Abstract
Background:
The present understanding of the clinical course, complications, and outcome of myasthenic crisis (MC) is based chiefly on observational studies and retrospective case series.
Aim:
To study the baseline demographic and clinical variables, risk factors, complications, outcome, and mortality in patients of MC.
Materials and Methods:
All patients of myasthenia gravis (MG) who presented with myasthenic crisis between July 2009 and December 2010 were included.
Results:
Ten patients of MC were included in this study. The median age of the patients was 40.5 years (range 14-71 years). Seven were females and three were males. Nine had generalized MG and one patient had oculobulbar involvement only. Median duration of disease was 3 years (range 1 month to17 years). Two patients had thymoma. Two patients had history of thymectomy in the past. Infection was the most common triggering factor accounting for five cases (50%) followed by inadequate treatment/drug withdrawal in three (30%) and steroid initiation and hypokalemia in the remaining two patients (20%). Median duration of MC was 12 days (range 3-28 days). Mortality was in 3 out of 10 (30%) during MC. Management in the intensive care unit (ICU) and treatment with plasma exchange/intravenous immunoglobulins were associated with good outcome.
Conclusions:
Ventilator support and management in intensive care unit are the most important components in the management of MC. The high mortality rate seen in present study may be more reflective of the actual ground reality in resource constrained developing countries, however, larger prospective studies are needed to confirm these findings.
Keywords: Intravenous immunoglobulins, mechanical ventilation, myasthenic crisis, myasthenia gravis, plasma exchange
Introduction
Myasthenic crisis (MC), traditionally defined as myasthenic weakness leading to respiratory failure requiring intubation and mechanical ventilation is a potentially life-threatening complication that affects about 15-20% of all patients with generalized autoimmune myasthenia gravis (MG).[1–3] It is a life-threatening medical emergency requiring early diagnosis and respiratory assistance. Mortality in MC has improved considerably largely due to improvement in respiratory care and intensive care unit management.[3–5]
The widespread use of immunotherapy, such as plasmapheresis and intravenous immunoglobulin has substantially altered the management of MC.[3–5] Our current understanding of the clinical course, complications, and outcome of MC is based chiefly on reports of patients treated in the 1970s and early 1980s[4,6–9] and retrospective case series reported after the 1990’s.[3,10,11–13] However, few prospective studies of MC are available.[3,11,14] In this prospective study of MC patients, various factors leading to MC and influencing the course during hospital stay and final outcome were studied.
Materials and Methods
All consecutive patients of MG who presented with myasthenic crisis between July 2009 and December 2010 were included. Inclusion criteria included (a) patients of MG diagnosed clinically and confirmed by at least one of the following tests: Response to neostigmine, presence of acetylcholine receptor (AChR) antibodies and repetitive nerve stimulation and (b) presence of MC defined as respiratory failure requiring intubation and mechanical ventilation. Myasthenic patients intubated for respiratory failure due to severe congestive heart failure, acute respiratory distress syndrome (ARDS), and hypoxic-ischemic coma were excluded. All MC patients were evaluated with regard to various demographic factors and clinical information like age, sex, duration from onset of MG. Clinical features of crisis including possible precipitating factors and factors associated with functional outcome and mortality were studied.
Statistical analysis
All quantitative variables were estimated using measures of central location (mean, median), measures of dispersion (standard deviation and standard error) and interquartile range. As sample size was very small, means were compared using Mann-Whitney test. Qualitative or categorical variables were described as frequencies and proportions. Proportions were compared using Chi square or Fisher’s exact test, whichever was applicable. All statistical tests were two-sided and performed at a significance level of α = 0.05.
Results
Ten patients of MG were admitted with MC during the study period. Baseline characteristics are shown in Tables 1 and 2. Mean age of patients was 42.5 years with a range of 14-71 years. Male:Female ratio was 1:2.3. Eight patients were young onset MG (< 50 years). In young onset MC (< 50 years) seven out of eight patients (87.5%) were females. In delayed onset MC both patients were males. In young onset myasthenia patients median age was 35.5 years comprising mainly of women, (seven out of eight) and in late onset myasthenia comprising of two males and no females, median age was 70.5 years. Average duration of disease was 4.52 years (median-3 years) with minimum duration of 1 month to maximum of 17 years. For young onset myasthenia patients it was 3.50 years and for old onset patients, it was 8.58 years.
Table 1.
