Abstract
Nummular headache (NH) is a newly categorized headache disorder characterized by a consistent clinicographics in each attack. Currently, it is considered as a primary headache disorder due to epicranial neuralgia but the pathomechanism is still unknown. We report a woman, whose recurrent NH subsided after trans-sphenoidal surgery for her pituitary oncocytoma. The recovery of NH in this patient encourages the central mechanism for NH occurrence. After a review of literature concerning, NH and intracranial secondaries we propose that central NH is a referral pain from pain-sensitive structures, such as meninges, superimposing by pre-existing lower pain threshold or pain modulation.
Keywords: Headache, nummular headache, oncocytoma, pituitary, referred pain
Introduction
Nummular headache (NH) is a newly headache disorder firstly reported at 2002 by Pareja et al.[1] According to the 2nd edition of International Classification of Headache Disorders, the diagnostic criteria of NH included a consistent rounded or elliptical shape of painful area typically 2-6 cm in diameter, and chronic and either continuous or interrupted by spontaneous remissions lasting weeks to months.[2] Besides, a consistent location of pain usually in the parietal region, lancinating exacerbations, and delineated boundary of painful area are characteristic features of NH.[2] Although, NH is proposed primary headache disorder due to epicranial neuralgia,[2–6] there is still no strong evidence to support this proposal, such as an absence of neuropathic change in painful area.[7]
Secondary headache can provide notable information for understanding the pathomechanism of its primary headache mimics. In contrast to a putative suggestion of peripheral neuralgia in NH, a few intracranial disorders, such as meningioma[8] or arachnoidal cyst,[9] have been found in patients presenting with a typical course of NH. The causal relation is further encouraged when pain subsides after a removal of meningioma in a patient.[8] Herein, we report a patient whose NH subsided after trans-sphenoidal microsurgery for pituitary macroadenoma. We review, the literature concerning for pituitary lesions and NH and make a proposal for central mechanism of NH.
Case Report
A 54-year-old woman was diagnosed the Behçet’s disease (BD) for more than 10 years and was remitted in recent 2 years. Since, 1 year ago, she suffered from a cyclic recurrence of headache; the pain was mild to moderate in intensity, lancinating or electric in nature, exclusively, located at vertex and left parasagittal area, circular in shape, 3.0 cm × 3.0 cm in size, and exhibited a clear-cut boundary from non-painful surrounding. It peaked and subsided spontaneously. There was no aura before or associated discomfort during attack. The severity of pain was intensified when she combed the hair or touched the painful area during pain attack. The intensity of pain did not significantly fluctuate substantially. In recent 1 month, she found herself easy for hitting objects at either side during walking without unsteadiness. She denied antecedent craniofacial trauma, migraine, herb or supplement usage, or consumption of alcohol or cigarette before.
On presentation, her vital signs were stable. She described her pain being continuing for 3 days without remission and also she was oriented and co-operative. Visual field showed a defect at the lateral part of temporal visual fields at both sides, or an incomplete chiasmal visual defect. No papilledema was seen. Neurological examination did not reveal abnormal finding. Within the painful area, there was no trophic change, erythema, or edema; however, mild hyperesthesia to pinprick pain was detected. When the hair was stretched or scalp was pressed, pain was exacerbated. Temporal artery was not tender or engorged. Bruit was not heard. Percussion pain was not elicited at temporomandibular joint, sinus or cervical spine. Clinically, her headache was not compatible with migraine, tension headache, non-structural headache of Behçet, or any known primary or secondary headache disorder.[2] Finally, NH was interpreted.
Head magnetic resonance imaging revealed a huge mass at the suprasellar region [Figure 1a and b]. The serum adenohypophyseal hormones were within reference range. Trans-sphenoidal microsurgery was performed after consent. Pathological study revealed adenoma cells with weakly eosinophilic staining. Immunohistochemical staining did not disclose a positive staining of prolactin, growth hormone, corticotropin, thyrotropin, or gonadal hormones. Electro microscopy showed a few secretory vesicles whereas an abundance of intracellular mitochondria [Figure 1c], compatible with non-functional oncocytoma. Patient subjectively, stated that the intensity and frequency of pain rapidly subsided after surgical procedure. On a follow-up of 6 years, headache did not recur.
Figure 1.
