Abstract
Aims:
To evaluate clinical profile and short-term outcome of psychogenic non-epileptic seizures (PNES) in Indian adult population.
Setting and Design:
A prospective observational study, conducted at tertiary teaching institute at New Delhi.
Materials and Methods:
Sixty-three patients with confirmed PNES were enrolled. The diagnosis was based on witnessing the event during video-electroencephalography (Video-EEG) monitoring. A detailed clinical evaluation was done including evaluation for coexistent anxiety or depressive disorders. Patients were divided into two groups on the basis of excessive or paucity of movements during PNES attacks. Patients were followed-up to 12 months for their PNES frequency.
Statistical Analysis:
Means and standard deviations were calculated for continuous variables. Chi-square and Students t-test were used to compare categorical and continuous variables respectively.
Results:
The mean age at onset of PNES was 25.44 years; with F:M ratio of 9.5:1. Coexistent epilepsy was present in 13 (20.63%) cases. Twenty-two patients (44%) with only PNES (n = 50) had received antiepileptic drugs. Out of 63 patients of PNES 24 (38.1%) had predominant motor phenomenon, whereas 39 (61.9%) had limp attacks. The common features observed were pre-ictal headache, ictal eye closure, jaw clenching, resistant behavior, ictal weeping, ictal vocalization, and unresponsiveness during episodes. Comorbid anxiety and depressive disorders was seen in 62.3% and 90.16% patients, respectively. Short-term (6-12 months) outcome of 45 patients was good (seizure freedom in 46.66% and >50% improvement in 24.44% cases).
Conclusion:
PNES is common, but frequently misdiagnosed and treated as epileptic seizures. A high index of suspicion is required for an early diagnosis. Proper disclosure of diagnosis and management of the psychiatric comorbidities can improve their outcome.
Limitation:
Limited sample size and change in seizures frequency as the only parameter for the assessment of the outcome are the two major limitations of our study.
Keywords: Psychogenic non-epileptic seizures, Coexistent epilepsy, video-electroencephalography
Introduction
Non-epileptic seizures (NES) are episodes of altered movement, sensation, or experience resembling epileptic seizures, without ictal electrical discharges in the brain.[1] The physiological causes of NES include convulsive syncope, paroxysmal vertigo, sleep disorders, panic attacks, movement disorders, transient ischemic attacks, complicated migraine, metabolic disorders, and drug intoxication or withdrawal.[2,3] Psychogenic non-epileptic seizures (PNES) are the commonest non-epileptic events; represents 20-30% of patients being referred for epilepsy surgery evaluation.[4] The incidence of PNES is estimated to be 1.5-3/lakh/year[5,6] and the prevalence ranges from 2-33 cases/lakh persons in the general population.[7,8] PNES occur more frequently in women (75-85%); with onset in young adulthood.[1,9] The prevalence data of coexistent epilepsy and PNES is variably estimated between 5% and 40%.[10–12]
PNES are categorized as Dissociative (International Classification of Diseases; ICD-10) or Conversion disorder (Diagnostic and Statistical Manual of Mental Disorders; DSM-IV). The major psychiatric comorbidities are somatoform, depressive, anxiety, post-traumatic stress, and personality disorders.[13,14] There are various pre-disposing (sexual or physical abuse, childhood trauma, head injury, intellectual disability, dysfunctional family, borderline-like personality, other psychopathology, defective illness perceptions and coping styles etc.), precipitating (emotional or physical stress, onset of new illnesses, life events, relationship conflicts etc.) and perpetuating factors (frequent health-care contacts, social or financial gain) have been associated with PNES.[15–19]
PNES can imitate any type of seizure event and sometimes may be confused with frontal lobe seizures.[20–23] Owing to clinical variability there is no well-established diagnostic criteria and clinical classification. The historical facts, ability to be induced by suggestion, psychological tests, serum prolactin, and ambulatory EEG are insufficient for the diagnosis of PNES.[24–27] The demonstration of clinical event without concomitant EEG changes during video-EEG is the diagnostic gold standard.[23,28]
PNES requires a very different approach for management and misdiagnosis places the patient at risk of increased morbidity and mortality. The indirect effect through loss of quality of life is much more than direct costs of drugs and investigations. Early diagnosis, proper disclosure of diagnosis, and comprehensive management by psychotherapy and pharmacotherapy can improve the outcome. The outcome of PNES in a given patient is variable, with good short-term outcome.[29–34] However, on long-term follow-up, most patients had recurrences of events.[35–39] The mean latency between manifestation and the diagnosis is about 7-16 years; indicating lack of awareness among patients, their family members as well as among treating doctor.[11,40,41]
Among adults there are very few studies from India.[42–44] Aim of our study was to diagnose PNES, study their clinical and video-EEG profile, and psychiatric comorbidity in adult population.
