Abstract
Background and Purpose:
The purpose of this study was to assess the effectiveness of modified constraint induced movement therapy (m-CIMT) in stroke subjects.
Materials and Methods:
A total of forty sub-acute stroke subjects were randomly assigned to either a m-CIMT (n = 20) or in a control group (n = 20). The m-CIMT group (14 men, 6 women; mean age = 55.2 years) consisted of structured 2 h therapy sessions emphasizing affected arm use, occurring 5 times/week for 2 weeks. A mitt was used to restrain the unaffected arm for 10 h/day for 2 week. The control group (11 men, 9 women; mean age = 56.4 years) consisted of conventional rehabilitation for time-matched exercise program. The outcome measures were evaluated at pre- and post-intervention by using the Wolf Motor Function Test (WMFT) and Fugl-Meyer assessment (FMA) of motor recovery after stroke.
Results:
After intervention significant effects were observed in m-CIMT group on WMFT (pre-test and post-test score was 28.04 ± 6.58, 13.59 ± 2.86; P =0.003). Similarly on FMA (pre- and post-test score was 31.15 ± 6.37, 55.7 ± 6.4; P = 0.00).
Conclusion:
There is a significant improvem ent in upper extremity function so it indicates that m-CIMT is effective in improving the motor function of the affected arm in stroke subjects. However, its long-term effect has not proved since there was no follow-up after intervention.
Keywords: Constraint induced movement therapy, exercise, rehabilitation, stroke
Introduction
Stroke presents one of the most disabling neurological disorders. For many subjects, the impact of stroke symptoms on activities of daily living is important.[1] Substantial efforts to develop rehabilitation protocols that minimize brain damage and improve outcomes in subjects with ischemic stroke are currently being pursued. Subjects with stroke often fail to develop full functional use of the affected upper extremity due to residual muscle weakness, spasticity and decrease in bone strength.[2]
Constraint induced movement therapy (CIMT) is a technique used in physical rehabilitation to treat individuals with decreased upper extremity function. Early research on CIMT was initiated by Dr. Edward Taub on monkeys’.[3] CIMT is based on the theory of “learned non-use,” which develops, during the early stages following a stroke as the subjects begins to compensate for difficulty using the impaired limb by increased reliance on the intact limbs.[4] This compensation has been shown to hinder recovery of the function in the impaired limb. It is also based on neuro-plasticity and cortical reorganization. Nudo and Milliken describe the cortical representation shrinking after lesion or sensory or motor deprivation. Changes in representational areas were prevented or reversed by focused motor training in primates with concurrent improvements in motor function.[5–7] In addition, functional imaging studies in humans with stroke have found recovery to be associated with shifts of activation during motor tasks involving the affected hand to ipsilateral secondary and tertiary motor areas and to contra lateral homologous motor areas.[8]
CIMT encourages, the use of the affected upper extremity with the goal of maximizing or restoring motor function, a technique known as “shaping”.[9] It is a form of operant or instrumental conditioning (associating a reward with a correct response as a basis for reinforcing the correct response) characterized by repetitions of a defined movement, such as picking up blocks and moving them toward a pail, in a series of trials. The objective of shaping is to alter motor behaviors by repetitive use of basic movement tasks, the difficulties of which are progressively increased. The subject should be motivated to perform even more optimally on the basis of the progressive improvement over trials.[10]
Although, efficacious, findings from survey[11] measuring subjects‘ and therapists’ opinions of CIMT suggest that its clinical implementation is limited. Specifically, when CIMT was described in an excerpt from case report[12] (1) 68% of the subjects with stroke said that they would not want to participate in the protocol; (2) two-thirds of the subjects who said that they would participate in CIMT conceded that they were somewhat or extremely unlikely to adhere to the CIMT protocol; (3) more than 80% of the patients felt that, if the protocol lasted for more weeks, with shorter physical therapy sessions and/or fewer hours wearing the restrictive devices, they would participate. Among therapists surveyed, more than 60% felt that subjects were extremely unlikely to adhere to such a protocol, with the primary reasons being the amount of time wearing the restrictive device and the number of practice hours.[12]
So Page et al,[13,14] modified the CIMT, which combines structured, 30 min, functional practice sessions using more-affected arm, with restriction of the less-affected arm 5 days/week for 5 h/day. Modified Constraint induced movement therapy (m-CIMT) has been shown to increase affected arm use and function in case studies,[15] and randomized controlled pilot studies enrolling subjects with acute stroke,[16] sub-acute stroke,[9,13] and chronic stroke.[17] Besides its reimbursement within existing current procedural terminology codes, CIMT and m-CIMT effects appear to be comparable.[18] Other data suggest that cortical reorganizations, brought about by increased arm use during m-CIMT, are responsible for the motor changes.[19] Long duration in the past studies poses great hindrance in practical application of this result oriented intervention. It is practically not possible for the therapist to treat single subject for 6-7 h and hence the present study aims to determine the effect of m-CIMT in improving the upper extremity function of stroke subjects in 2 h.
