Abstract
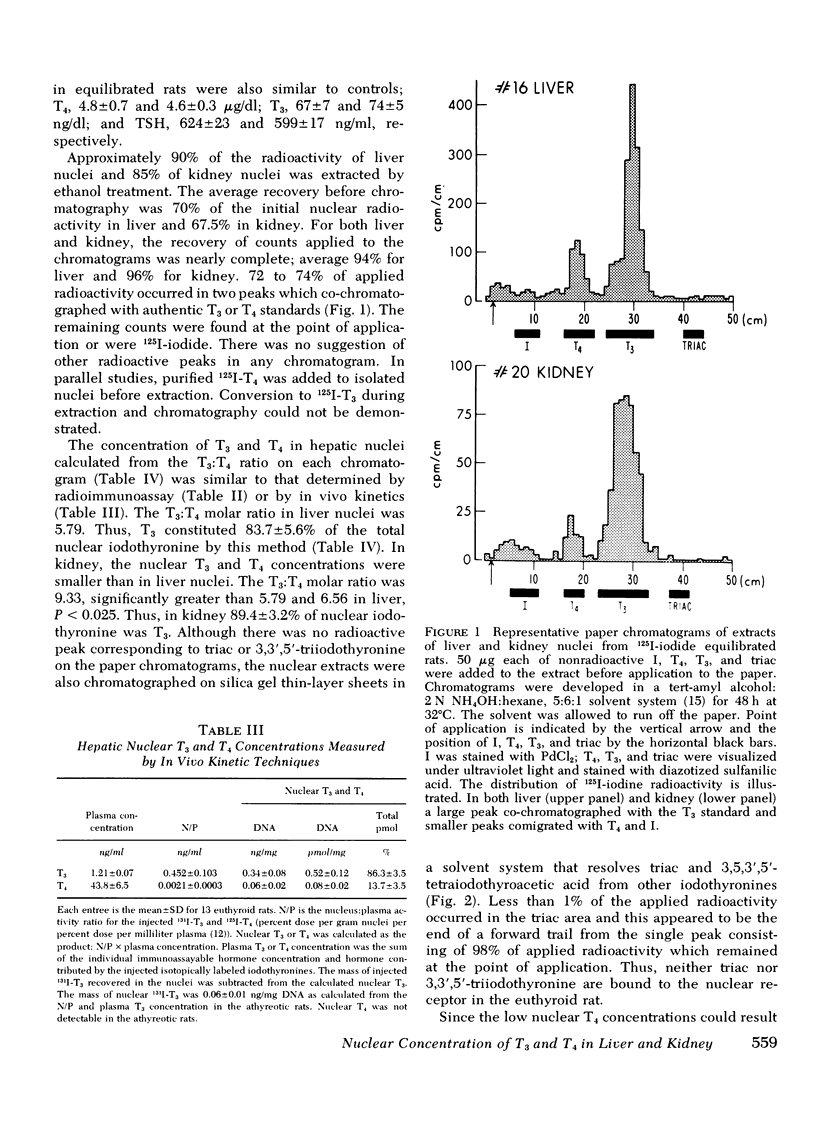
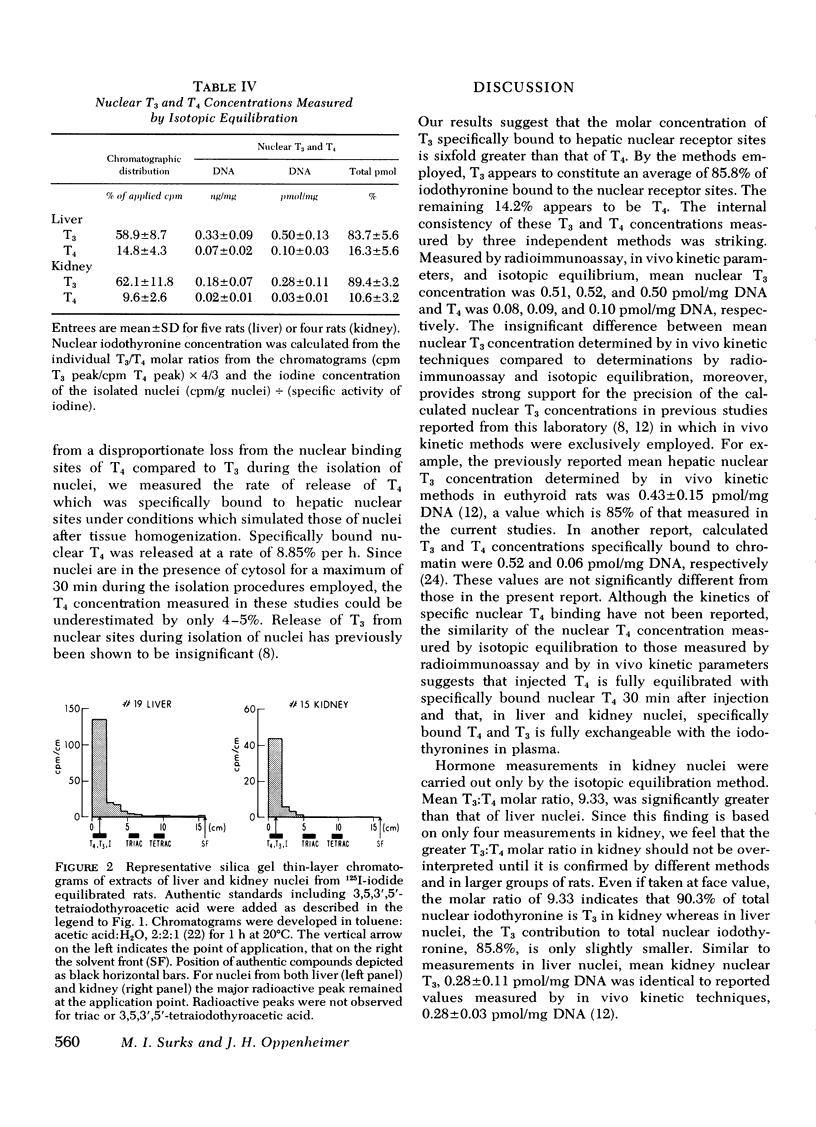
To estimate the relative contribution of l-triiodothyronine (T3) and l-thyroxine (T4) to thyroidal effects, we have measured the concentration of iodothyronine bound to specific hepatic nuclear receptor sites by three different techniques: (a) specific radioimmunoassay after separation of T3 and T4 by preparative paper chromatography; (b) in vivo kinetic approaches as reported previously; and (c) isotopic equilibration. By these three methods, receptor concentration of T3 and T4 in liver was 0.51±0.19 (SD) and 0.08±0.06; 0.52±0.12 and 0.08±0.02; and 0.50±0.13 and 0.10±0.03 pmol/mg DNA, respectively. The percentage contribution of T3 and T4 to total receptor iodothyronine was thus 86.8±9.0 and 13.2±9.4; 86.3±3.5 and 13.7±3.5; and 83.7±5.6 and 16.3±5.6%, respectively. In kidney, specifically bound nuclear T3 and T4 were estimated both by isotopic equilibration and by in vivo kinetic techniques to be 0.28±0.11 and 0.03±0.01 pmol/mg DNA, respectively. Thus, T3 constituted 89.4±3.2% of total receptor iodothyronine in this tissue. No other iodothyronines or analogs were bound to the nuclear sites in either tissue. Kidney and liver nuclear T3 concentrations also were identical to values previously reported with in vivo kinetic techniques. Other studies from this laboratory have suggested that thyroid effect is related to the molar concentration of iodothyronine bound to specific nuclear sites, that the sites are similar in various tissues, and that iodothyronine in plasma is in equilibrium with nuclear T3. If these relationships are assumed, T3 contributes between 85 and 90% of thyroidal effects in the euthyroid rat. The remaining 10-15% of thyroidal effect appears to result from the intrinsic activity of T4.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman W., Surks M. I., Oppenheimer J. H. Quantitative aspects of iodothyronine binding by cytosol proteins of rat liver and kidney. Endocrinology. 1974 Aug;95(2):492–498. doi: 10.1210/endo-95-2-492. [DOI] [PubMed] [Google Scholar]