Demographic profile of patients
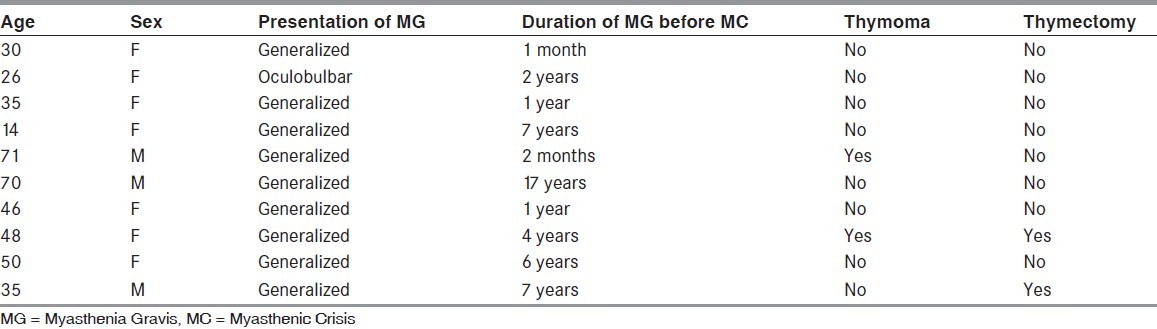
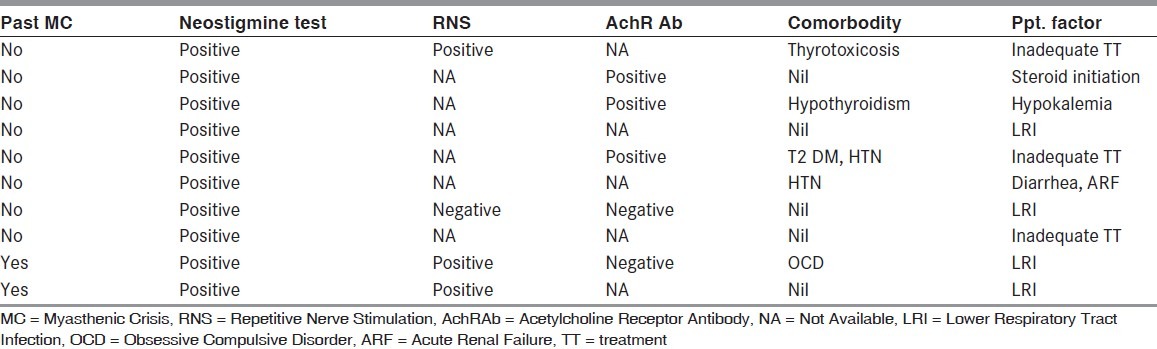
Table 2.
Clinical characteristics of patients
Trigger event
Infection was the most common triggering factor accounting for five cases (50%) followed by inadequate treatment/ drug withdrawal in three (30%) and steroid initiation and hypokalemia in the remaining two patients (20%). Among infections respiratory tract infection was the commonest (75%) followed by gastrointestinal infection (25%).
Treatment received
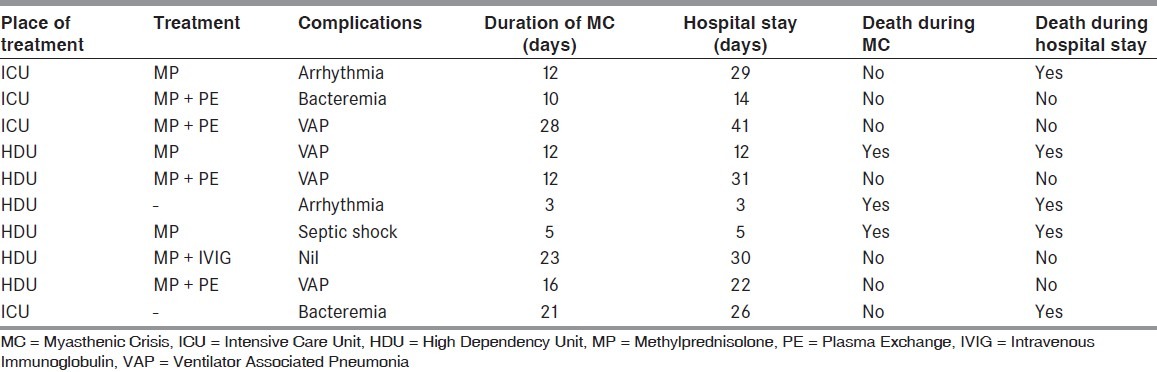
Acetyl cholinesterase inhibitors were stopped in all patients and restarted when weaning was planned. Three patients (30%) received pulsed IV methylprednisolone therapy alone (1 g IV for 5 days) [Table 3]. Four patients received methylprednisolone pulse and plasma exchange (five cycles over 10 days). One patient received methylprednisolone pulse and intravenous immunoglobulin (IVIG). Two patients did not receive methylprednisolone or plasma exchange/IVIG because of severe sepsis and unstable hemodynamic status. One of these two patients required hemodialysis for renal failure.