Materials and Methods
Stroke subjects were recruited from Central Referral Hospital and STNM Hospital in Sikkim, India by simple random sampling method. Institutional ethics committee approved the study. The subjects who fulfilled the following inclusion criteria were selected: (1) Ischemic or hemorrhagic first ever sub-acute stroke subjects referred by physician between 2 weeks and 4 weeks after the onset, (2) both genders of any age, (3) having at least 10° of active extension of each metacarpophalengeal joints, inter-phalengeal joints of all the digits and 10° wrist extension of the affected limb, (4) spasticity grade ≥1 according to Modified Ashworth Scale, (5) Mini Mental State Examination ≥17. We also applied the following exclusion criteria: Subjects with severe aphasia, severe shoulder pain affecting therapy or any comorbid condition that could limit upper extremity function. The baseline measures were collected after informed consent was obtained. Subjects were individually randomized into intervention and control groups by using lottery method. There was total number of 20 subjects in each group. Since no follow-up and less time was kept for restraint of the unaffected upper extremity so no drop out during the study. The baseline data regarding name, age, sex, hospital number, post stroke duration, and the side of involvement were taken for all subjects.
The WMF and Fugl-Meyer assessment (FMA) were administered as an outcome measure for the both groups and the scores were documented by the tester. The subjects in both groups were made to sit in comfortable position in a calm and well-ventilated room before treatment.
m-CIMT group
In intervention group, a mitt was used to restrain the unaffected arm which prevented the use of unaffected limb. It was made of cotton material which was extending till the forearm. The subjects were encouraged to wear a mitt during treatment and post treatment as 10 h/day for 2 weeks except for activities like toileting, washing etc., The mitt allowed the unaffected upper extremity to assist in transfers and ambulation, but it prevented use of the unaffected fingers to manipulate objects and necessitated use of the affected hand to perform daily activities. The total duration for mitt was recorded by subjects in the log book, which was provided by the therapist.
The shaping technique for m-CIMT was given for 2 h/day for 2 weeks at the frequency of 5 days a week. “Shaping” is a commonly used operant conditioning method in which a behavioral objective (in this case, movement) is approached in small steps of progressively increasing difficulty. The participant is rewarded with enthusiastic approval for improvement, but never blamed or punished for failure. Tasks were individually selected according to motor ability, to ensure successful experience, and prevent frustration leading to learned non-use. Task difficulty was progressively increased using behavioral techniques of shaping and successive approximation.
Control group
The control group received standard physical therapy treatment that included compensatory technique for ADLs (all daily living activity), upper extremity strength, and range of motion and traditional positioning for affected arm. To equalize treatment intensity, the same number of therapy sessions was provided to both groups. No restraint was used and subjects were free to use either hand for daily activities.
Outcome measure
Wolf Motor Function Test (WMFT) is a 17 item instrument consisting of 15 timed and 2 strength task. Tasks 1-6 of the WMFT involve timed joint segment movement and tasks 7-15 consisted of timed integrated functional movements. FMA is a cumulative numerical scoring system for measurement of motor recovery, balance, sensation, and joint range of motion in subjects who have sustained stroke. The functions of wrist and hand were assessed separately. Pre-intervention and at the end of 2nd week outcome measures, WMFT and FMA were taken for both the group for determination of motor function.
Data analysis
Data analysis was performed using SPSS Windows, version 16. Comparisons of differences within each group before and after therapy sessions in items WMFT and FMA were performed by using the paired t-test.
