Abstract
Obesity is a metabolic disorder and fundamental cause of other fatal diseases including atherosclerosis and cancer. One of the main factor that contributes to the development of obesity is high-fat (HF) consumption. Lipid ingestion will initiate from the gut feedback mechanisms to regulate glucose and lipid metabolisms. But these lipid-sensing pathways are impaired in HF-induced insulin resistance, resulting in hyperglycemia. Besides that, duodenal lipid activates mucosal mast cells, leading to the disruption of the intestinal tight junction. Lipopolysaccharide that is co-transited with dietary fat postprandially, promotes the release of cytokines and the development of metabolic syndrome. HF-diet also alters microbiota composition and enhances fat storage. Although gut is protected by immune system and contains high level of antioxidants, obesity developed presumably when this protective mechanism is compromised by the presence of excessive fat. Several therapeutic approaches targeting different pathways have been proposed. There may be no one single most effective treatment, but all aimed to prevent obesity. This review will elaborate on the physiological and molecular changes in the gut that lead to obesity, and will provide a summary of potential treatments to manage these pathophysiological changes.
OVERVIEW
The increased prevalence of obesity and obesity-associated complications continues to be a major global health issue. This is due mainly to the cause of obesity that is multifactorial. High-fat (HF), caloric-dense diets, sedentary lifestyles, increased urbanization and psychosocial stress are the most common contributing factors.1 Obesity itself predisposes one to other diseases such as type 2 diabetes mellitus, hypertension, dyslipidemia, metabolic syndrome (MetS), atherosclerosis, degenerative joint disorders and cancer.2 The prevalence of MetS increases with the severity of obesity and reaches 50% in morbidly obese youngsters.3 Effective treatment with better prognosis can be achieved if we have a comprehensive understanding of the pathogenesis of the metabolic disorder. Most studies so far focus on relating aberrant gene expression in the liver and adipose tissues to obesity, while less correlation is made between pathophysiological changes in the gut and obesity. In actual fact, emerging evidence have suggested that intestinal inflammation occurs during the development of obesity.
Inflammation in obese patients corresponds with increased plasma levels of C-reactive protein, tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemotactic protein-1, IL-8, leptin and osteopontin.4 Suggested by Spagnuolo et al.,5 a significant positive correlation between body mass index Z-score and C-reactive protein may lead to obesity-related complications. Obesity and diabetes alter the glucose-stimulated gut-to-hypothalamic nitric oxide signaling pathway. The disrupted enteric glucose detection is associated with a significant increase in inflammatory, oxidative/nitric oxide and endoplasmic reticulum stress gene expression.6 Signaling mechanisms reported to promote inflammation have also been found to be initiated and/or mediated by luminal lipid and gut microbiota. These observations have collectively suggested the importance of preserving gut function, and a greater understanding of the pathological state of the gut may be useful in finding new strategy to treat and to prevent obesity.
The review will first present different mechanisms in the gut that promote directly or mediate the development of obesity under the influence of HF-diet. These include descriptions of a number of established and hypothetical gut lipid-sensing systems that are present in the body to regulate glucose production. This is followed by addressing the impact of lipid-induced inflammation, and reporting data that linked these pathological changes to obesity. The review will then discuss the role of microbiota, which has been associated with inflammation-driven insulin resistance. Finally, the importance of gut antioxidants will be highlighted. This topic is of particular interest because the presence of antioxidants in the gut appears to be less appreciated compared with its other physiological functions such as metabolism and absorption. The second part of this review will summarize potential treatments proposed to manage these pathophysiological changes, namely to point out the effectiveness and feasibility of these therapies, and the use of dietary antioxidants as a probable direction for future treatment.
GUT LIPID-SENSING SYSTEMS
This section aimed to provide evidence about the presence of several lipid-sensing systems in the body, which has been linked to obesity and MetS development. Among the different types of stimulus, cholecystokinin (CCK) is the most established peptide hormone that initiates a gut–brain–liver neuronal mechanism. Lipids in the lumen stimulate the release of CCK from I cells that line the mucosa of the duodenum,7 which then binds to CCK-A receptor in the gastrointestinal system.8 The impact of CCK release was demonstrated in an in vivo experiment using CCK-8, which is an active form of CCK. By cannulating the gut and using pancreatic clamp techniques, intraduodenally administered CCK-8 reduces hepatic glucose production. The effect was reversed when CCK-A receptor inhibitor MK-329 was co-administered with CCK-8. The importance of CCK-A receptor in regulating glucose production is further evidenced when receptor-deficient rats become glucose intolerant, obese and eventually diabetic. The neuronal network involving CCK is the most well studied. Briefly, hepatic vagotomy, inhibition of gut vagal afferent neurons by anesthetic tetracaine administered into the duodenum, and inhibition of glutamatergic neurotransmission in the nucleus of the solitary tract by direct infusion of N-methyl-𝒟-aspartate receptor inhibitor MK-801 have all been shown to negate the ability of CCK-8 to lower glucose production. Clearly, the nucleus of the solitary tract is relaying a signal from the intestine to the liver for the effect on glucose production. Stimulation of this network is independent of changes in food intake, but a mere 3 days of HF feeding attenuates this lipid-sensing mechanism.9
Besides CCK, lipid was suggested to induce the production of oleoylethanolamide (OEA) in the mucosal layer of the duodenum and jejunum. OEA is then taken up by CD36, a membrane glycoprotein, which may act both as a receptor and a transporter. OEA within the enterocytes activates peroxisome proliferator-activated receptor-α and promotes satiety.10 It is reported recently that lipid-induced OEA mobilization in the gut is controlled by sympathetic nervous system. Surgical removal of the sympathetic innervation and pharmacological blockade of β2-adrenergic receptor abolish the effect of OEA.11 Taken together, if this hypothetical model is correct, OEA is hence an endogenous proliferator-activated receptor-α ligand where its synthesis can be modulated by pharmacological intervention. The effect of HF on this mechanism has not been demonstrated but HF alone is known to suppress proliferator-activated receptor-α.