Materials and Methods
A total of 82 patients, age >14 years with clinical suspicion of PNES with or without any coexisting epilepsy attending neurology outpatient in a tertiary care hospital in North India were enrolled over a period of 2 years (August 2009-July 2011). Clinical suspicion was based on the following criteria:
Refractory epilepsy or change in symptomatology
Episodes of variably prolonged unresponsiveness and lying motionless
Multiple or non-stereotypical seizure patterns
Occurrence in situations as in front of audience, doctor or in waiting room
Seizure provoked by emotional stress
Associated psychiatric disorders.
After a thorough clinical history and detailed neurological examination, all the cases were subjected to video-EEG monitoring. Of 82 patients, 63 (76.82%) had confirmed diagnosis of PNES. The diagnosis of PNES was based on witnessing the event, that occurred either spontaneously or on induction by hyperventilation and verbal suggestion during video-EEG. Based on the clinical semiology, the patients were divided into two groups; type A characterized by excessive motor phenomenon or type B with limp attacks with/out minimal motor phenomenon. All patients were further evaluated for any coexisting anxiety or depressive disorders using DSM-IV criteria and their severities were assessed using Hamilton Depression/Anxiety rating scales. After confirming the diagnosis of PNES, all the patients and the family members were properly explained about the diagnosis; that these episodic events were not due to epilepsy and such phenomenon may occur due to various kinds of stress, lack of coping skills or existent psychological disturbances. All the patients were thoroughly interrogated to identify the possible precipitating factors if any; counseled to increase their self-confidence and coping skills and encouraged to remain socially and occupationally active. Along with counseling, patients were started on antidepressants with/without anxiolytics; according to the psychiatric comorbidity. Antiepileptic drugs were slowly withdrawn in patients with only PNES. All patients were followed-up at 6 and 12 months either by hospital visits or telephonically regarding frequency of PNES. A good outcome was defined as achieving seizure freedom state or >50% improvement.
Statistical methods
Data were entered into Microsoft Excel spreadsheet and statistically analyzed. Means and standard deviations were calculated for all continuous variables. Chi-square test was used to compare categorical variables, and Students t-test for continuous variables. P < 0.05 was considered the level of statistical significance.
Results
A total of 63 patients were included in the study. Majority of patients were women (90.46%), with the mean age at onset being 25.44 ± 10.22 years. Most of our patients were literate (84.12%), resident of urban areas (55.55%), from nuclear families (58.73%), and were belonging to either upper lower or lower middle class (93.65%) (Modified Kuppuswamy scale 2007). Of the 57 female patients; 33 were house wives, 10 students, and the rest 14 were widows and unmarried females. Of the six male patients, four were gainfully employed, one was unemployed, and one was a student [Table 1].
Table 1.
Clinical characteristics of psychogenic non-epileptic seizures patients
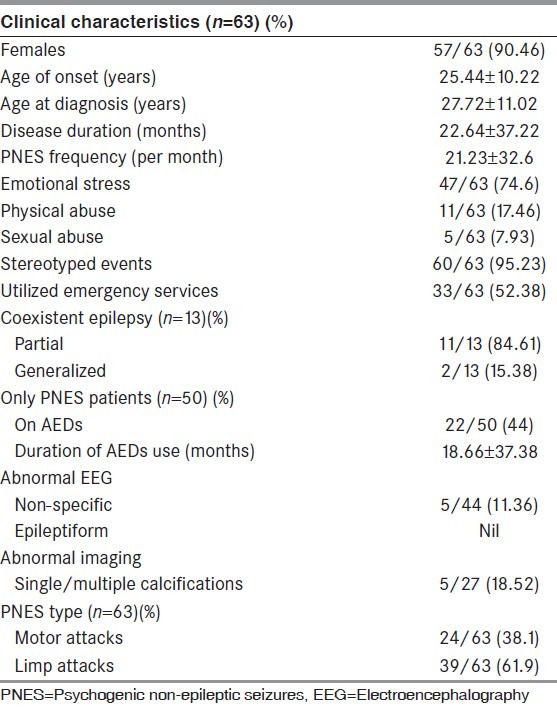
The main reason for evaluation was the confirmation of primarily suspected PNES in 50/63 (79.36%) patients; of which 22/50 (44%) were initially diagnosed and treated as epilepsy. In the remaining 13/63 (20.63%) patients with coexisting epilepsy; the reasons were; recurrence of seizures in previously controlled epilepsy in seven patients and uncontrolled epilepsy in six patients. Neuroimaging and EEG data of patients with epilepsy coexisting PNES were not been analyzed. Among the patients with only PNES (n = 50), we were able to perform inter-ictal EEG in 44 patients, and neuroimaging computed tomography/magnetic resonance imaging (CT/MRI) in 27 patients. Of them, we found 11.36% patients with non-specific EEG abnormalities and none with epileptiform discharges. Single or multiple calcifications were observed in 18.52% patients with only PNES who underwent neuroimaging.