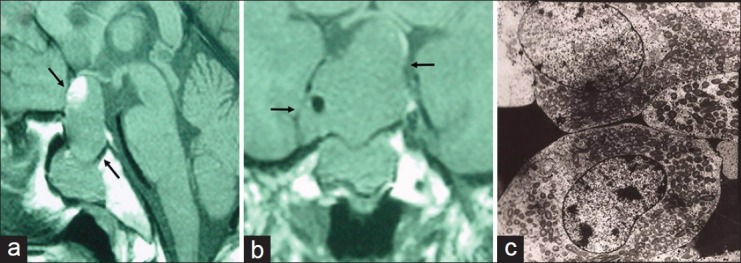
The magnetic resonance imaging showed a huge suprasellar macroadenoma with central necrosis (a and b) (arrow). The electro microscopy of the macroadenoma revealed an abundance of mitochondria in cytoplasm, compatible with pituitary oncocytoma (c)
Discussion
The consistent clinicographics of pain and cyclic recurrence in our patient are compatible with the diagnostic criteria and typical course of NH.[2–4] In previous, a concurrent sensory alteration or motor atrophic change within the painful area is considered supportive evidence for peripheral involvement in NH. However, current knowledge has acknowledged that allodynia and hyperesthesia within the painful area is not unique to neuropathic change of distal nerve in epicranial tissue but can also occur in central sensitization.[10] Moreover, focal trophic change within the painful area is, factually, infrequently found in NH patients.[3] Accordingly, central source of NH cannot be completely excluded simply by a presence of allodynia/hyperesthesia within the painful area as in our patient.
An involvement of the sellar and regional structures associated with NH as our patient is not an exception. Three such patients have been previously reported in literature. Grosberg et al.,[11] described a patient who suffered NH shortly after sinus surgery. Alvaro et al.,[12] mentioned a woman who suffered NH a few days after trans-sphenoidal surgery for the pituitary growth hormone adenoma. The drug response and prognosis in these two patients was not further discussed. Yin et al.,[13] reported a man in whom NH occurred shortly after trans-sphenoidal surgery for prolactinoma. The pain responded favorably to gabapentin. The main distinction between our patient and previously reported three patients is an occurrence of NH without antecedent head trauma or surgery. Nevertheless, the findings in these four patients suggest that NH is not a post-craniotomy headache in previously reported patients, and a tight relation between NH and an involvement of the sellar and regional structures is believed.
Clinically, pituitary tumor can provoke symptomatic headache radiating to vertex in 10-31% of patients.[14,15] This remote pain is recognized a form of referred pain conveyed by the trigeminosensory nerve. In previously reported three NH patients[11–13] and ours, their pain exclusively occurred at the vertex, suggesting that their pain share a common pain-referral pathway with non-NH pain. Since, NH is an uncommon painful complication in sellar disorders; other mechanism is believed to participate for its characteristic clinicographics.
Reviewing the literature, we find two important points probably relating to the occurrence of central NH. Firstly, besides of the previously reported three patients and our patient associated with involvement of sellar and regional structures,[11–13] there are another three NH patients in whom intracranial secondaries are found diffusely at cerebellar tentorium,[8] high parietal cortical area,[9] and parietotemporal cortical area,[9] respectively. These intracranial secondaries are exclusively located at the ipsilateral juxtameninges and are also close to the painful area in them.[8,9] Taken together, central NH is reasonably suggested a form of referral pain from pain-sensitive structures, especially meninges, at elsewhere. The intracranial secondary is likely nearby the painful area. Secondly, pre-existing factor, such as craniofacial surgery,[11] antecedent head trauma[1,11,12] or migraine,[1,3] is present in these central NH patients. Accordingly, dural involvement, superimposing by a pre-existing lower threshold of pain or trigger of pain response,[15] may co-operatively trigger painful event in central NH patients.
Our patient also has had BD. Headache occurs in 60% of BD patients, especially in case of acute type of BD.[16] Migraine and tension type of headache constitute over half of them, and the nonstructural headache of Behçet is approximately 20%.[17] The remainders are other primary headache syndromes, direct or indirect secondary headache consequence to BD, and unclassified headache syndromes.[17] Until now, NH has not been mentioned in BD patients in literature yet. If a causal relation exists between BD and NH is far from conclusion in our patient herein. However, BD may perhaps modulate the pain threshold or pain response to facilitate NH occurrence in our patient.