Table 3.
Treatment received and outcome of patients
Duration of hospital stay
Of the 10 patients 4 patients were managed in intensive care unit (ICU) and 6 patients in high dependency unit (HDU) where nurse patient ratio was 6:1 as compared with 1:1 in ICU. Mean duration of ventilatory support was 14.20 days (median-12 days) with range of 3-28 days. Average duration of hospital stay was 21.3 days (median 24 days) with a range of 3-41 days. Ventilator associated pneumonia was seen in four patients. In addition, hospital acquired blood stream infections were seen in three patients.
Outcome
Mortality was in 3 out of 10 cases (30%) during MC [Table 3]. Overall mortality during hospital stay was in 5 out of 10 cases (50%). Seven patients recovered from MC and were weaned off ventilator but two of these died later during hospital stay after being weaned off from ventilator. Patients who received plasma exchange/IVIG had significantly better outcome (P = 0.04). There was no statistically significant difference in other parameters (viz., age, sex, duration of MG, trigger event, biochemical parameters, or duration of ventilation) between patients who survived and who succumbed to MC.
Cause of death
Of the three patients who succumbed to MC, one patient had bronchopneumonia and refractory septic shock. Another patient had fulminant diarrhea followed by renal failure and had cardiac arrest during hemodialysis. Third patient had sudden cardiac death probably secondary to cardiac arrhythmia.
Among the two patients who expired after being weaned off ventilator, one had sudden cardiac death due to cardiac arrhythmia. Other patient had thyrotoxicosis and had suffered cardiac arrest while on ventilator; she was revived but had hypoxic ischemic brain injury. She succumbed after being weaned off ventilator.
Discussion
MC, defined as respiratory failure requiring ventilatory support, is a potentially life-threatening complication that occurs in approximately 15-20% of patients.[7,8,11] In this prospective study consecutive patients of MC were included who presented between July 2009 and December 2010.
Demographic profile
The demographic profile reflects that young onset MC is predominantly a disease of young women in their third and fourth decade where as no female preponderance is seen in late onset myasthenia. This is in agreement with earlier studies where preponderance of female patients has been noted in young MC patients and equal sex distribution in delayed onset myasthenia patients, though overall female predominance was noted.[4,7,15] The MC therefore has a bimodal age of distribution with an early peak affecting primarily women, and a later peak affecting both sexes equally.
The median interval from onset of myasthenic symptoms to crisis was 3.0 years. Seventy percent experienced the initial crisis within 2 years of disease onset. This data supports the concept that MG is most severe during early 2-3 years. Earlier studies have reported median interval ranging from 8 months to 5-6 years[3,4,9] with more recent studies reporting shorter interval as compared with earlier ones.[12–14] Improved disease control, with fewer episodes of crisis in patients with longstanding MG, may explain why the interval from onset to first crisis has fallen in recent years.
Clinical features of crisis
Ninety percent patients had generalized MG and 10% patients had oculobulbar disease. Median duration of crisis was 12 days and median duration of hospital stay was 24 days. This is in agreement with earlier studies. An uncomplicated MC therefore usually recovers over 2 weeks. Thomas et al.[3] identified three independent predictors of prolonged intubation: Pre-intubation serum bicarbonate 230 mg/dl, peak vital capacity day 1-6 post-intubation < 25 ml/kg and age >50 years. The proportion of patients remaining intubated after 14 days was 0% (0/11) with no risk factors, 21% (4/19) with one risk factor, 46% (7/15) with two risk factors, and 88% (7/8) with three risk factors. In the present study due to small sample size the confidence interval was too large to study the statistical significance of various parameters leading to prolonged intubation.
Despite advances in the management of MC and tremendous improvement in the mortality rate the duration of crisis has changed little over past 50 years ever since mechanical ventilation became available. This observation suggests that ventilator support is the most important aspect in the management of MC. Other drug therapies though effective in improving survival have not resulted in further reduction in the duration of crisis.
Precipitants of crisis
Similar to previous studies[3,4,7,9,15] infection, especially respiratory tract infection was the most common precipitant of MC accounting for 50% of crisis episodes followed by inadequate treatment/drug withdrawal. Inadequate treatment and drug withdrawal were also a frequent cause of crisis accounting for 3 patients out of 10. A previous Indian study by Panda et al.[12] had also reported drug withdrawal as the frequent trigger of MC in their patients being responsible for crisis in 3 out of 11 patients. This trigger may be as important as infection as a precipitant to MC in developing countries like India due to poor awareness on the part of patients in addition to drug nonaffordability.