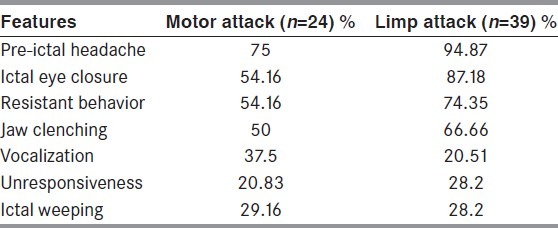
Three patients (4.76%) had spontaneous PNES events, whereas in rest 60 patients the diagnosis was confirmed by inducing the clinical event during video-EEG monitoring. The most common PNES type was limp attacks seen in 39 patients (61.9%); followed by motor attacks in 24 patients (38.1%). The common semiological characteristics observed (in either type) are listed below [Table 2]. Historically, six patients had significant injuries (forehead or occipital bruises) whereas four patients had urinary incontinence and bite on tip of the tongue during the episodes, but not observed during the witnessed episodes.
Table 2.
Semiological characteristics
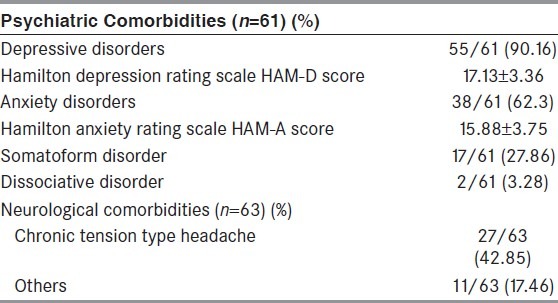
The comorbid anxiety and depressive disorders were seen in 62.3% and 90.16% cases, respectively. On the assessment of severity, majority of these patients had moderate-severe scores on Hamilton depression scales (65.56%), and mild-moderate scores on Hamilton anxiety scales (81.76%) respectively [Table 3]. Neurological comorbidities were seen in 38 (60.31%) patients (six had coexistent epilepsy), commonest being chronic tension type headache in 27 patients, migraine in 2, mild mental retardation in 2, alcohol dependence in 2, tubercular meningitis in 1, drug addiction in 1, operated brain tumor in 1, history of significant head injury in 1, and Neuromyelitis Optica in 1 patient respectively.
Table 3.
Comorbidities
We were able to follow-up 32 patients (50.8%) for 12 months, 13 patients (20.63%) for 6 months and 9 patients (14.28%) for 3 months. Nine patients (14.28%) were lost to follow-up. A total of 45 patients with follow-up of 6-12 months were included in analysis. Of these, 31 patients (68.88%) had good outcome (46.66% seizure free and 24.44% had >50% improvement). Among the 14 patients, (31.11%) with poor outcome; six patients (13.33%) had <50% improvement, another four (8.88%) had persistent PNES with same frequency whereas the remaining four (8.88%) patients had increase in PNES frequency from baseline. In our study, good outcome predictors were younger age (P = 0.54), short duration of illness (P = 0.44), less frequent episodes (P = 0.66), patients with limp attacks (P = 0.27). However, effect of gender, coexistent epilepsy, and psychiatric comorbidity could not be assessed because of incomparable data.
Discussion
Similar to the previous studies majority of our patients were women (90.46%) in late 2nd-3rd decade (72%) of their life; with a mean age of onset and presentation of PNES 25.44 years and 27.72 years respectively. In one previous Indian study of 71 adult patients with PNES (Lazarus 2003), compared to our study there was a younger population group (mean age at onset 19.68 years) and a lower proportion of women (78.9%).[42] The likely reason is the fact that majority of patients in their study were students whereas married females constituted majority of our patients.