In another study, when lipids are infused into the duodenal segment, the elevated long-chain fatty acyl-coenzyme A (LCFA-CoA) inhibits hepatic glucose production. With gut and hepatic vagotomy diminishing this effect12 support the existence of another gut–brain–liver sensing system. Carnitine palmitoyltransferase-1 (CPT1), located in the outer mitochondrial membrane, is regulating entry of LCFA-CoA into the organelle, where LCFA-CoA undergoes β-oxidation. However, upregulation of intestinal CPT1 as a result of HF feeding13 may not be helpful because the signaling effect of LCFA-CoA is disrupted in HF-diet-induced insulin-resistant and obese rodents.12 As for CPT1 expressed in the hypothalamus, an inhibition on the enzyme decreases food intake and glucose production.14 Nonetheless, the link between intestinal LCFA-CoA and hypothalamic CPT1 in the regulation of glucose homeostasis is currently unclear.
N-acylphosphatidylethanolamine (NAPE) is a class of phospholipids readily synthesized by small intestinal tissues when lipid is consumed. Circulatory NAPE at physiological doses reduces food intake in a dose-dependent manner.15 Via the method of radioactive labeling, NAPE was found capable of entering the central nervous system and accumulated in the hypothalamus. NAPE acts through the central nervous system because its hypophagia effect was observed in vagotomized rats.15 In the intestine, NAPE is hydrolyzed to N-acylethanolamines by enzyme NAPE-specific phospholipase D. However, animals fed with HF-diet containing variable percentages of fat have N-acylethanolamines decrease in a dose- and time-dependent manner.16 Similarly, chronic HF feeding significantly reduces postprandial NAPE secretion.15 In short, HF-diet attenuates the secretion of NAPE and N-acylethanolamines, which are supposed to be a feedback response to the presence of intestinal lipid.
Different from other sensing molecules, which are lipids, acyl CoA-monoacylglycerol acyltransferase-2 (MGAT2) is an interesting enzyme that controls obesity development. This enzyme catalyzes the conversion of fatty acyl CoA and monoacylglycerol to diacylglycerol. It is highly expressed in the proximal region of the small intestine, and is responsible for about 75% of triacylglycerol synthesis in the intestine.17 Chronic feeding with HF-diet increases intestinal expression of MGAT2. Conversely, MGAT2-deficient mice are protected from obesity, hyperinsulinemia, glucose intolerance, hypercholesterolemia and hepatic steatosis.13 Although the underlying mechanism leading to these effects are poorly understood, specific identification of this enzyme with its relevance to MetS parameter is an important finding for obesity treatment.
In summary, sufficient data have demonstrated that central nervous system bridges the function of two visceral organs in the regulation of blood glucose level. Despite the fact that extrapolating these animal data to human condition is not straightforward, it is only through animal study that one can understand the mechanisms involved and identify important targets for the development of more effective therapies for obesity. The presence of multiple gut–brain–liver mechanisms shows the complexity of the lipid-sensing system, which may have potentially increased the difficulty of managing obesity and obesity-related complications.
LUMINAL LIPID AND INFLAMMATION
Gastrointestinal tract (GIT) is the most common route of food and drug administration, which means GIT is generally the first organ to be exposed to attacks by pro-oxidants from the diet,18, 19, 20, 21, 22, 23, 24, 25, 26, 27 phagocytes activated by diet-derived bacteria and toxins,28 and the effects of HF-diet. All these factors cause inflammation. HF-diet in particular, is reported to activate mast cells and Toll-like receptors (TLRs).
In BALB/c mice, an in situ duodenal administration of butter rich in saturated fat has markedly increased the production of TNF-α by lamina proprial macrophages. TNF-α enhances lymphocyte adherence to microvessels of the mucosa.29 Cytokine at low level is beneficial as it modulates barrier functions and protects the epithelial layer. However, various LCFAs micelles (range between 10 and 50 μℳ) administered to lymphocytes (200 μl, 4 × 105 per well) have dose-dependently inhibited the production of interferon-γ.30 Incubation of LCFAs (0.01 to 0.1 mℳ) with epithelial cells increases the secretion of growth-regulated oncogene/cytokine-induced neutrophil chemoattractant-1 and IL-6,31 also in a dose-dependent manner. This means that LCFAs correlate positively with cytokine production, thereby onset of inflammation.
