT-CLPTM1L [5p15] and two from DMRT1 locus [9p24]) to 14% and 21% of the risk to brothers and sons, respectively. Based on the high heritability of TGCT, more than one hundred additional loci are expected to be discovered24. Notably, the allelic ORs associated with these novel loci are in the range of 1.2 to 1.3, continuing the trend of identifying loci with higher odds ratios for TGCT than for other cancer types23.
Interestingly, each locus harbors biologically plausible candidate genes implicating several pathways – most strikingly, spermatogenesis and male germ cell development (HPGDS, SMARCAD1, SEPT4, TEX14, RAD51C, PPM1E, TRIM37), chromosomal segregation (MAD1L1, TEX14, SKA2), and DNA damage response (SMARCAD1, RFWD3, RAD51C). None of the four newly identified loci have been previously implicated in GWAS of other cancers, further supporting that there are distinct pathways and regions implicated in TGCT susceptibility; however rare mutations in RAD51C have been implicated in ovarian cancer susceptibility53. TGCT susceptibility is particularly unique in that many of the associated genes affect male germ cell development and differentiation, thus emphasizing the potential detrimental effect that inherited variation in this developmental process can have on the tumorigenic potential of the primordial germ cell.
This study is the first to implicate variation within genes involved in chromosomal segregation as associated with cancer susceptibility. TGCT karyotypes are unique among cancers, in that nearly all carry the same chromosomal aberration, a gain of 12p, most often in the form of an isochromosome, which is considered essential for tumor development58–60. Variation in these genes may lead to chromosomal instability and facilitate the development of aneuploidy. Numerous potential regulatory SNPs were identified, suggesting that newly identified associations might be mediated by plausible genes within each locus, which warrant further fine-mapping and functional studies to elucidate the biological bases of the TGCT susceptibility regions. Studies of the genetic basis of TGCT continue to provide novel insights into this unique disease with high heritability.
ONLINE METHODS
Studies
Detailed characteristics of the study populations are described in both the Supplementary Note and Supplementary Table 3. Subjects used in the current study are all of European descent and data from each study were collected and analyzed in accordance with local ethical permissions and informed consent. Three studies (STEED, FTCS, and UPENN) were included in the discovery meta-analysis, and six studies contributed to replication by de novo genotyping (TestPAC, ATLAS, OUHRH, and MDA) or in silico look-up in existing data (UKTCC and USC).
Genotyping and quality control
Genotype quality control metrics for the reported GWAS scans (UPENN and UKTCC) were previously described18,19. Genotype quality control metrics for STEED, FTCS, and USC are described in Supplementary Note61. OUHRH and MDA studies were genotyped using the 5′ exonuclease assay (TaqMan™) and the ABI prism 7900HT sequence detection system, all according to the manufacturer’s instructions, across several genotyping centers. Primers and probes were supplied directly by Applied Biosystems as Assays-By-Design™. Technical validation was performed in the HapMap samples (n=270) with greater than 99% genotype concordance. TestPAC and ATLAS studies conducted genotyping using the iPLEX mass array platform (Sequenom, Inc.) following manufacturer’s protocol. Assays at all genotyping centers included at least four negative controls and 2–5% duplicates on each plate. Standard quality control protocol was implemented; SNP call rate > 95%, no deviation from Hardy-Weinberg equilibrium in controls at P<0.00001, <2% discordance between genotypes in duplicate had to be fulfilled and cluster plots for SNPs that were close to failing any of the QC criteria were re-examined centrally.
Statistical analysis
Two genome-wide scans from the National Cancer Institute (STEED and FTCS) were analyzed as a combined dataset using a logistic regression model for trend effect adjusted for age, study, and additionally for one eigenvector (only one with p < 0.05) to account for population stratification in this European population. From the top 500 SNPs by trend P values from the NCI scan excluding previously reported ones, 340 SNPs were selected based on the availability of surrogates (r2 > 0.6) in the previous TGCT GWAS scan from the University of Pennsylvania. Since SNP content differs between the Illumina and Affymetrix platforms, the best correlated surrogate per each marker was paired to perform a discovery meta-analysis (111 SNPs, direct match; 229 SNPs, surrogate match). From the discovery meta-analysis, 17 of 40 SNPs with P values < 10−4 were selected for follow up in the remaining studies. In silico follow-up was done in the USC and UKTCC scans, whereas additional genotyping was done in TestPAC, ATLAS, OUHRH and MDA studies (Supplementary Table 3). Not all markers were available for replication efforts from all sites (see Supplementary Table 4).