Most of our patients came from nuclear families with poor emotional and peer support. Emotional stress was the precipitating factor in 74.6% of our patients, but only 17.46% of our patients had reported physical abuse. The lower reporting of sexual abuse (7.93%) by our patients as compared to previous studies (11-67%) was likely due to our social structure, fear of earning disrepute and legal issues.[14,19,32,45,46] The data about comorbid psychiatric illnesses is highly variable because of the use of different nomenculture, criteria, and classifications.
The majority (79.36%) of our patients were having only PNES. Whereas 20.63% of our patients had coexistent PNES and epileptic seizures, of them the commonest being partial seizures (84.61%). The available data of coexistence of PNES and epilepsy is highly variable (5-40%); because of the differences in study population, study type, and reasons for video-EEG monitoring.[10–12] Forty-four percent of our patients with only PNES (n = 50) were misdiagnosed and treated as epileptic seizures; which is not an uncommon observation (31-75.5%) in older studies.[32,42,47–49] Utilization of emergency services for management of PNES is common; seen in 52.38% of our patients, similar to observations by McKenzie (49.7%).[32] Historically, 20.63% of our patients had presentation as pseudostatus; similar to that reported by Reuber (20.1%).[36]
In our study, the mean delay in diagnosis of PNES was nearly 2 years (22.64 months); whereas most studies have reported a mean delay of 1.5-9 years.[14,31,32,41–43] The possible reasons for the delay in diagnosis was lack of awareness both among patients and treating doctor, use of alternative system of medicine, and belief that these events were due to supernatural causes. Shorter duration of video-EEG monitoring and early use of induction protocol, are the reasons for lower rates of spontaneous PNES events (4.76%) in our patients. As expected, more spontaneous events were seen with prolonged monitoring; as reported in 71-96.2% cases within 48 h of video-EEG monitoring in previous studies.[48,50,51]
In contrast to older belief that a PNES patient had multiple or changing semiology; recent studies have shown that majority of PNES attacks are similar in a patient.[42,52] Historically, 95.23% of our patients were having stereotyped events that were confirmed by eye witness of previous event and event during V-EEG monitoring. There is no uniform clinical semiological classification of PNES; and in most of the studies patients were classified on the basis of either excessive or paucity of motor phenomenon. In most of the studies, the commonest PNES subtypes were attacks with excessive motor phenomenon (52-77%).[48,51–55] In contrast, limp attacks were the most common PNES type (61.9%) seen in our patients; similar to other Indian studies (Lazarus 2003: 59.2%; Dhanraj 2005: 53.3%).[42–44] The reason behind this is not clear; but probably it is the cultural difference and poor emotional support in our patients, especially among the females.
Apart from classical ictal features of a typical motor or limp attack; a significant number of our patients had pre-ictal headache (87.3%), ictal eye closure (74.6%), resistant behavior (66.66%), jaw clenching (60.31%), ictal weeping (28.57%), ictal vocalization (26.98%), and unresponsiveness (25.4%) during episodes. A few studies had reported significant injuries, urinary incontinence and tongue bite with PNES episodes.[40,53,56] Historically, six of our patients had significant injuries over forehead or occipital areas; whereas four each had urinary incontinence and bite over tip of the tongue.
Abnormal non-epileptiform inter-ictal EEG (46-54%),[41,52,53,57] and abnormal neuroimaging (30-43%)[30,53] are not uncommon in PNES patients without coexisting epilepsy. None of our patients with only PNES had epileptiform discharges; although 11.36% had non-specific inter-ictal EEG abnormality. Similarly, 18.52% our patients with only PNES had single or multiple calcified lesions on CT brain. It should be kept in mind that neurocysticercosis is endemic in India, and asymptomatic calcified lesions in brain are not uncommon in our population. Our main focus was to identify comorbid depressive and anxiety disorders. We found a high incidence of depressive (90.16%) and anxiety disorders (62.3%) in our patients. Most of them had moderate-severe depression and mild-moderate anxiety scores respectively. Chronic tension type headache (42.85%) was the commonest neurological comorbidity.
