Abstract
Objectives:
Sympathetic skin response (SSR) is a test for evaluation of the sympathetic sweat gland pathways, and it has been used to study the central sympathetic pathways in spinal cord injury (SCI). This study aimed to assess the autonomic pathways according to normal or abnormal SSR in urinary incontinence patients due to incomplete spinal cord injury.
Materials and Methods:
Suprapubic, palmar, and plantar SSR to the peripheral nerve electrical stimulation were recorded in 16 urinary incontinence patients with incomplete spinal cord injury at various neurological levels and in 30 healthy control subjects.
Results:
All the recordings of SSR from the incomplete SCI patients with urinary incontinence as compared with their counterparts in the control group showed significantly reduced amplitudes with more prominent reduction in the suprapubic area recording site (P value < 0.0004). SSR with significantly prolonged latencies were recorded from palm and plantar areas in response to suprapubic area and tibial N stimuli, respectively (P value < 0.02). In this study, a significantly higher stimulus intensity (P value < 0.01) was needed to elicit SSR in the cases compared with the control group.
Conclusion:
This study showed abnormal SSR in urinary incontinence patients due to incomplete SCI. In addition, for the first time we have described recording of abnormal SSR from the suprapubic area as another way to show bladder sympathetic system involvement.
Keywords: Spinal cord injury, sympathetic skin response, urinary incontinence
Introduction
Injury, vascular lesions, infection, and tumors of the spinal cord cause the majority of suprasacral neurogenic bladder problems.[1] Spinal cord injury (SCI) affects both spinal autonomic and somatic functions.[1] Due to SCI, functions of the lower urinary tract are lost. This may be due to deterioration of the urinary autonomic system, somatic system or both.[1] Urinary incontinence is one of the common presenting symptoms of this bladder dysfunction.[1] Moreover, diseases of the genitourinary system are currently the cause of death in some patients with SCI, requiring advancement in diagnostic tools and urologic management in these patients.[1]
Testing autonomic nervous system function is an important but difficult area of clinical neurophysiology but sympathetic skin response (SSR) is a fast, simple and obtainable test for possibility of evaluating autonomic system dysfunctions.[2–7] SSR has been studied in the peripheral as well as central autonomic nervous system dysfunctions,[2,8] such as neurogenic bladder. There are no contra-indications for recording of SSRs that is specific to SCI.[6,9] Some studies report the use of SSR in SCI and reported correlations with other autonomic and sensory disorders in these patients.[6,9]
Neurophysiologic and clinical application of SSR was first described by Shahani.[2] SSR is defined as the change of the electrical potential of the skin, showing sympathetic cholinergic function.[10] SSR is a somato-sympathetic reflex with a spinal, a bulbar, and a suprabulbar component. Reactive sites for evoked SSR extend from the posterior hypothalamus through the ventrolateral reticular formation of the pons and medulla, to the spinal cord, but until now, the definite central pathways in humans has not been precisely defined.[4–6]
The sympathetic innervations to the bladder neck or internal urethral sphincter, which modulates relaxation of the body of the bladder and narrowing of the bladder neck to inhibit voiding, are provided by the Illiohypogastric nerves, which exit from the spinal cord at T11-L2 segments. Due to SCI, this spinal sympathetic pathway can be deteriorated, causing incompetent bladder neck, and urinary incontinence.[1] An assessment of the sympathetic reflex with Sympathetic Skin Response test (SSR) in patients with incomplete SCI would be one of the ways to know neurophysiologic basis of urinary incontinence.
To the best of our knowledge, there is no pervious study to record SSR from the suprapubic, as a new technique, from homogenous population of patients with incomplete SCI with urinary incontinence in the literature. This study was conducted to investigate the effect of incomplete SCI on sympathetic skin response as a clue to the sympathetic nervous system involvement, in urinary incontinence patients due to incomplete SCI.
Subjects and Methods
Twenty-two urinary incontinence patients due to incomplete SCI were recruited sequentially in a 10-month interval from those who referred to Urodynamic clinic. We received the approval of ethics committee of our University and each subject (both case and control groups) gave verbal and written informed consent prior to participation in the study.
Exclusion criteria consisted of complete SCI, spinal shock, spinal cord hemi-section syndrome (Brown-Squared syndrome), cauda equina syndrome, traumatic brain injury, limb amputation, age over 60 years, positive history of peripheral neuropathy, diabetes mellitus, clinical depression or consumption of any drug affecting autonomic nervous system, open wounds, peripheral nerve lesions, casts, or dressings that could make SSR unobtainable technically or increase the risk of infection for the patient.[10–12] They underwent a nerve conduction study for evaluation of the median and tibial nerves. Of these, six patients that failed to show normal nerve conduction were excluded from the study. The other 16 patients were included in the study as the case group.