- FLOCK E. V., BOLLMAN J. L. The metabolism of thyroxine and triiodothyronine in the eviscerated rat. J Biol Chem. 1955 Jun;214(2):709–721. [PubMed] [Google Scholar]
- Goslings B., Schwartz H. L., Dillmann W., Surks M. I., Oppenheimer J. H. Comparison of the metabolism and distribution of L-triiodothyronine and triiodothyroacetic acid in the rat: a possible explanation of differential hormonal potency. Endocrinology. 1976 Mar;98(3):666–675. doi: 10.1210/endo-98-3-666. [DOI] [PubMed] [Google Scholar]
- HENINGER R. W., LARSON F. C., ALBRIGHT E. C. IODINE-CONTAINING COMPOUNDS OF EXTRATHYROIDAL TISSUES. J Clin Invest. 1963 Nov;42:1761–1768. doi: 10.1172/JCI104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Koerner D., Surks M. I., Oppenheimer J. H. In vitro demonstration of specific triiodothyronine binding sites in rat liver nuclei. J Clin Endocrinol Metab. 1974 Apr;38(4):706–709. doi: 10.1210/jcem-38-4-706. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Dockalova J., Sipula D., Wu F. M. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973 Aug;37(2):177–182. doi: 10.1210/jcem-37-2-177. [DOI] [PubMed] [Google Scholar]
- MONEY W. L., KUMAOKA S., RAWSON R. W., KROC R. L. Comparative effects of thyroxine analogues in experimental animals. Ann N Y Acad Sci. 1960 Apr 23;86:512–544. doi: 10.1111/j.1749-6632.1960.tb42827.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Dillman W., Surks M. I. Effect of thyroid hormone analogues on the displacement of 125I-L-triiodothyronine from hepatic and heart nuclei in vivo: possible relationship to hormonal activity. Biochem Biophys Res Commun. 