At short term follow-up (<1 year), about 19-67% patients were seizure free and additional 21-56% had significant reduction in seizure frequency.[29–34] However, many patients with good initial outcome had recurrence of events on long term follow-up. The long term (1-14 years) follow-up studies variably reported seizure freedom rates from 16-40%.[11,35–39] In the recently published largest series (260 patients) of short term (6-12 months) outcome of PNES patients by McKenzie (2010); 38% of patients had been free of spells and additional 23% patients had at least 50% reduction in spell frequency.[32] To the best of our knowledge, there are no studies addressing prognosis of PNES and their factors in adult population from India. Similar to older studies, the short term (6-12 month) outcome was good in our study; which included cessation of seizures in 46.66% and >50% reduction in seizure frequency in 24.44% patients. The most important prognostic factor probably is the acceptance of the diagnosis of PNES by patients and their family members. The other favorable prognostic factors reported are young age at onset,[36,47] male[32] or female,[37] better education,[47,55] good social, family, and security support system,[31,32,34,37] employment, and independent lifestyle,[34,35,37] low intelligence quotient[32,58] shorter duration of condition,[39,47] less frequent episodes,[43] accepting the diagnosis,[31,34] motionless spells,[39,47,55] lack of comorbid epilepsy,[37,47,59] continued antiepileptic drugs (AEDs) use,[35] lack of physical or sexual abuse,[30] and lack of psychiatric comorbidities.[30,32,47] The possible good prognostic factors in our study were young age at onset (P = 0.54), shorter duration of illness (P = 0.44), less frequent episodes (P = 0.66), and limp attacks (P = 0.27).; although none was statistically significant. The effect of gender, literacy status, socioeconomic status, coexisting epilepsy and comorbid psychiatric illnesses on the outcome could not be analyzed because of inadequate sample size.
Apart from small sample size and incomparable data, the other potential limitation of our study was that the outcome assessment was based only upon reduction in frequency of seizures, and other parameters as use of AEDs, employment, quality of life, etc., were not studied. So, larger studies with longer follow-up and addressing these issues are required to find out the outcome factors in Indian population.
Conclusion
PNES is common among young women. PNES is frequently misdiagnosed and wrongly treated as epileptic seizures. A high index of suspicion is required for an early diagnosis. Proper disclosure of diagnosis and management of the psychiatric comorbidities can improve the outcome and overall quality of life of patients with PNES.
Limitation
There are two major limitation of our study, first is limited sample size. Secondly, follow-up is only in terms of change in seizures frequency; while other parameters as use of AEDs, socio-occupational functioning, psychiatric comorbidity outcome, and quality of life was not assessed.
Acknowledgment
We acknowledge all our electrophysiological technical staff of department of Neurology, G. B. Pant hospital New Delhi; for their technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Lesser RP. Psychogenic seizures. Neurology. 1996;46:1499–507. doi: 10.1212/wnl.46.6.1499. [DOI] [PubMed] [Google Scholar]
- 2.Binder LM, Salinsky MC. Psychogenic nonepileptic seizures. Neuropsychol Rev. 2007;17:405–12. doi: 10.1007/s11065-007-9047-5. [DOI] [PubMed] [Google Scholar]
- 3.Andermann F. Nonepileptic paroxysmal neurological events. In: Gates JR, Rowan AJ, editors. Nonepileptic Seizures. 2nd ed. Boston: Buterworth-Heinemann; 2000. pp. 51–69. [Google Scholar]
- 4.Rowan AJ. Diagnosis of nonepileptic seizures. In: Gates JR, Rowan AJ, editors. Nonepileptic Seizures. 2nd ed. Boston: Buterworth-Heinemann; 2000. pp. 15–30. [Google Scholar]