Intraduodenal infusion of polyunsaturated fatty acid-rich lipid emulsion activates intestinal mucosal mast cells, causing the release of histamine, rat mast cell protease-II and prostaglandin D2.32 The physiological significance of the activation of mast cells during fat absorption is not known. It is possible that by increasing lymph flow and vascular permeability, histamine facilitates the passage of chylomicrons particles through the lamina propria.32 Rat mast cell protease-II may increase epithelial permeability by suppressing the expression of tight junction-associated proteins zonula occludens-1 and occludin.33 Moreover, rat mast cell protease-II is specific for mucosal mast cells and selectively attacks type IV collagen in the basement membranes.34 Of note, an interrupted tight junction has been observed in obesity.35
Bacterial endotoxin or lipopolysaccharide (LPS) is present in large quantity in the gut. It is hypothesized by Erridge et al.36 that a small amount of LPS may co-transit with dietary fat from the gut postprandially, and activates leukocyte and endothelial cells. Subcutaneous LPS infusion induces several features of MetS in mice and triggers the release of inflammatory factors.37 In addition, obese-prone rats treated with HF-diet exhibit an upregulation of TLR4 in the gut wall and a decrease in the intestinal alkaline phosphatase activity.35 The intestinal alkaline phosphatase is a duodenal brush-border enzyme that detoxifies LPS.38 Binding of LPS39 and non-esterified free fatty acids40 to TLR4 initiates the secretion of various pro-inflammatory cytokines from the macrophages, adipocytes and liver. Conversely, TLR4-deficient mice are partially protected from non-esterified free fatty acid-induced insulin resistance.40 Similarly, CD14 mutant mice challenged with a HF-diet or given an LPS treatment, resisted cytokine production, hypertriglyceridemia, insulin resistance, obesity and visceral adiposity.37 With CD14 being a co-receptor of TLR4 suggests the presence of two binding sites on this receptor complex that controls insulin sensitivity as well as the development of MetS parameters.
Overall, luminal lipid by direct action on the enterocytes and through activation of mast cells, causes the release of cytokines. There is sufficient evidence to show that lipid induces inflammation in the course of obesity development. Further to this, Ding et al.41 are convinced that intestinal inflammation precedes and correlates with diet-induced obesity, adiposity and insulin resistance. They suggest that HF interact with gut microbiota to trigger the expression of TNF-α and nuclear factor-kappaB. The absence of microbiota in germ-free (GF) mice has blunted upregulation of these biomarkers, which are primary indicators of inflammation. With regard to this, our understanding on the onset of inflammation is not complete without mentioning the role and the effect of gut microbiota.
GUT MICROBIOTA
Already been reported almost a decade ago that although food consumption was significantly lesser, conventionally raised specific-pathogen free (CONV) mice have a 40% higher total body fat content than the GF mice. Interestingly, GF mice developed a 60% increase in their total body fat when being colonized with microbiota harvested from the cecums of CONV mice.42 The resistance of GF mice to diet-induced obesity is due mainly to the animal having a different genetic make up for lipid processing in the intestine.
Microbiota via carbohydrate response element-binding protein enhances uptake of monosaccharides and hepatic triglycerides production. Through the suppression of angiopoietin-like protein 4 (Fiaf), which is a lipoprotein lipase inhibitor, microbiota promotes incorporation of triglycerides into the adipocytes.42 On the contrary, Fiaf is highly expressed in GF mice. Fiaf induces peroxisomal proliferator-activated receptor coactivator-1α in the gastrocnemius muscle whereby this nuclear receptor increases mitochondrial fatty acid oxidation.43 High levels of phosphorylated AMP-activated protein kinase in GF mice also promote fatty acid oxidation in the skeletal muscle and insulin sensitivity.43
Furthermore, microbiota increases energy and lipid metabolism by upregulating genes involved in the tricarboxylic acid cycle and pyruvate metabolism in the liver.44 By acting via G protein-coupled receptor 41 (Gpr41), microbiota increases adiposity and leptin production. Gpr41-deficient mice demonstrate a decrease in peptide YY (PYY) secretion, and reductions in intestinal absorption and delivery of short-chain fatty acids, as well as hepatic lipogenesis.44 Including Gpr41, several other Gpr expressed on enteroendocrine cells have been identified as fat sensor that mediates the secretion of GI peptides. Activation of Gpr41 and Gpr43 by short-chain fatty acids leads to the release of PYY,45 whereas Gpr4046 and Gpr12047 via LCFA as ligand of these receptors, have been implicated in CCK release. Although these recent data shed light on the mechanism responsible for gut hormone release, precise mechanisms by which fat is sensed, and subsequent signaling pathways that lead to changes in GI function, energy intake, hence body weight, are still not well understood.
Microbiota is shaped by an innate immune system that helps to defend the host against infection by pathogenic microbes. TLR5, a component of the system and a transmembrane protein, is highly expressed on the intestinal mucosa. TLR5-deficient (T5KO) mice have significantly higher body masses than age-matched wild-type mice, exhibited sign of hyperphagia, have high production of pro-inflammatory cytokines in the adipose tissue, and developed other hallmark features of MetS. A broad-spectrum of antibiotics introduced to T5KO mice at the time of weaning for a period of 12 weeks, attenuated HF-diet-exaggerated MetS development. When T5KO microbiota is transplanted into the intestine of wild-type GF mice, the latter developed T5KO MetS phenotype.48 These data indicate that a change in microbiota composition increases the risk of metabolic disorder.