Results
Total 40 subjects were recruited, out of which 20 were in intervention group and 20 in control group. Mean age in intervention group was 55.2 ± 9.27 and in control group, it was 56.4 ± 11.4. Out of 20 subjects in intervention group, 14 were males and 6 were females. In control group, 11 were males and 9 were females. Baseline characteristics of all subjects are given in Table 1.
Table 1.
Subjects baseline characteristics of subjects in intervention and control group
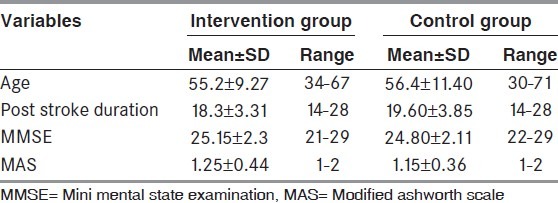
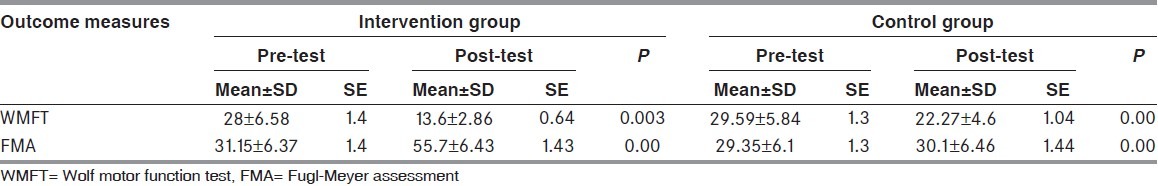
Table 2 shows the pre- and post-test score of WMFT and FMA in intervention and control group. The pre-test score for WMFT in intervention group was 28.04 ± 6.58 and post-test score was 13.59 ± 2.86 (P =0.003). In control group, the pre-test score was 29.59 ± 5.84 and after 2 weeks of conventional therapy, it was 22 ± 4.68 (P =0.00). In intervention group, the pre-test score of FMA was 31.15 ± 6.37 and after 2 weeks of intervention it was 55.7 ± 6.4 (P =0.0). In control group, pre-test score was 29.3 ± 6.10 and post-test score was 39.1 ± 6.4 after 2 weeks (P =0.0).
Table 2.
Pre- and post-score of Wolf motor function test and Fugl-Meyer assessment in intervention and control group
Pre- and post-test difference of WMFT and FMA in intervention and control group has shown in Table 3. The difference of WMFT in intervention group was 14.75 ± 4.83 and in control group it was 7.21 ± 2.01. In intervention group, the difference of FMA was 24.95 ± 3.74 and in control group it was 9.5 ± 2.7.
Table 3.
Pre- and post-difference of Wolf motor function test and Fugl-Meyer assessment in intervention and control group

Discussion
The outcome measures in this study showed improvements, after 2 week training period in stroke subjects. So, the results indicate that m-CIMT is improving the function of the affected upper extremity in stroke subjects. According to Fritz et al,[20] and Wolf et al.[13] WMFT was used as an outcome measure to determine the motor function of the upper extremity of the affected side. FMA upper extremity portion was also used to measure the motor function of upper extremity.
The pre-test score for WMFT in intervention group was 28.04 ± 6.58 and post-test score was 13.59 ± 2.86 (P = 0.003). In control group, the pre-test score was 29.59 ± 5.84 and after 2 weeks of conventional therapy, it was 22 ± 4.68 (P =0.00). In intervention group, the pre-test score of FMA was 31.15 ± 6.37 and after 2 weeks of intervention it was 55.7 ± 6.4 (P =0.0) and in control group, pre-test score was 29.3 ± 6.10 and post-test score was 39.1 ± 6.4 after 2 weeks (P =0.0). This shows that there is a significant improvement in upper extremity function and thus, it indicates that m-CIMT is effective in improving the motor function of the affected arm in stroke subjects.
Findings of our study are in correlation with the findings of Wolf et al,[21,22] and Taub et al,[23] showed improvements in motor skills and the use of the affected arm and hand in daily activities after CIMT. Prior studies carried out by Wolf et al,[24] and Morris et al,[25] reported that WMFT is an useful measure to examine and measure the effectiveness of CIMT for stroke survivors and Page et al,[13] saw considerable changes among CIMT subjects between pre-test and post-test sessions on the WMFT, both in terms of rating of arm use, and in terms of time taken to complete the task. Subjects in CIMT group showed especially, strong improvements on “shaping” tasks on WMFT. The greater improvement in the scores of FMA was seen in m-CIMT group than traditional rehabilitation group corresponded with those of previous studies carried out by Page et al.[13,17] The substantial improvement pattern reflected by FMA, in the m-CIMT group suggested that m-CIMT reversed impairments rather than simply helped patients to adapt to residual impairments.