In conclusion, we report a woman who exhibited a typical course of NH was identified the pituitary oncocytoma finally. The NH was favorably responded to tumor resection. The findings of this patient suggest that NH can be a referred pain from intracranial secondary involving the nearby pain-sensitive structures, especially, the meninges. NH should be alerted for central source even when sensory algometry is present within the painful area, or an additional neurological deficit is detected, such as visual defect in our patient.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Pareja JA, Caminero AB, Serra J, Barriga FJ, Barón M, Dobato JL, et al. Numular headache: A coin-shaped cephalgia. Neurology. 2002;58:1678–9. doi: 10.1212/wnl.58.11.1678. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Subcommittee of the International Headache Society. The International classification of headache disorders: 2nd ed. Cephalalgia. 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen WH. Nummular headache: A review of world literature [Chin] TSMH Med Nurs J. [In press] [Google Scholar]
- 4.Pareja JA, Montojo T, Alvarez M. Nummular headache update. Curr Neurol Neurosci Rep. 2012;12:118–24. doi: 10.1007/s11910-011-0247-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen WH, Chen YT, Lin CS, Li TH, Lee LH, Chen CJ. A high prevalence of autoimmune indices and disorders in primary nummular headache. J Neurol Sci. 2012;320:127–30. doi: 10.1016/j.jns.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen WH, Li TH, Lee LH, Huang CC. Varicella-zoster virus infection and nummular headache: A possible association with epicranial neuralgia. Intern Med. 2012;51:2439–41. doi: 10.2169/internalmedicine.51.7998. [DOI] [PubMed] [Google Scholar]
- 7.Pareja JA, Cuadrado ML, Fernández-de-las Peñas C, Nieto C, Sols M, Pinedo F. Nummular headache with trophic changes inside the painful area. Cephalalgia. 2008;28:186–90. doi: 10.1111/j.1468-2982.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- 8.Guillem A, Barriga FJ, Giménez-Roldán S. Nummular headache secondary to an intracranial mass lesion. Cephalalgia. 2007;27:943–4. doi: 10.1111/j.1468-2982.2007.01328.x. [DOI] [PubMed] [Google Scholar]
- 9.Guillem A, Barriga FJ, Giménez-Roldán S. Nummular headache associated to arachnoid cysts. J Headache Pain. 2009;10:215–7. doi: 10.1007/s10194-009-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosberg BM, Pavlovic J, Robbins MS, Napchan U, Solomon S, Lipton RB. Posttraumatic and postsurgical nummular headache (abstract). 52nd Annual Scientific Meeting American Headache Society; Los Angeles, CA. 2010. [Google Scholar]
- 12.Alvaro LC, García JM, Areitio E. Nummular headache: A series with symptomatic and primary cases. Cephalalgia. 2009;29:379–83. doi: 10.1111/j.1468-2982.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 13.Yin HL, Chui C, Tung WF, Chen WH. Nummular headache after trans-sphenoidal microsurgery: A referred pain-based headache syndrome. Polish J Neurol Neurosurg. doi: 10.5114/ninp.2013.36764. [In press] [DOI] [PubMed] [Google Scholar]
- 14.Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. The clinical characteristics of headache in patients with pituitary tumours. Brain. 2005;128:1921–30. doi: 10.1093/brain/awh525. [DOI] [PubMed] [Google Scholar]
- 15.Wang SJ, Hung CW, Fuh JL, Lirng JF, Hwu CM. Cranial autonomic symptoms in patients with pituitary adenoma presenting with headaches. Acta Neurol Taiwan. 2009;18:104–12. [PubMed] [Google Scholar]
- 16.Ideguchi H, Suda A, Takeno M, Kirino Y, Ihata A, Ueda A, et al. Neurological manifestations of Behçet’s disease in Japan: A study of 54 patients. J Neurol. 2010;257:1012–20. doi: 10.1007/s00415-010-5454-2. [DOI] [PubMed] [Google Scholar]
- 17.Monastero R, Mannino M, Lopez G, Camarda C, Cannizzaro C, Camarda LK, et al. Prevalence of headache in patients with Behçet’s disease without overt neurological involvement. Cephalalgia. 2003;23:105–8. doi: 10.1046/j.1468-2982.2003.00476.x. [DOI] [PubMed] [Google Scholar]