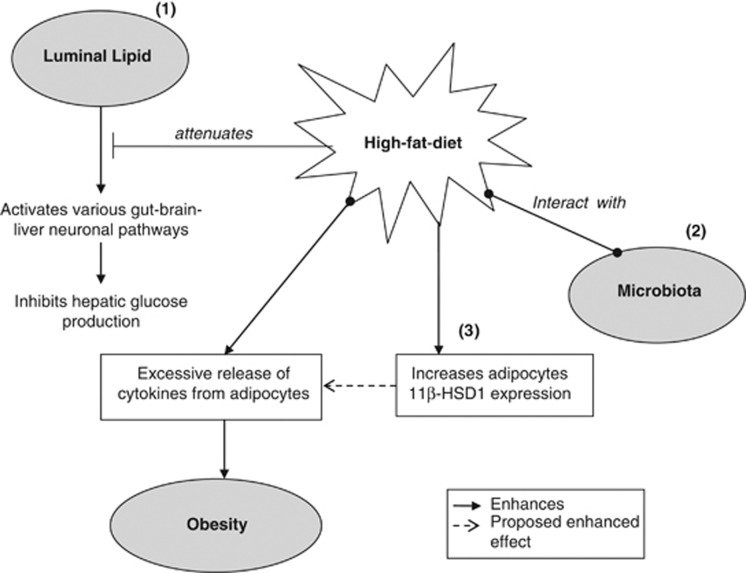
Recently, Serino et al.49 reported that metabolic adaptation to HF-diet is associated with a change in metabolic phenotype-specific gut microbiota, which is independent of genetic factor and diet. Effects exerted by this signature microbiota, such as increased production of adipokine, adipose cell size and stroma vascular fraction cell number, are facilitated by increased paracellular permeability in the ileum and cecum of the HF-fed mice. There has also been suggestion that microbiota activates vagal sensory neurons and satiety center. However, data describing the effect of microbiota-brain axis on feeding behavior is still lacking,50 thus will not be discussed in this review. Figure 1 highlights the effects of HF-diet, by which HF through multiple mechanisms, disrupts glucose homeostasis and mediates the development of obesity.
Figure 1.
A multipronged mechanism that contributes to obesity development. (1) Luminal lipid activates gut lipid-sensing mechanisms to lower hepatic glucose production and maintain glucose homeostasis. These signaling pathways are suppressed by high-fat (HF) diet. (2) Interaction between microbiota and HF leads to the excessive release of adipocytokines, consequently obesity development. (3) As HF-diet increases 11β-HSD1 expression in the adipose tissues, which in turn promotes visceral obesity, it is likely that 11β-HSD1 enhances the release of adipocytokines, resulting in an effect similar to that observed between HF and microbiota. 11β-HSD1, 11β-hydroxysteroid dehydrogenase-1.
One of the components of the gut that is frequently neglected in most reviews is gut antioxidants. As GIT is the first organ exposed to exogenous compound, microorganism and free radicals, other than the immune system, GIT is expected to possess high levels of endogenous antioxidants, which act to protect itself against free radicals and to filter out pro-oxidants from entering the circulation.23 With many studies reporting the effectiveness and the use of natural antioxidants to prevent and ameliorate obesity, it might be worthy to better understand and explore the role of gut antioxidants in this context.
GUT ANTIOXIDANTS
Enterochromaffin cells of the mucosal epithelium are the major source of melatonin in the body.51 The level observed was about 400 times greater than the pineal gland.52 Melatonin is a powerful antioxidant, a free radical scavenger, and probably the only endogenous gut antioxidant having well-defined properties. Its ability to scavenge a myriad of radical species protects GIT against lipid peroxidation, oxidative DNA and protein damages.53 Melatonin has anti-aging, anti-carcinogenic and antitumor effects,54 and it enhances immune function. Majority of these effects is mediated through MT1 receptor.55
Theoretically, gastroduodenal mucosal layer is protected from acid-induced injury by the existing protective mechanism, namely bicarbonate and mucus secreted by the superficial epithelial cells. Intracerebroventricular infusion of the α1-selective adrenoceptor agonist, phenylephrine, causes significant release of melatonin56 and bicarbonate56, 57 from the duodenal mucosa. MT2 inhibition by luzindole (600 nmol/kg, intravenous), blocks bicarbonate56, 58 but not melatonin secretion.56 This suggests that melatonin-stimulated bicarbonate secretion is mediated via the central nervous system.56 Melatonin injection (10 mg/kg, intraperitoneal) before physical and psychological stress59, 60 protects the animals from mucosal degeneration, inflammatory cell infiltration and generation of free radicals in the gastrointestinal tissues. Daily injection of melatonin (0.5 mg/kg, subcutaneous) for 12 days also reverses body weight gain and food intake, which were significantly higher in pinealectomy than sham-operated rats.61 These favorable outcomes have important impact because psychosocial stress has increasingly been linked to inflammation and obesity.
Apart from melatonin and possibly many other less characterized endogenous antioxidants, gut may gain protection from dietary antioxidants. Flavonoids are the most widely distributed plant antioxidants. They have low bioavailability, with high concentration being observed in the stomach and intestinal lumen.62, 63, 64, 65 The low bioavailability of flavonoids has long been known to be attributed to the compounds hydrophilicity. The glycosylated flavonoids need to be hydrolyzed by bacteria before absorption.66 Non-flavonoids such as vitamin E and carotenoids, and water-soluble antioxidants are also found at high concentration in the mucosa of the avian species.67 Flavonoids and other phenolic compounds exert direct protective effects on GIT by scavenging reactive oxygen and the chlorine species.68
FUTURE TREATMENT
A wide range of treatments has been proposed to correct pathophysiological changes in the gut as a result of HF consumption. This will be discussed in order following the etiology of the disorder presented above.