In-line with a previous report, statistically significant improvements in hand function after CIMT was observed. Furthermore, analysis showed that more of the subjects scored relatively higher for hand improvement. Several possibilities may account for this. First is an inclusion criterion. In-line with methodologies described in previous CIMT studies, only subjects capable of at least 10° extension at the finger joints were enrolled. This inclusion criterion is further supported by a recent article, which suggests that finger extension ability predicts the effects of CIMT. Another influence is the “shaping” tasks that the subjects practiced during CIMT. In this study, graded shaping programs were used and the neural mechanism underlying the effects of CIMT is also likely to affect results. The motor learning literature suggests that massed practice has only a neutral or negative effect on the learning of continuous tasks and a variable effect on the learning of discrete tasks. However, CI Therapy employs massed practice to increase the tendency of patients to use their more-impaired limb, and thereby induces a use-dependent functional reorganization of brain structures.[26]
Recent studies using brain imaging and transcranial magnetic stimulation techniques show that after CIMT there is reorganization in the motor representations of upper extremities. Penfield’s brain mapping shows that the motor areas for the hands are much larger than for shoulder, elbow, and forearm. Correspondingly, cortical reorganization induced by intensive training involving the upper extremity has been reported most frequently for the hand representation areas within the primary motor cortex. The consequent increase in more affected arm use, involving sustained and repeated practice of functional arm movements, induces expansion of the contralateral cortical area controlling movement of the more-affected arm and recruitment of new ipsilateral areas. This use-dependent cortical reorganization may serve as the neural basis for the permanent increase in use of the affected arm. This kind of evidence suggests that body elements with the most extensive cortical representation areas are more likely to show training-induced neural changes.
It is believed that stroke subjects express greater motor disability on their more affected sides than that which actually exists. Over time, this movement suppression or learned non-use becomes so habitual that subjects use the less affected side for most ADLs. Data from this study provide further support for contention that learned non-use after stroke can be overcome by m-CIMT.[13,15] Furthermore, WMFT and FMA data support the contention that m-CIMT participation can elicit functional changes.
Our study and previous ones demonstrate that implementation of m-CIMT during early stoke rehabilitation is safe and feasible. No significant adverse events occurred during the intervention period, and no loss of motor function caused by restraint of unaffected arm could be detected. Therefore, available evidence in human stroke subjects undergoing m-CIMT does not bear out the potential adverse consequences of early overutilization of the affected limb as seen in animal studies. Given that, the previous CIMT studies reported use dependent cortical reorganizations[27] and given that remarkably short training protocols have induced cortical and functional changes[28] increases in affected limb use that we observe are believed to have caused cortical reorganization that resulted in functional improvement. The m-CIMT protocol emphasizes intensive practice and use of functional tasks to train the affected arm. Intensive practice of the affected arm might provide sufficient proprioceptive and visual feedback to develop the internal models for feed forward control of movement. Thus, subjects are able to preplan motor patterns more efficiently after m-CIMT. However, m-CIMT did not improve efficiency of movement execution (normalized movement time) or movement smoothness (normalized movement units) as it happens in conventional rehabilitation.[29]
The original CIMT protocol advocates restriction of the unaffected upper extremity for 90% of waking hours for 2 week.[30] This original protocol however, is mentally challenging and often results in poor compliance. In this study, to promote clinical compliance, modified version of CIMT was employed in which the duration of restraint was combined total of 10 h/day and intermission was freely allowed on request. Moreover, results of previous study suggest that, while at least 3 h of restraint per day is effective, it is less effective than 6 h.[31] To clarify this issue, further studies are needed. There are few limitations of our study like: Small sample size due to limited stroke subjects, the rater who was not blinded to the study. Another limitation could be no follow-up after the intervention and hence the improvement of upper extremity function in two groups could not be better compared.
Conclusion
Authors conclude that m-CIMT is effective in improving function and use of the affected upper extremity in stroke subjects.