- 5.Sigurdardottir KR, Olafsson E. Incidence of psychogenic seizures in adults: A population-based study in Iceland. Epilepsia. 1998;39:749–52. doi: 10.1111/j.1528-1157.1998.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 6.Szaflarski JP, Ficker DM, Cahill WT, Privitera MD. Four-year incidence of psychogenic nonepileptic seizures in adults in Hamilton county, OH. Neurology. 2000;55:1561–3. doi: 10.1212/wnl.55.10.1561. [DOI] [PubMed] [Google Scholar]
- 7.Benbadis SR, Allen Hauser W. An estimate of the prevalence of psychogenic non-epileptic seizures. Seizure. 2000;9:280–1. doi: 10.1053/seiz.2000.0409. [DOI] [PubMed] [Google Scholar]
- 8.Hitiris N, Leach JP, Mohanrav R, Mallik A, Greene J, Duncan R. Usefulness of investigation in a first seizure clinic. J Neurol Neurosurg Psychiatry. 2005;76:1316. [Google Scholar]
- 9.Chabolla DR, Krahn LE, So EL, Rummans TA. Psychogenic nonepileptic seizures. Mayo Clin Proc. 1996;71:493–500. doi: 10.4065/71.5.493. [DOI] [PubMed] [Google Scholar]
- 10.Benbadis SR, Agrawal V, Tatum WO., 4th How many patients with psychogenic nonepileptic seizures also have epilepsy? Neurology. 2001;57:915–7. doi: 10.1212/wnl.57.5.915. [DOI] [PubMed] [Google Scholar]
- 11.Bodde NM, Brooks JL, Baker GA, Boon PA, Hendriksen JG, Aldenkamp AP. Psychogenic non-epileptic seizures-diagnostic issues: A critical review. Clin Neurol Neurosurg. 2009;111:1–9. doi: 10.1016/j.clineuro.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay RE, Cohen A, Brown MC. Coexisting epilepsy and nonepileptic seizures. In: Gates JR, Rowan AJ, editors. Nonepileptic Seizures. 1993. pp. 73–84. [Google Scholar]
- 13.Bowman ES. Nonepileptic seizures: Psychiatric framework, treatment, and outcome. Neurology. 1999;53:S84–8. [PubMed] [Google Scholar]
- 14.Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153:57–63. doi: 10.1176/ajp.153.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Duncan R, Oto M. Predictors of antecedent factors in psychogenic nonepileptic attacks: Multivariate analysis. Neurology. 2008;71:1000–5. doi: 10.1212/01.wnl.0000326593.50863.21. [DOI] [PubMed] [Google Scholar]
- 16.Fiszman A, Alves-Leon SV, Nunes RG, D’Andrea I, Figueira I. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: A critical review. Epilepsy Behav. 2004;5:818–25. doi: 10.1016/j.yebeh.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Westbrook LE, Devinsky O, Geocadin R. Nonepileptic seizures after head injury. Epilepsia. 1998;39:978–82. doi: 10.1111/j.1528-1157.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 18.Reuber M, Qurishi A, Bauer J, Helmstaedter C, Fernandez G, Widman G, et al. Are there physical risk factors for psychogenic non-epileptic seizures in patients with epilepsy? Seizure. 2003;12:561–7. doi: 10.1016/s1059-1311(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 19.Reuber M, Howlett S, Khan A, Grünewald RA. Non-epileptic seizures and other functional neurological symptoms: Predisposing, precipitating, and perpetuating factors. Psychosomatics. 2007;48:230–8. doi: 10.1176/appi.psy.48.3.230. [DOI] [PubMed] [Google Scholar]
- 20.Kanner AM, Morris HH, Lüders H, Dinner DS, Wyllie E, Medendorp SV, et al. Supplementary motor seizures mimicking pseudoseizures: Some clinical differences. Neurology. 1990;40:1404–7. doi: 10.1212/wnl.40.9.1404. [DOI] [PubMed] [Google Scholar]
- 21.Morris HH, 3rd, Dinner DS, Lüders H, Wyllie E, Kramer R. Supplementary motor seizures: Clinical and electroencephalo-graphic findings. Neurology. 1988;38:1075–82. doi: 10.1212/wnl.38.7.1075. [DOI] [PubMed] [Google Scholar]
- 22.Saygi S, Katz A, Marks DA, Spencer SS. Frontal lobe partial seizures and psychogenic seizures: Comparison of clinical and ictal characteristics. Neurology. 1992;42:1274–7. doi: 10.1212/wnl.42.7.1274. [DOI] [PubMed] [Google Scholar]