There are several approaches to overcome suppression of the gut–brain–liver sensing mechanism. These include upregulation of CCK-A receptor expression, using a CCK-A receptor agonist and modulate signaling proteins that act along the CCK/CCK-A receptor G protein-coupled signaling pathway.9 With regard to the OEA-mediated local signaling pathway, there have been suggestions to increase the expression of CD36 and magnify OEA, either from diet or through inhibition of OEA degradation.10 Alternatively, central or intestinal inhibition of CPT1 by genetic and biochemical modification,14 and increases dietary LCFA-CoA.12 Other proposals include the use of long-acting NAPE analogs15 and MGAT2 inhibitor.13 However, since the concept of lipid-sensing-regulated glucose production is relatively new, more study is required to demonstrate the effectiveness and practicality of these therapies. Study should establish both the toxicity and pharmacokinetics profiles of new drugs that act on the neuronal axis. Other studies such as verification of whether regulating a singular sensing pathway is more ideal or combining pathways will give an additive effect; and comparing the efficacy between established treatments and regulation of lipid-sensing system remained to be explored.
One of the main consequence of HF ingestion is causing significant disruption to the tight junction,69, 70 possibly through activation of mast cells. In this regard, the use of prebiotics and probiotics has some advantages. Prebiotics are mainly non-digestible fibers. They improve the distribution of occludin and zonula occludens-1, and promote normalization of the endocannabinoid system responsiveness in the gut, thereby reduce gut permeability, metabolic endotoxemia and fat mass development.71 Similarly, probiotics, the live microorganisms, are capable of maintaining epithelial permeability. They avert the re-arrangement of tight junction proteins, upregulate the expression of claudins and occludins, and normalize the barrier function.72, 73, 74 However, both prebiotics and probiotics do not improve metabolic profiles in obese rodents.75, 76 Mast cells stabilizers disodium cromoglycate and ketotifen have been shown to reduce body weight gain and abdominal fat mass in diet-induced obese mice. These animals also have reduced inflammatory cytokines, chemokines and proteases in the serum and white adipose tissues, and have shown better glucose tolerance and insulin sensitivity. Similar observations are found in mast cell-deficient mice fed a Western diet.77
There are multiple ways to tackle changes in the composition of microbiota. Bifidobacteria are beneficial to the gut because they reduce intestinal LPS and improve mucosal barrier function.78 Prebiotics restore the number of bifidobacteria,79 which was decreased by HF feeding.78 Young male rats treated for 10 weeks with diets containing 10 and 20% of prebiotic inulin:oligofructose (w/w) had a dose-dependent increase in Bifidobacterium spp. and Lactobacillus spp.76 The effects of prebiotics on improving gut barrier function and inflammation are partly through upregulation of GLP2, thus suggesting the potential use of GLP2 to prevent metabolic endotoxemia and metabolic disorders.80 Co-secretion of GLP1 and GLP2 is implicated to decrease circulatory LPS and cytokines.71 On the other hand, vancomycin75 significantly corrects microbiota composition altered in HF-treated animals. The subsequent effects include restoration of intestinal permeability, lowering of plasma LPS, attenuation of adipocyte inflammation and oxidative stress, and improvement in MetS parameters.81 Despite these therapies, it is necessary to note that the composition of the gut microbiome is dynamic and adaptable. To achieve high efficacy, treatment needs to be specific. This also poses the question of whether targeting microbiota is a realistic approach. There are suggestions that antibiotics affect epithelial integrity, and widespread use of antibiotics in early life may cause obesity.82 Moreover, as to whether long-term ingestion of a broad spectrum of antibiotics will lead to other disorders is still not known. Hence, it is probably preferable to specifically target on proteins that regulate the development of obesity. For example, Fiaf and proliferator-activated receptor coactivator-1α to modulate fat storage;42, 43 Gpr41 (ref. 44), TLR4, CD14 (refs 37,39) and TLR5 (ref. 48) to prevent low-grade inflammation.
In terms of enzymatic approach, the more established therapeutic target is 11β-hydroxysteroid dehydrogenase-1 (11β-HSD1). This enzyme converts inactive cortisone and 11-dehydrocorticosterone to the active cortisol in human and corticosterone in rats, respectively.83 In order to understand the mechanism of excessive glucocorticoids-induced growth of visceral fat, Matsuzaki et al.84 conducted an experiment using mice, which overexpress 11β-HSD1 selectively in the adipose tissues. These transgenic mice have increased corticosterone in the adipose tissues, developed visceral obesity and exhibited pronounced symptoms of MetS. There has been intensive research to identify and to produce highly selective 11β-HSD1 inhibitor. Activities include screening of potential compounds from natural resources and synthesizing the inhibitor through computer modeling strategy. A selective and specific reductase inhibitor will prevent side effects85 and visceral obesity.86 On a different note, carbohydrate metabolism has been linked to glucocorticoids formation and modification of 11β-HSD1 activity, whereby targeted inhibition of adipocyte glucose-6-phosphate transport and pentose pathway, reduce 11β-HSD1 activity. In other words, glucocorticoids formation may be controlled by carbohydrate metabolism.87 Taking this into account, it is probably necessary to simultaneously monitor energy expenditure when developing an 11β-HSD1 inhibitor.