Acknowledgments
My sincere thanks to DEAN, SMIMS, Gangtok, Sikkim for providing support and guidance throughout the study, and also I would like to thank all my patients as it was not possible to do this endeavor without their cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Jones SP, Leathley MJ, McAdam JJ, Watkins CL. Physiological monitoring in acute stroke: A literature review. J AdvNurs. 2007;60:577–94. doi: 10.1111/j.1365-2648.2007.04510.x. [DOI] [PubMed] [Google Scholar]
- 2.Pang MY, Ashe MC, Eng JJ. Muscle weakness, spasticity and disuse contribute to demineralization and geometric changes in the radius following chronic stroke. Osteoporos Int. 2007;18:1243–52. doi: 10.1007/s00198-007-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- 4.Grotta JC, Noser EA, Ro T, Boake C, Levin H, Aronowski J, et al. Constraint-induced movement therapy. Stroke. 2004;35:2699–701. doi: 10.1161/01.STR.0000143320.64953.c4. [DOI] [PubMed] [Google Scholar]
- 5.Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: Compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15:173–89. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- 6.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–5. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 7.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 8.Sabatini U, Toni D, Pantano P, Brughitta G, Padovani A, Bozzao L, et al. Motor recovery after early brain damage. A case of brain plasticity. Stroke. 1994;25:514–7. doi: 10.1161/01.str.25.2.514. [DOI] [PubMed] [Google Scholar]
- 9.Page SJ, Sisto S, Johnston MV, Levine P. Modified constraint-induced therapy after subacute stroke: A preliminary study. Neurorehabil Neural Repair. 2002;16:290–5. doi: 10.1177/154596830201600307. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SL. Revisiting constraint-induced movement therapy: Are we too smitten with the mitten. Is all nonuse“learned”? And other quandaries. Phys Ther. 2007;87:1212–23. doi: 10.2522/ptj.20060355. [DOI] [PubMed] [Google Scholar]
- 11.Page SJ, Levine P, Sisto S, Bond Q, Johnston MV. Stroke patients’ and therapists’ opinions of constraint-induced movement therapy. Clin Rehabil. 2002;16:55–60. doi: 10.1191/0269215502cr473oa. [DOI] [PubMed] [Google Scholar]
- 12.Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys Ther. 1999;79:847–53. [PubMed] [Google Scholar]
- 13.Page SJ, Sisto SA, Levine P, Johnston MV, Hughes M. Modified constraint induced therapy: A randomized feasibility and efficacy study. J Rehabil Res Dev. 2001;38:583–90. [PubMed] [Google Scholar]
- 14.Page SJ, Sisto SA, Levine P. Modified constraint-induced therapy in chronic stroke. Am J Phys Med Rehabil. 2002;81:870–5. doi: 10.1097/00002060-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Page SJ, Sisto S, Johnston MV, Levine P, Hughes M. Modified constraint-induced therapy in subacute stroke: A case report. Arch Phys Med Rehabil. 2002;83:286–90. doi: 10.1053/apmr.2002.28007. [DOI] [PubMed] [Google Scholar]
- 16.Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: A randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- 17.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: A single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14–8. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 18.Hakkennes S, Keating JL. Constraint-induced movement therapy following stroke: A systematic review of randomised controlled trials. Aust J Physiother. 2005;51:221–31. doi: 10.1016/s0004-9514(05)70003-9. [DOI] [PubMed] [Google Scholar]
- 19.Szaflarski JP, Page SJ, Kissela BM, Lee JH, Levine P, Strakowski SM. Cortical reorganization following modified constraint-induced movement therapy: A study of 4 patients with chronic stroke. Arch Phys Med Rehabil. 2006;87:1052–8. doi: 10.1016/j.apmr.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. 2005;36:1172–7. doi: 10.1161/01.STR.0000165922.96430.d0. [DOI] [PubMed] [Google Scholar]
- 21.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: The EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- 24.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. ExpNeurol. 1989;104:125–32. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris DM, Uswatte G, Crago JE, Cook EW, 3rd, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–5. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt RA. Motor Control and Learning. 2nd ed. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 27.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–6. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 28.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–23. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 29.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends CognSci. 2000;4:423–31. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 30.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: Results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–75. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 31.Sterr A, Elbert T, Berthold I, Kölbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: An exploratory study. Arch Phys Med Rehabil. 2002;83:1374–7. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]