The meta-analysis was conducted using the suite of tools in GLU (Genotyping Library and Utilities) software, combining study-specific odds ratio (OR) estimates using a fixed effects model, which used the inverse-variance method to estimate the combined OR and its 95% confidence intervals (CIs). To assess existence of heterogeneity among studies, Cochran’s Q statistic was used to calculate P for heterogeneity.
Recombination hotspots were identified in the vicinity of the novel TGCT associated loci using SequenceLDhot62, a program that uses the approximate marginal likelihood method63 and calculates likelihood ratio statistics at a set of possible hotspots. We tested five unique sets of 100 control samples drawn from STEED. PHASE v2.1 program was used to calculate background recombination rates64,65 and LD heatmap was visualized in r2 using snp.plotter program66.
The relative risk attributable to a set of SNPs (λ) was estimated using the following formula67
where qt is the minor allele frequency of SNPi and pi = 1 − qi. SNP specific risks for rare homozygotes, heterozygotes, and common homozygotes are denoted by r0i, r1i, and r2i, respectively. The NCI controls (n=1,140) were used to estimate minor allele frequencies and odds ratio estimates from SNP association analyses were used to estimate relative risks. This formula assumes the effects of all SNPs in the set are multiplicative. The proportion of familial risk attributable to a set of SNPs was calculated as , where λ0 is the familial relative risk estimated from TGCT epidemiological studies (λ0 =4 for affected father, λ0 =8 for affected brother)68.
Genomic annotation
Genomic annotation on high LD surrogates (r2 ≥ 0.8, 1000 Genomes CEU) of 5 SNPs (rs17021463, rs12699477, rs4888262, rs9905704, and rs7221274) from the four TGCT susceptibility loci identified in the current study was conducted using ENCODE tools – HaploReg69 and RegulomeDB70 (Supplementary Table 5). rs12699477 did not have surrogates with r2 ≥ 0.8 threshold, thus we lowered the threshold to 0.7 for surrogates, and then conducted annotation. All surrogates were queried in RegulomeDB browser to cross examine predicted regulatory DNA elements such as regions of DNase hypersensitivity, binding sites of transcription factors, and promoter regions that have been biochemically characterized to regulation transcription. Summaries of each SNP analysis by RegulomeDB browser expressed in scores are added to Supplementary Table 5. To predict potential regulatory SNPs, we assessed SNPs that meet one of the following criteria - 1) conserved (GERP and/or Siphy); 2) present in a promoter or DNase hypersensitivity region; or 3) predicted to have a cis eQTL or having a RegulomeDB score of ≤ 3. Twenty-nine SNPs that passed one of these criteria also changed a motif, and are annotated further with the motif of interest and their log-odds (LOD) motif score for the specific SNP of interest in Supplementary Table 6. Two SNPs in 3′-UTR regions were evaluated using SNP Function Prediction for changes in miRNA binding sites and are included in Supplementary Table 6.
Supplementary Material
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products or organization indicate endorsement by the U.S. Government. The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, NCI, the screening center investigators and staff of the PLCO Cancer Screening Trial, Mr. Thomas Riley and staff at Information Management Services, Inc., and Ms. Barbara O’Brien and staff at Westat, Inc. for their contributions to the PLCO Cancer Screening Trial. The authors thank Stephanie Ciosek, Monique McDermoth and Kurt D’Andrea for expert assistance with the conduct of TestPAC. The authors thank Laurence Kolonel and Loic Le Marchand for providing access to the Multi-Ethnic Cohort aggressive prostate cancer scan, as well as Juan Pablo Lewinger, Malcolm Pike, David J. Van Den Berg, and Kimberly Siegmund for technical and scientific contributions to the parent study at USC.