Last but not least, the potential use of dietary antioxidants. The ability of antioxidants to restore and maintain normal gut physiology is receiving much attention lately. As reported by Jurgonski et al.,88 antioxidants improve glycemia, atherogenic index, antioxidant status and gut function. The authors show that ethanol extract of the chicory seeds containing 9.6% caffeoylquinic acids normalizes the total content of short-chain fatty acids in the cecal digesta, which was decreased in animals receiving high cholesterol and fructose diet. In another study, grape, which is rich in polyphenols, stimulates the proliferation of various species of Lactobacillus. Outcome of the study suggests that plant foods with high dietary fiber and polyphenols content enhance GIT health by promoting a beneficial microbiota profile.89 Meanwhile, antioxidant resveratrol given to HF-fed mice has helped to re-adjust the level of active GLP1 in the portal vein and intestine back to basal levels. Resveratrol also normalizes HF-diet-induced modification of cecal bacterial composition and decreases the elevated colonic TNF-α.90 Moreover, because melatonin increases the secretion of mucosa-protecting agents and prevents intestinal inflammation, food rich in serotonin and tryptophan may well be a non-pharmacological treatment to alleviate obesity. Table 1 summarizes treatments that may potentially counteract the effect of HF-diet on gut physiology.
Table 1. The pathophysiology of gut in obesity development and the proposed treatments for each disorder.
| Pathophysiology | Treatment options |
|---|---|
| Impaired gut lipid-sensing systems | To restore pathways by normalizing the specific protein and enzyme expression |
| Altered microbiota composition | Antibiotics |
| Prebiotics | |
| Disrupted barrier function | Prebiotics |
| Probiotics | |
| Antibiotics | |
| Mast cells stabilizers | |
| Increased 11β-HSD1 | Selective 11β-HSD1 inhibitor |
| Modulate carbohydrate metabolism pathway | |
| Altered microbiota status overrides the effects of gut antioxidants? | Dietary antioxidants? |
| Reported to increase: | |
| Lactobacillus | |
| Bifidobacteria | |
| Melatonin synthesis? | |
| Short-chain fatty acids | |
| Glucagon-like peptide-1 | |
| Reported effects: | |
| Reduce inflammation | |
| Preserve mucosal cell integrity | |
| Form mucosal protective layer | |
| Prevent obesity | |
| Reduce appetite | |
| Improve glycemia | |
| Improve atherogenic index | |
| Improve microbiota profile |
Abbreviation: 11β-HSD1, 11β-hydroxysteroid dehydrogenase-1.
It was hypothesized that the altered microbiota status has outcompeted the effects of gut antioxidants. With the widespread beneficial effects of antioxidants, increased consumption of dietary antioxidants may be effective to overcome the adversity and to prevent obesity.
PERSPECTIVES AND CONCLUSIONS
HF diet is one of the main causes of obesity development. Since gut is the first line of absorption, modulating molecular pathways that are activated by luminal lipids is probably a more effective mechanism than targeting other tissues in the body. Studies of the gut–brain–liver neuronal axis have shown that excessive fat impairs the signaling mechanism and hepatic glucose homeostasis. In addition, HF diet increases the release of TNF-α from the gut, alters mucosal immunity, activates mast cells, increases vascular permeability, and disrupts the intestinal basement membranes. Absorption of dietary fat enables uptake of LPS, which further increases the secretion of pro-inflammatory cytokines and intestinal permeability. Gut microbiota have received considerable interest over the last decade, seemingly due to increasing evidence of microbiota regulating fat storage, and are playing a pivotal role in governing obesity development. More important, HF diet promotes inflammation and obesity by interacting with and altering gut microbiota composition.
With reference to studies of gut microbiota, antibiotics are without doubt an obvious and straightforward choice of treatment to restore the altered microbiota profile. However, as the first line of body defense against harmful pathogens, GIT is arguably the largest immune system in our body. Gut is also presumably having high levels of endogenous and exogenously acquired antioxidants. These findings collectively indicate the coexistence of intestinal defense mechanisms and microbiota, working in concert in maintaining mucosal homeostasis. Therefore, it is tempting to suggest that one of the reasons for obesity development is the changes in the microbiota composition superceding the protective mechanism that the gut possesses. This may be similar to gastric ulcer that occurs as a result of an imbalance between acid and bicarbonate secretion. Unless the cause of obesity is confounded by genetic factors, dietary antioxidants, which may preserve gut function and restore the anatomical and physiological changes in the gut, might be an area that is worthwhile to explore further. The effectiveness of dietary antioxidants in this context is shown in the recent literature. In addition to their ability to promote the normal physiological function of the gut, dietary antioxidants are expected to be devoid of side effects that may accompany the use of antibiotics.
Study Highlights

Guarantor of the article: Chooi Y. Lee, PhD.
Specific author contributions: Chooi Y. Lee is the sole author of the manuscript.
Financial support: None.
Potential competing interests: None.