- 23.Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18:497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- 24.Iriarte J, Parra J, Urrestarazu E, Kuyk J. Controversies in the diagnosis and management of psychogenic pseudoseizures. Epilepsy Behav. 2003;4:354–9. doi: 10.1016/s1525-5050(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 25.Scott DF. Recognition and diagnostic aspects of nonepileptic seizures. In: Riley TL, Roy A, editors. Pseudoseizures. Baltimore: Williams and Wilkins Co.; 1982. pp. 21–33. [Google Scholar]
- 26.Reuber M. Psychogenic nonepileptic seizures: Diagnosis, etiology, treatment and prognosis. Schweiz Arch Neurol Psych. 2005;156:47–57. [Google Scholar]
- 27.Cragar DE, Berry DT, Fakhoury TA, Cibula JE, Schmitt FA. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev. 2002;12:31–64. doi: 10.1023/a:1015491123070. [DOI] [PubMed] [Google Scholar]
- 28.Cascino GD. Video-EEG monitoring in adults. Epilepsia. 2002;25:231–48. doi: 10.1046/j.1528-1157.43.s.3.14.x. [DOI] [PubMed] [Google Scholar]
- 29.Walczak TS, Papacostas S, Williams DT, Scheuer ML, Lebowitz N, Notarfrancesco A. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia. 1995;36:1131–7. doi: 10.1111/j.1528-1157.1995.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanner AM, Parra J, Frey M, Stebbins G, Pierre-Louis S, Iriarte J. Psychiatric and neurologic predictors of psychogenic pseudoseizure outcome. Neurology. 1999;53:933–8. doi: 10.1212/wnl.53.5.933. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger AB, Dhoon A, Weisbrot DM, Devinsky O. Predictive factors for outcome of nonepileptic seizures after diagnosis. J Neuropsychiatry Clin Neurosci. 1999;11:458–63. doi: 10.1176/jnp.11.4.458. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie P, Oto M, Russell A, Pelosi A, Duncan R. Early outcomes and predictors in 260 patients with psychogenic nonepileptic attacks. Neurology. 2010;74:64–9. doi: 10.1212/WNL.0b013e3181c7da6a. [DOI] [PubMed] [Google Scholar]
- 33.Riaz H, Comish S, Lawton L, Scheepers B. Non-epileptic attack disorder and clinical outcome: A pilot study. Seizure. 1998;7:365–8. doi: 10.1016/s1059-1311(05)80004-4. [DOI] [PubMed] [Google Scholar]
- 34.Silva W, Giagante B, Saizar R, D’Alessio L, Oddo S, Consalvo D, et al. Clinical features and prognosis of nonepileptic seizures in a developing country. Epilepsia. 2001;42:398–401. doi: 10.1046/j.1528-1157.2001.45299.x. [DOI] [PubMed] [Google Scholar]
- 35.Carton S, Thompson PJ, Duncan JS. Non-epileptic seizures: Patients’ understanding and reaction to the diagnosis and impact on outcome. Seizure. 2003;12:287–94. doi: 10.1016/s1059-1311(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 36.Reuber M, Pukrop R, Bauer J, Helmstaedter C, Tessendorf N, Elger CE. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol. 2003;53:305–11. doi: 10.1002/ana.3000. [DOI] [PubMed] [Google Scholar]
- 37.Meierkord H, Will B, Fish D, Shorvon S. The clinical features and prognosis of pseudoseizures diagnosed using video-EEG telemetry. Neurology. 1991;41:1643–6. doi: 10.1212/wnl.41.10.1643. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan SS, Spillane JE, McMahon EM, Sweeney BJ, Galvin RJ, McNamara B, et al. Clinical characteristics and outcome of patients diagnosed with psychogenic nonepileptic seizures: A 5-year review. Epilepsy Behav. 2007;11:77–84. doi: 10.1016/j.yebeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Selwa LM, Geyer J, Nikakhtar N, Brown MB, Schuh LA, Drury I. Nonepileptic seizure outcome varies by type of spell and duration of illness. Epilepsia. 2000;41:1330–4. doi: 10.1111/j.1528-1157.2000.tb04613.x. [DOI] [PubMed] [Google Scholar]
- 40.Reuber M, Elger CE. Psychogenic nonepileptic seizures: Review and update. Epilepsy Behav. 2003;4:205–16. doi: 10.1016/s1525-5050(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 41.Reuber M, Fernández G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology. 2002;58:493–5. doi: 10.1212/wnl.58.3.493. [DOI] [PubMed] [Google Scholar]
- 42.Lazarus JP, Bhatia M, Shukla G, Padma MV, Tripathi M, Shrivastava AK, et al. A study of nonepileptic seizures in an Indian population. Epilepsy Behav. 2003;4:496–9. doi: 10.1016/s1525-5050(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 43.Dhanaraj M, Rangaraj R, Arulmozhi T, Vengatesan A. Nonepileptic attack disorder among married women. Neurol India. 2005;53:174–7. doi: 10.4103/0028-3886.16403. [DOI] [PubMed] [Google Scholar]