A portion of this work was supported by the Intramural Research Program of the National Cancer Institute and by support services contract HHSN261200655004C with Westat, Inc. The Penn GWAS (UPenn) and replication effort for the TestPAC study was supported by the Abramson Cancer Center at the University of Pennsylvania and National Institute of Health grant R01CA114478 to Drs. Kanetsky and Nathanson. Replication effort for the ATLAS study was supported by the National Institute of Health grant R01CA085914 to Dr. Schwartz. USC GWAS controls were supported by the Multiethnic Cohort Study (NCI U01-CA98758). USC GWAS testicular cases and Familial Study were supported by the California Cancer Research Program (99-00505V-10260, 03-00174VRS-30021) and National Cancer Institute (R01CA102042) grants to Dr. Cortessis and a Whittier Foundation award to the Norris Comprehensive Cancer Center. The study at MD Anderson was supported by the Center for Translational and Public Health Genomics of the Duncan Family Institute for Cancer Prevention and Risk Assessment and by MD Anderson Senior Research Trust Fellowship to Dr. Wu. The UK testicular cancer study was supported by the Institute of Cancer Research, Cancer Research UK and the Wellcome Trust and made use of control data generated by the Wellcome Trust Case Control Consortium (WTCCC) 2. Support was provided by the Norwegian Cancer Society to Drs. Lothe and Skotheim, Health Region South-Eastern Norway to Drs. Lothe and Fosså and the Norwegian ExtraFoundation for Health and Rehabilitation to Dr. Fosså.
Footnotes
Author Contributions
S.J.C. and K.L.N. supervised the overall study. P.A.K., M.A.T.H., C.P.K., V.K.C., A.C.B., D.T.B., M.B.C., R.L.E., S.D.F., L.A.K., S.M.K., N.R., E.C.S., X.W., M.H.G., S.M.S., K.A.M., and K.L.N. contributed to recruitment, study and data management. C.C.C., P.A.K., Z.W, M.A.T.H., R.K., R.I.S., C.T., K.B.J., R.A.L., J.T.L., D.C.T., M.Y, and F.R.S., contributed to genotyping or association analysis of individual studies. C.C.C., Z.W. and R.K. carried out the meta-analysis and the additional reported ENCODE analyses. C.C.C. and K.L.N. prepared the manuscript together with P.A.K., R.K., and S.J.C and all authors reviewed and contributed to the manuscript.
Conflict of Interest
There are no conflicts of interest.
URLs
GLU, http://code.google.com/p/glu-genetics/
SequenceLDhot, http://www.maths.lancs.ac.uk/~fearnhea/Hotspot/
snp.plotter, http://cbdb.nimh.nih.gov/~kristin/snp.plotter.html
PHASE v2.1, http://www.stat.washington.edu/stephens/phase/download.html
InPower, http://dceg.cancer.gov/bb/tools/INPower/readme
HaploReg, http://www.broadinstitute.org/mammals/haploreg/haploreg.php
RegulomeDB, http://regulome.stanford.edu
References
- 1.Rosen A, Jayram G, Drazer M, Eggener SE. Global trends in testicular cancer incidence and mortality. Eur Urol. 2011;60:374–9. doi: 10.1016/j.eururo.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) based on November 2011 SEER data submission, posted to the SEER web site, 2012. National Cancer Institute; Bethesda MD: 2012. [Google Scholar]
- 3.Stang A, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973–2007. Int J Androl. 2012;35:616–25. doi: 10.1111/j.1365-2605.2011.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol. 2012;9:339–49. doi: 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daling JR, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115:1215–23. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2011;117:848–53. doi: 10.1002/cncr.25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacson JC, et al. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118:5374083. doi: 10.1002/cncr.27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromen K, et al. Testicular, other genital, and breast cancers in first-degree relatives of testicular cancer patients and controls. Cancer Epidemiol Biomarkers Prev. 2004;13:1316–24. [PubMed] [Google Scholar]
- 9.Chia VM, et al. Risk of cancer in first- and second-degree relatives of testicular germ cell tumor cases and controls. Int J Cancer. 2009;124:952–7. doi: 10.1002/ijc.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimdal K, et al. Risk of cancer in relatives of testicular cancer patients. Br J Cancer. 1996;73:970–3. doi: 10.1038/bjc.1996.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonneveld DJ, et al. Familial testicular cancer in a single-centre population. Eur J Cancer. 1999;35:1368–73. doi: 10.1016/s0959-8049(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 12.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99:260–6. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 13.Neale RE, Carriere P, Murphy MF, Baade PD. Testicular cancer in twins: a meta-analysis. Br J Cancer. 2008;98:171–3. doi: 10.1038/sj.bjc.6604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow AJ, De Stavola BL, Swanwick MA, Maconochie NE. Risks of breast and testicular cancers in young adult twins in England and Wales: evidence on prenatal and genetic aetiology. Lancet. 1997;350:1723–8. doi: 10.1016/s0140-6736(97)05526-8. [DOI] [PubMed] [Google Scholar]