Management
Ventilator support is the most important component in the management of MC. This requires management of patient in an ICU set up. In a resource limited set up of developing countries it may not be always possible to admit each and every patient in an ICU. In this study also only 4 out of 10 patients could find a place in ICU rest all were managed in HDU. Place of treatment was, however, very important determinant of outcome as all the patients managed in ICU recovered from MC, where 50% patients (3/6) managed in HDU expired. This data emphasizes the role of intensive care as reported in literature where with improved respiratory care alone, including the use of positive pressure ventilation, mortality fell from 43% to 17% from the mid-1950s to 1962 at Mt. Sinai Medical Center and from 42% to 6% from 1960 to 1979 at Columbia-Presbyterian Medical Center.[3] Despite a dramatic reduction in mortality in the last 50 years, the median duration of intubation for MC (2 weeks) has changed little.
Drug therapy
Plasma exchange and intravenous immune globulin (IVIG)
In this study in addition to pulsed IV methylprednisolone, four patients received plasma exchange and one patient received IVIG. All patients who received plasma exchange or IVIG recovered from MC. Since only one patient received IVIG, comparison between plasma exchange and IVIG is not possible due to small sample size. Of the three patients who received pulsed IV methylprednisolone alone, two expired whereas all the four patients who received plasma exchange or IVIG recovered from crisis. Therefore plasma exchange/IVIG does appear superior to methylprednisolone alone. Although the trials do not show a clear difference between IVIG and plasmapheresis in the treatment of MC, there is suggestive evidence and personal clinical experience that plasmapheresis works more quickly than IVIG in seriously ill patients with myasthenia.[16–18]
Complications during treatment
As expected in critically ill patients on life support systems, hospital acquired infection was the commonest complication seen. Ventilator associated pneumonia was seen in four patients (40%) and blood stream infections in two patients (20%). However, cardiac arrhythmias and sudden cardiac death were also seen. Cardiac arrhythmias seen were atrial fibrillation in one patient and narrow complex tachycardia and sinus bradycardia in another patient, this patient was also suffering from thyrotoxicosis. Two other patients had sudden cardiac death possibly due to cardiac arrhythmia. Cardiac involvement in MG has been suggested.[19] In addition to acetylcholine receptors, heart and skeletal muscles are also speculated to be autoimmune targets in MG.[20] These are considered antistriational antibodies and include autoantibodies to titin, ryanodine receptor, and muscular voltage-gated potassium channel.[21,22] Cardiac involvement in MG may take several forms, ranging from asymptomatic ECG changes to ventricular tachycardia, myocarditis, conduction disorders, heart failure, and sudden death.[23]
Outcome
In this study three patients (30%) succumbed to MC. All these patients were being managed in HDU. All the patients who were managed in ICU recovered from MC. This underscores the importance of intensive care in the management of MC. In addition two more patients who recovered from MC died later during the hospital stay, both of whom had cardiac arrhythmia. Thomas et al.[3] reported a mortality rate of 4% (3/73) in their case series. Other studies have reported estimates of mortality in MG crisis as high as 6-16.7%.[3,12,13,24] Among the Indian studies Aggarwal et al.[14] reported no mortality in their case series of six patients, Murthy et al.[13] reported 2 deaths (9.5%) in a case series of 21 patients, and Panda et al.[12] reported only 1 death (9%) in their case series of 11 patients. However, all these Indian studies were retrospective case series of patients admitted in ICU. The higher fatality rate seen in this study speaks for the fact that this was a prospective study which included all consecutive patients presenting in the emergency department with MC irrespective of their place of management, for example, ICU or HDU. The present study may be more reflective of the actual ground reality in resource constrained developing countries.