References
- McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Nguyen XT, Lane J, Smith BR, et al. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance – a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Spagnuolo MI, Cicalese MP, Caiazzo MA, et al. Relationship between severe obesity and gut inflammation in children: what's next. Ital J Pediatr. 2010;36:66–71. doi: 10.1186/1824-7288-36-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duparc T, Naslain D, Colom A, et al. Jejunum inflammation in obese and diabetic mice impairs enteric glucose detection and modifies nitric oxide release in the hypothalamus. Antioxid Redox Signal. 2011;14:415–423. doi: 10.1089/ars.2010.3330. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- Wank S.Cholecystokinin receptors Am J Physiol 1995269Gastrointest Liver Physiol 32G628–G646. [DOI] [PubMed] [Google Scholar]
- Cheung GWC, Kokorovic A, Lam CKL, et al. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Fu J, Astarita G, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, DiPatrizio NV, Guijarro A, et al. Sympathetic activity controls fat-induced oleoylethanolamide signaling in small intestine. J Neurosci. 2011;31:5730–5736. doi: 10.1523/JNEUROSCI.5668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PYT, Caspi L, Lam CKL, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- Yen CLE, Cheong ML, Grueter C, et al. Deficiency of the intestinal enzyme acyl CoA: monoacylglycerol acyltransferease-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Arduini A, et al. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- Gillum MP, Zhang D, Zhang X, et al. N-acylphosphatidylethanolamine, a gut-derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep TA, Madsen AN, Holst B, et al. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 2011;25:765–774. doi: 10.1096/fj.10-166595. [DOI] [PubMed] [Google Scholar]
- Yen CE, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- Srigiridhar K, Nair KM. Supplementation with alpha-tocopherol or a combination of alpha-tocopherol and ascorbic acid protects the gastrointestinal tract of iron-deficient rats against iron-induced oxidative damage during iron repletion. Br J Nutr. 2000;84:165–173. [PubMed] [Google Scholar]
- Brown ED, Morris VC, Rhodes DG, et al. Urinary malondialdehyde equivalents during ingestion of meat cooked at high or low temperatures. Lipids. 1995;30:1053–1056. doi: 10.1007/BF02536291. [DOI] [PubMed] [Google Scholar]
- Aw TY. Determinants of intestinal detoxication of lipid hydroperoxides. Free Radic Res. 1998;1925:637–646. doi: 10.3109/10715769809065819. [DOI] [PubMed] [Google Scholar]
- Gopaul NK, Zacharowski K, Halliwell B, et al. Evaluation of the postprandial effect of a fast-food meal on human plasma F2-isoprostane and lipid peroxide levels. Free Radic Biol Med. 2000;28:806–814. doi: 10.1016/s0891-5849(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Kanner J, Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31:1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Zhao K, Whiteman ML. The gastrointestinal tract: a major site of antioxidant action. Free Radic Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- Zhao K, Whiteman M, Spencer J, et al. DNA damage by nitrite and peroxynitrite: protection by dietary phenols. Methods Enzymol. 2001;335:296–307. doi: 10.1016/s0076-6879(01)35252-7. [DOI] [PubMed] [Google Scholar]
- Long LH, Lan ANB, Hsuan FTY, et al. Generation of hydrogen peroxide by ‘antioxidant' beverages and the effect of milk addition: is cocoa the best beverages. Free Radic Res. 1999;31:67–71. doi: 10.1080/10715769900300611. [DOI] [PubMed] [Google Scholar]
- Long LH, Halliwell B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human subjects. Free Radic Res. 2000;32:463–467. doi: 10.1080/10715760000300461. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Li X, Makimoto M. Identification of hydroxy-hydroquinone in coffee as a generator of reactive oxygen species that break DNA single strands. Mutat Res. 2001;419:43–51. doi: 10.1016/s1383-5718(98)00123-5. [DOI] [PubMed] [Google Scholar]
- Chamulitrat W. Activation of the superoxide-generating NADPH oxidase of intestinal lymphocytes produces highly reactive free radicals from sulfite. Free Radic Biol Med. 1999;27:411–421. doi: 10.1016/s0891-5849(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Fujiyama Y, Hokari R, Miura S, et al. Butter feeding enhances TNF-α production from macrophages and lymphocyte adherence in murine small intestinal microvessels. J Gastroenterol Hepatol. 2007;22:1838–1845. doi: 10.1111/j.1440-1746.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- Hara Y, Miura S, Komoto S, et al. Exposure to fatty acids modulates interferon production by intraepithelial lymphocytes. Immunol Lett. 2003;86:139–148. doi: 10.1016/s0165-2478(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Miura S, Kishikawa H, et al. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131:2943–2950. doi: 10.1093/jn/131.11.2943. [DOI] [PubMed] [Google Scholar]
- Ji Y, Sakata Y, Tso P. Nutrient-induced inflammation in the intestine. Curr Opin Clin Nutr Metab Care. 2011;14:315–321. doi: 10.1097/MCO.0b013e3283476e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore CL, Jepson MA, Hirst BH, et al. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occluding. Eur J Cell Biol. 1998;75:321–330. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- Patrick MK, Dunn IJ, Buret A, et al. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988;94:1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Austen WG, Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yeh W, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled reeptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Covasa M. Current and emerging concepts on the role of peripheral signals in the control of food intake and development of obesity. Br J Nutr. 2012;108:778–793. doi: 10.1017/S0007114512000529. [DOI] [PubMed] [Google Scholar]
- Liou AP, Lu X, Sei Y, et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Katsuma S, Adachi T, et al. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani AB, Nezami BG, Gewirtz A, et al. Obesity and its associated disease: a role for microbiota. Neurogastroenterol Motil. 2012;24:305–311. doi: 10.1111/j.1365-2982.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhlin N, Kvetnoy I. The synthesis of melatonin in the enterochromaffin cells. Archs Path. 1976;38:21–25. [PubMed] [Google Scholar]
- Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- Zavodnik IB, Domanski AV, Lapshina EA, et al. Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: chemiluminescence measurements and theoretical calculations. Life Sci. 2006;79:391–400. doi: 10.1016/j.lfs.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Popovich IG, Zabezhinski MA, et al. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006;1757:573–589. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Chen C, Fichna J, Bashashati M, et al. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011;17:3888–3898. doi: 10.3748/wjg.v17.i34.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom M, Flemstrom G. Central nervous α1-adrenoceptor stimulation induces duodenal luminal release of melatonin. J Pineal Res. 2004;36:103–108. doi: 10.1046/j.1600-079x.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- Larson GM, Jedstedt G, Nylander O, et al. Intracerebral adrenoceptor agonists influence rat duodenal mucosal bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 1996;271:G831–G840. doi: 10.1152/ajpgi.1996.271.5.G831. [DOI] [PubMed] [Google Scholar]
- Sjoblom M, Jedstedt G, Flemstrom G. Peripheral melatonin mediates neural stimulation of duodenal mucosal bicarbonate secretion. J Clin Invest. 2001;108:625–633. doi: 10.1172/JCI13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan F, Cetinel S, Contuk G, et al. Role of melatonin in reducing water avoidance stress-induced degeneration of the gastrointestinal mucosa. J Pineal Res. 2004;37:113–121. doi: 10.1111/j.1600-079X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- Khan R, Daya S, Potgieter B. Evidence for a modulation of the stress response by the pineal gland. Experientia. 1990;46:860–862. doi: 10.1007/BF01935539. [DOI] [PubMed] [Google Scholar]
- Canpolat S, Aydin M, Yasar A, et al. Effects of pinealectomy and exogenous melatonin on immunohistochemical ghrelin staining of arcuate nucleus and serum ghrelin levels in the rat. Neurosci Lett. 2006;410:132–136. doi: 10.1016/j.neulet.2006.09.071. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Yamaguchi M, Sobue T, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128:1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- Garsetti M, Pellegrini N, Baggio C, et al. Antioxidant activity in human faeces. J Nutr. 2000;84:705–710. [PubMed] [Google Scholar]
- Gee JM, Johnson IT. Polyphenolic compounds: interactions with the gut and implications for human health. Curr Med Chem. 2001;8:1245–1255. doi: 10.2174/0929867013372256. [DOI] [PubMed] [Google Scholar]
- Asfar S, Abdeen S, Dashti H, et al. Effect of green tea in the prevention and reversal of fasting-induced intestinal mucosal damage. Nutrition. 2003;19:536–540. doi: 10.1016/s0899-9007(02)01097-3. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser VD, Shackleton CHL, Winter J. Hydrolysis of dietary flavoniod glycosides by strains of intestinal Bacteroides from humans. Biochem J. 1987;248:953–956. doi: 10.1042/bj2480953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JA, Karadas F, Surai PF, et al. Lipid-soluble and water-soluble antioxidant activities of the avian intestinal mucosa at different sites along the intestinal tract. Comp Biochem Physiol Part B. 2005;141:366–372. doi: 10.1016/j.cbpc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not. Am J Clin Nutr. 2005;81 (Suppl:268S–276SS. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- Ventura MT, Polimeno L, Amoruso AC, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38:732–736. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Duckworth CA, Guan Y, et al. Mechanisms of epithelial cell shedding in the Mammalian intestine and maintenance of barrier function. Ann N Y Acad Sci. 2009;1165:135–142. doi: 10.1111/j.1749-6632.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microbial Cell Factories. 2011;10 (Suppl 1:S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigen R, Bruewer M. Effect of probiotics on intestinal barrier function. Ann N Y Acad Sci. 2009;1165:183–189. doi: 10.1111/j.1749-6632.2009.04059.x. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert SC, Barrett KE. Modulation of intestinal barrier properties by probiotics: role in reversing colitis. Ann N Y Acad Sci. 2009;1165:175–182. doi: 10.1111/j.1749-6632.2009.04042.x. [DOI] [PubMed] [Google Scholar]
- Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EF, Cotter PD, Hogan A, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 62:220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilisation of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xiao G, Yao Y, et al. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, de Wiele T Van, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt T, Chen R, Wang X, et al. Effect of diabetes on enzymes involved in rat hepatic corticosterone production. J Diabetes. 2010;2:275–281. doi: 10.1111/j.1753-0407.2010.00087.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- Sahni-Arya B, Flynn MJ, Bergeron L, et al. Cofactor-specific modulation of 11β-hydroxysteroid dehydrogenase 1 inhibitor potency. Biochim Biophys Acta. 2007;1774:1184–1191. doi: 10.1016/j.bbapap.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Bader T, Zoumakis E, Friedberg M, et al. Human adipose tissue under in vitro inhibition of 11β-hydroxysteroid dehydrogenase type 1: differentiation and metabolism changes. Horm Metab Res. 2002;34:752–757. doi: 10.1055/s-2002-38255. [DOI] [PubMed] [Google Scholar]
- McCormick KL, Wang X, Mick GJ. Evidence that the 11β-Hydroxysteroid dehydrogenase (11β-HSD1) is regulated by pentose pathway flux. J Biol Chem. 2006;281:341–347. doi: 10.1074/jbc.M506026200. [DOI] [PubMed] [Google Scholar]
- Jurgonski A, Juskiewicz J, Zdunczyk Z, et al. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition. 2012;28:300–306. doi: 10.1016/j.nut.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Pozuelo MJ, Agis-Torres A, Hervert-Hernandez D, et al. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. J Food Sci. 2012;77:H59–H62. doi: 10.1111/j.1750-3841.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- Anh Dao T, Waget A, Klopp P, et al. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS ONE. 2011;6:e20700. doi: 10.1371/journal.pone.0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]