- 15.Braun MM, Ahlbom A, Floderus B, Brinton LA, Hoover RN. Effect of twinship on incidence of cancer of the testis, breast, and other sites (Sweden) Cancer Causes Control. 1995;6:519–24. doi: 10.1007/BF00054160. [DOI] [PubMed] [Google Scholar]
- 16.Kanetsky PA, et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet. 2009;41:811–5. doi: 10.1038/ng.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapley EA, et al. A genome-wide association study of testicular germ cell tumor. Nat Genet. 2009;41:807–10. doi: 10.1038/ng.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbull C, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42:604–7. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanetsky PA, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20:3109–17. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlin A, et al. Variants in KITLG predispose to testicular germ cell cancer independently from spermatogenic function. Endocr Relat Cancer. 2012;19:101–8. doi: 10.1530/ERC-11-0340. [DOI] [PubMed] [Google Scholar]
- 21.Dalgaard MD, et al. A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation. J Med Genet. 2012;49:58–65. doi: 10.1136/jmedgenet-2011-100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajpert-de Meyts E, Hoei-Hansen CE. From gonocytes to testicular cancer: the role of impaired gonadal development. Ann N Y Acad Sci. 2007;1120:168–80. doi: 10.1196/annals.1411.013. [DOI] [PubMed] [Google Scholar]
- 23.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum Genet. 2011;130:59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42:570–5. doi: 10.1038/ng.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moniot B, et al. Hematopoietic prostaglandin D synthase (H-Pgds) is expressed in the early embryonic gonad and participates to the initial nuclear translocation of the SOX9 protein. Dev Dyn. 2011;240:2335–43. doi: 10.1002/dvdy.22726. [DOI] [PubMed] [Google Scholar]
- 26.Park JM, et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Res. 2007;67:881–9. doi: 10.1158/0008-5472.CAN-05-3767. [DOI] [PubMed] [Google Scholar]
- 27.Rowbotham SP, et al. Maintenance of silent chromatin through replication requires SWI/SNF-like chromatin remodeler SMARCAD1. Mol Cell. 2011;42:285–96. doi: 10.1016/j.molcel.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Costelloe T, et al. The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature. 2012;489:581–4. doi: 10.1038/nature11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoor M, Schuster-Gossler K, Roopenian D, Gossler A. Skeletal dysplasias, growth retardation, reduced postnatal survival, and impaired fertility in mice lacking the SNF2/SWI2 family member ETL1. Mech Dev. 1999;85:73–83. doi: 10.1016/s0925-4773(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 30.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–70. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–82. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Cheng L, et al. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 34.Clark AT. The stem cell identity of testicular cancer. Stem Cell Rev. 2007;3:49–59. doi: 10.1007/s12015-007-0002-x. [DOI] [PubMed] [Google Scholar]
- 35.Koster R, et al. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest. 2010;120:3594–605. doi: 10.1172/JCI41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skotheim RI, et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65:5588–98. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 37.Altshuler DM, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–8. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Sun H, Tomchick DR, Yu H, Luo X. Structure of human Mad1 C-terminal domain reveals its involvement in kinetochore targeting. Proc Natl Acad Sci U S A. 2012;109:6549–54. doi: 10.1073/pnas.1118210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, et al. Functional evaluation of missense variations in the human MAD1L1 and MAD2L1 genes and their impact on susceptibility to lung cancer. J Med Genet. 2010;47:616–22. doi: 10.1136/jmg.2009.074252. [DOI] [PubMed] [Google Scholar]
- 40.Iwanaga Y, Kasai T, Kibler K, Jeang KT. Characterization of regions in hsMAD1 needed for binding hsMAD2. A polymorphic change in an hsMAD1 leucine zipper affects MAD1-MAD2 interaction and spindle checkpoint function. J Biol Chem. 2002;277:31005–13. doi: 10.1074/jbc.M110666200. [DOI] [PubMed] [Google Scholar]