- 44.Deka K, Chaudhury PK, Bora K, Kalita P. A study of clinical correlates and socio-demographic profile in conversion disorder. Indian J Psychiatry. 2007;49:205–7. doi: 10.4103/0019-5545.37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lancman ME, Brotherton TA, Asconapé JJ, Penry JK. Psychogenic seizures in adults: A longitudinal analysis. Seizure. 1993;2:281–6. doi: 10.1016/s1059-1311(05)80141-4. [DOI] [PubMed] [Google Scholar]
- 46.Moore PM, Baker GA. Non-epileptic attack disorder: A psychological perspective. Seizure. 1997;6:429–34. doi: 10.1016/s1059-1311(97)80016-7. [DOI] [PubMed] [Google Scholar]
- 47.An DM, Wu XT, Yan B, Mu J, Zhou D. Clinical features of psychogenic nonepileptic seizures: A study of 64 cases in southwest China. Epilepsy Behav. 2010;17:408–11. doi: 10.1016/j.yebeh.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Mari F, Di Bonaventura C, Vanacore N, Fattouch J, Vaudano AE, Egeo G, et al. Video-EEG study of psychogenic nonepileptic seizures: Differential characteristics in patients with and without epilepsy. Epilepsia. 2006;47:64–7. doi: 10.1111/j.1528-1167.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 49.Brooks JL, Goodfellow L, Bodde NM, Aldenkamp A, Baker GA. Nondrug treatments for psychogenic nonepileptic seizures: What’s the evidence? Epilepsy Behav. 2007;11:367–77. doi: 10.1016/j.yebeh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Perrin MW, Sahoo SK, Goodkin HP. Latency to first psychogenic nonepileptic seizure upon admission to inpatient EEG monitoring: Evidence for semiological differences. Epilepsy Behav. 2010;19:32–5. doi: 10.1016/j.yebeh.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parra J, Kanner AM, Iriarte J, Gil-Nagel A. When should induction protocols be used in the diagnostic evaluation of patients with paroxysmal events? Epilepsia. 1998;39:863–7. doi: 10.1111/j.1528-1157.1998.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 52.Seneviratne U, Reutens D, D’Souza W. Stereotypy of psychogenic nonepileptic seizures: Insights from video-EEG monitoring. Epilepsia. 2010;51:1159–68. doi: 10.1111/j.1528-1167.2010.02560.x. [DOI] [PubMed] [Google Scholar]
- 53.Hovorka J, Nezádal T, Herman E, Nemcová I, Bajacek M. Psychogenic non-epileptic seizures, prospective clinical experience: Diagnosis, clinical features, risk factors, psychiatric comorbidity, treatment outcome. Epileptic Disord. 2007;9:S52–8. doi: 10.1684/epd.2008.0156. [DOI] [PubMed] [Google Scholar]
- 54.D’Alessio L, Giagante B, Oddo S, Silva W W, Solís P, Consalvo D, et al. Psychiatric disorders in patients with psychogenic non-epileptic seizures, with and without comorbid epilepsy. Seizure. 2006;15:333–9. doi: 10.1016/j.seizure.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Arain AM, Hamadani AM, Islam S, Abou-Khalil BW. Predictors of early seizure remission after diagnosis of psychogenic nonepileptic seizures. Epilepsy Behav. 2007;11:409–12. doi: 10.1016/j.yebeh.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Fawi GH, El-Kholy SH, Sabry MM, Thabit MN. Diagnostic aids of psychogenic non-epileptic seizures. Egypt J Neurol Psychiat Neurosurg. 2006;43:371–80. [Google Scholar]
- 57.Reuber M, Fernández G, Bauer J, Singh DD, Elger CE. Interictal EEG abnormalities in patients with psychogenic nonepileptic seizures. Epilepsia. 2002;43:1013–20. doi: 10.1046/j.1528-1157.2002.52301.x. [DOI] [PubMed] [Google Scholar]
- 58.McDade G, Brown SW. Non-epileptic seizures: Management and predictive factors of outcome. Seizure. 1992;1:7–10. doi: 10.1016/1059-1311(92)90047-5. [DOI] [PubMed] [Google Scholar]
- 59.Irwin K, Edwards M, Robinson R. Psychogenic non-epileptic seizures: Management and prognosis. Arch Dis Child. 2000;82:474–8. doi: 10.1136/adc.82.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]