Conclusion
Myasthenic crisis has favorable outcome with timely respiratory support and management in ICU. However in resource constrained developing countries with many of the patients of myasthenic crisis still being managed outside ICU setting and prohibitive cost of immunomodulatory therapy, the prognosis of myasthenic crisis over all may not be as favorable. However, larger prospective studies from developing countries are needed to confirm these findings.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Fink ME. Treatment of the critically ill patient with myasthenia gravis. In: Ropper AH, editor. Neurological and neurosurgical intensive care. 3rd ed. New York: Raven Press; 1993. pp. 351–62. [Google Scholar]
- 2.Chaudhuri A, Behan PO. Myasthenic crisis. QJM. 2009;102:97–107. doi: 10.1093/qjmed/hcn152. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CE, Mayer SA, Gungor Y, Swarup R, Webster EA, Chang I, et al. Myasthenic crisis: Clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48:1253–60. doi: 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: Crisis and death. Ann N Y Acad Sci. 1981;377:670–7. doi: 10.1111/j.1749-6632.1981.tb33765.x. [DOI] [PubMed] [Google Scholar]
- 5.Juel VC. Myasthenia gravis: Management of myasthenic crisis and perioperative care. Semin Neurol. 2004;24:75–81. doi: 10.1055/s-2004-829595. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins RB, Witorsch P, Smyth NP. Aspects of treatment of crisis in myasthenia gravis. South Med J. 1970;63:1127–30. doi: 10.1097/00007611-197010000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson IT, Murphy RP, Lascelles RG. Ventilatory failure in myasthenia gravis. J Neurol Neurosurg Psychiatry. 1982;45:217–22. doi: 10.1136/jnnp.45.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gracey DR, Divertie MB, Howard FM., Jr Mechanical ventilation for respiratory failure in myasthenia gravis. Two-year experience with 22 patients. Mayo Clin Proc. 1983;58:597–602. [PubMed] [Google Scholar]
- 9.Sellman MS, Mayer RF. Treatment of myasthenic crisis in late life. South Med J. 1985;78:1208–10. doi: 10.1097/00007611-198510000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Phillips LH. The epidemiology of myasthenia gravis. Semin Neurol. 2004;24:17–20. doi: 10.1055/s-2004-829593. [DOI] [PubMed] [Google Scholar]
- 11.Jani-Acsadi A, Lisak RP. Myasthenic crisis: Guidelines for prevention and treatment. J Neurol Sci. 2007;261:127–33. doi: 10.1016/j.jns.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Goyal V, Behari M, Singh S, Srivastava T. Myasthenic crisis: A retrospective study. Neurol India. 2004;52:453–6. [PubMed] [Google Scholar]
- 13.Murthy JM, Meena AK, Chowdary GV, Naryanan JT. Myasthenic crisis: Clinical features, complications and mortality. Neurol India. 2005;53:37–40. doi: 10.4103/0028-3886.15050. discussion 40. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal AN, Gupta D, Behera D, Prabhakar S, Jindal SK. Intensive respiratory care in patients with myasthenic crisis. Neurol India. 2002;50:348–51. [PubMed] [Google Scholar]
- 15.Perlo VP, Poskanzer DC, Schwab RS, Viets HR, Osserman KE, Genkins G. Myasthenia gravis: Evaluation of treatment in 1,355 patients. Neurology. 1966;16:431–9. doi: 10.1212/wnl.16.5.431. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AI, Choudhry MA, Akbar MS, Mohammad Y, Chua HC, Yahia AM, et al. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology. 1999;52:629–32. doi: 10.1212/wnl.52.3.629. [DOI] [PubMed] [Google Scholar]
- 17.Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2008:CD002277. doi: 10.1002/14651858.CD002277.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann Neurol. 1997;41:789–96. doi: 10.1002/ana.410410615. [DOI] [PubMed] [Google Scholar]
- 19.Gibson TC. The heart in myasthenia gravis. Am Heart J. 1975;90:389–96. doi: 10.1016/0002-8703(75)90330-0. [DOI] [PubMed] [Google Scholar]
- 20.Aarli JA. Inflammatory myopathy in myasthenia gravis. Curr Opin Neurol. 1998;11:233–4. doi: 10.1097/00019052-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Satoh T, Yasuoka H, Hamaguchi Y, Tanaka K, Kawakami Y, et al. Novel autoantibodies to a voltage-gated potassium channel Kv1. 4 in a severe form of myasthenia gravis. J Neuroimmunol. 2005;170:141–9. doi: 10.1016/j.jneuroim.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Romi F, Skeie GO, Gilhus NE, Aarli JA. Striational antibodies in myasthenia gravis: Reactivity and possible clinical significance. Arch Neurol. 2005;62:442–6. doi: 10.1001/archneur.62.3.442. [DOI] [PubMed] [Google Scholar]
- 23.Calin C, Savu O, Dumitru D, Ghiorghiu I, Cålin A, Capraru C, et al. Cardiac involvement in myasthenia gravis--is there a specific pattern? Rom J Intern Med. 2009;47:179–89. [PubMed] [Google Scholar]
- 24.Werneck LC, Scola RH, Germiniani FM, Comerlato EA, Cunha FM. Myasthenic crisis: Report of 24 cases. Arq Neuropsiquiatr. 2002;60:519–26. doi: 10.1590/s0004-282x2002000400001. [DOI] [PubMed] [Google Scholar]