- 41.Fu X, et al. RFWD3-Mdm2 ubiquitin ligase complex positively regulates p53 stability in response to DNA damage. Proc Natl Acad Sci U S A. 2010;107:4579–84. doi: 10.1073/pnas.0912094107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, et al. RING finger and WD repeat domain 3 (RFWD3) associates with replication protein A (RPA) and facilitates RPA-mediated DNA damage response. J Biol Chem. 2011;286:22314–22. doi: 10.1074/jbc.M111.222802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Zeller T, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ihara M, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–52. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Kissel H, et al. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–64. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Greenbaum MP, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103:4982–7. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuznetsov S, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J Cell Biol. 2007;176:581–92. doi: 10.1083/jcb.200608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JY, et al. Spermiogenesis and exchange of basic nuclear proteins are impaired in male germ cells lacking Camk4. Nat Genet. 2000;25:448–52. doi: 10.1038/78153. [DOI] [PubMed] [Google Scholar]
- 51.Karlberg S, Tiitinen A, Lipsanen-Nyman M. Failure of sexual maturation in Mulibrey nanism. N Engl J Med. 2004;351:2559–60. doi: 10.1056/NEJM200412093512423. [DOI] [PubMed] [Google Scholar]
- 52.Meindl A, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–4. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 53.Loveday C, et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44:475–6. doi: 10.1038/ng.2224. author reply 476. [DOI] [PubMed] [Google Scholar]
- 54.Mondal G, Ohashi A, Yang L, Rowley M, Couch FJ. Tex14, a Plk1-regulated protein, is required for kinetochore-microtubule attachment and regulation of the spindle assembly checkpoint. Mol Cell. 2012;45:680–95. doi: 10.1016/j.molcel.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishida A, Sueyoshi N, Shigeri Y, Kameshita I. Negative regulation of multifunctional Ca2+/calmodulin-dependent protein kinases: physiological and pharmacological significance of protein phosphatases. Br J Pharmacol. 2008;154:729–40. doi: 10.1038/bjp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avela K, et al. Gene encoding a new RING-B-box-Coiled-coil protein is mutated in mulibrey nanism. Nat Genet. 2000;25:298–301. doi: 10.1038/77053. [DOI] [PubMed] [Google Scholar]
- 57.Karlberg S, et al. Testicular failure and male infertility in the monogenic Mulibrey nanism disorder. J Clin Endocrinol Metab. 2011;96:3399–407. doi: 10.1210/jc.2011-1493. [DOI] [PubMed] [Google Scholar]
- 58.Atkin NB, Baker MC. Specific chromosome change i(12p) in testicular tumors? Lancet. 1982;2:1349. doi: 10.1016/s0140-6736(82)91557-4. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez E, et al. Cytogenetic analysis of 124 prospectively ascertained male germ cell tumors. Cancer Res. 1992;52:2285–91. [PubMed] [Google Scholar]
- 60.Skotheim RI, et al. Novel genomic aberrations in testicular germ cell tumors by array-CGH, and associated gene expression changes. Cellular Oncology. 2006;28:315–26. doi: 10.1155/2006/219786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schumacher FR, et al. Testicular germ cell tumor susceptibility associated with the UCK2 locus on chromosome 1q23. Hum Mol Genet. doi: 10.1093/hmg/ddt109. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fearnhead P. SequenceLDhot: detecting recombination hotspots. Bioinformatics. 2006;22:3061–6. doi: 10.1093/bioinformatics/btl540. [DOI] [PubMed] [Google Scholar]
- 63.Fearnhead P, Donnelly P. Approximate likelihood methods for estimating local recombination rates. Journal of the Royal Statistical Society Series B-Statistical Methodology. 2002;64:657–680. [Google Scholar]
- 64.Crawford DC, et al. Evidence for substantial fine-scale variation in recombination rates across the human genome. Nat Genet. 2004;36:700–6. doi: 10.1038/ng1376. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Stephens M. Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism data. Genetics. 2003;165:2213–33. doi: 10.1093/genetics/165.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luna A, Nicodemus KK. snp.plotter: an R-based SNP/haplotype association and linkage disequilibrium plotting package. Bioinformatics. 2007;23:774–6. doi: 10.1093/bioinformatics/btl657. [DOI] [PubMed] [Google Scholar]
- 67.Cox A, et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet. 2007;39:352–8. doi: 10.1038/ng1981. [DOI] [PubMed] [Google Scholar]
- 68.Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br J Cancer. 2004;90:1765–70. doi: 10.1038/sj.bjc.6601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–7. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.