1973 Dec 10;55(3):544–550. doi: 10.1016/0006-291x(73)91177-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Newman E. S. Conversion of thyroxine to triiodothyronine in normal human subjects. Science. 1970 Sep 11;169(3950):1099–1100. doi: 10.1126/science.169.3950.1099. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O. Thyroid hormone binding by a component of mitochondrial membrane. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3225–3229. doi: 10.1073/pnas.72.8.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Koerner D., Dillman W., Oppenheimer J. H. Limited capacity binding sites for L-triiodothyronine in rat liver nuclei. Localization to the chromatin and partial characterization of the L-triiodothyronine-chromatin complex. J Biol Chem. 1973 Oct 25;248(20):7066–7072. [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Incomplete suppression of thyrotropin secretion after single injection of large L-triiodothyronine doses into hypothyroid rats. Endocrinology. 1976 Dec;99(6):1432–1441. doi: 10.1210/endo-99-6-1432. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Oppenheimer J. H. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972 Dec;51(12):3104–3113. doi: 10.1172/JCI107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Weinbach S., Volpert E. M. On the identfication of 4-hydroxy-3,5-diiodophenylpyruvic acid in rat thyroid glands. Endocrinology. 1968 Jun;82(6):1156–1162. doi: 10.1210/endo-82-6-1156. [DOI] [PubMed] [Google Scholar]
- Tata J. R. How specific are nuclear "receptors" for thyroid hormones? Nature. 1975 Sep 4;257(5521):18–23. doi: 10.1038/257018a0. [DOI] [PubMed] [Google Scholar]
- Westerfeld W. W., Richert D. A., Ruegamer W. R. New assay procedure for thyroxine analogs. Endocrinology. 1965 Nov;77(5):802–811. doi: 10.1210/endo-77-5-802. [DOI] [PubMed] [Google Scholar]