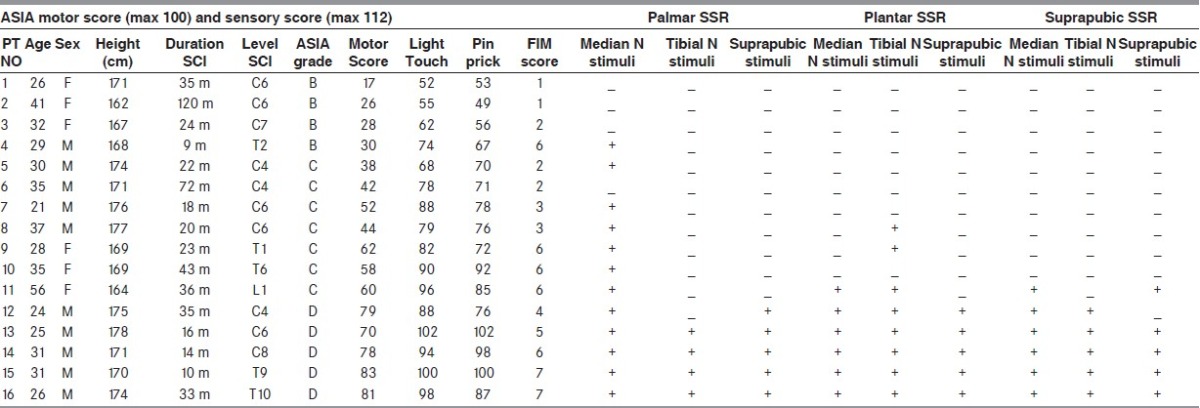
The American Spinal Injury Association (ASIA) impairment score was utilized for neurological classification of the level and severity of SCI in all cases. All the patients were investigated for the degree of independence in bladder management (elimination and perineal hygiene) with Functional Independence Measure (FIM).[1,11] A detailed clinical examination of all cases, including neurological level, ASIA motor and sensory scores, FIM grades, and duration from the onset of SCI is shown in Table 1.
Table 1.
SSR in incomplete SCI patients with urinary incontinence
Thirty healthy volunteers were selected as our control group; they had neither positive history of SCI nor other exclusion criteria for this study.
SSR: The tests were carried out on both patients and controls, sitting or supine and relaxed on a bed in a quiet dimly lit room, with the eyes open so as not to fall asleep; they were invited not to sigh, laugh, cough, or breathe deeply as far as possible during the study. Environmental and skin temperatures kept at 24°C and 32.8°C, respectively were controlled.[10–13] SSR was recorded by Synergy multi-linker EMG machine(viasys healthcare manor way, old woking surrey. GU22 9JU.UK, 2007). SSR was done for both upper limbs, both lower limbs, and suprapubic area, respectively and the recording was done separately for each recording site. For limbs SSR, active electrodes were placed on the palm of each hand and plantar surface of each foot, and the references were placed on the dorsum of the hand and foot, respectively. For suprapubic SSR, active electrode was placed in the midline just above the pubic bone and the reference was placed 4 cm distal (caudal) to it in the midline.
A band pass of 0.1-1 KHz, a base time of 500 ms/div and a sensitivity of 200 μV/div were used. The SSR was obtained using an electrical stimulation (the constant voltage stimulation) consisting of a single square-wave pulse of 0.1 ms duration and with an maximal intensity (target for considering absent SSR), just sufficient to produce a painful sensation in the control group and stimulate twice as much the motor threshold in SCI patient and recorded SSR from all recording sites with the same target stimulus intensity (we slowly increased the stimulus intensity to recording SSR or reach the maximal intensity). The electrical stimulation was applied to both median nerves, tibial nerves for each recording site (it was applied for the upper and lower limbs independently on both sides). Suprapubic stimulation was applied with 8 cm distance with active electrode below the umbilical area. The ground electrode was placed around the forearm and the leg, respectively.[14,15] It was positioned just 1 cm from the active electrode at the suprapubic area.
Ten stimuli were administered at random intervals of more than 60 s in the absence of distractions to avoid habituation for each stimulation site. Only reproducible responses without any movement artifact, that are consistent were selected for analysis (because each response can vary somewhat, then only responses that were consistent, with some variation in each subject, were selected for analysis). SSR latencies were measured from the origin of the trace to the first deflection of the trace from baseline. For each recording site, the mean latencies of 30 controls reproducible responses to each stimulation site were measured separately. The average of the peak-to-peak amplitude was calculated in the same manner for each recording site.[12–15]
Data were analyzed statistically using STATA (version 10, Stata Corp, Texas, USA.). Mann-Whitney test was used to compare the variables of interest between the case and the control groups. Spearman correlation coefficient was used to assess the linear relationship of ordinal variables in two groups. P Value < 0.05 was considered as statistically significant in this study. The values are presented as mean (+/- standard error of the mean or 95% confidence interval).
Results
Sixteen patients (10 males, with the mean age of 28.9 years and age range of 25.2-32.5 years and 6 females with the mean age of 36.3 years and age range of 26.7-45.9 years) were included in the study as the case group. Ten subjects had incomplete cervical SCI, five had thoracic incomplete SCI, and one had lumbar incomplete SCI. The cause of SCI was motor vehicle accidents in 11 patients, surgery in 2, trauma (falling) in 2 and infection in 1. The average time period between this investigation and the SCI was 33.87 (± 6.8) months, ranging from 9 to over 120 months. Thirty healthy volunteers (15 males, with the mean age of 27.8 years and age range of 24.5-31 years and 15 females with the mean age of 30.7 years and age range of 26.95-34.5 years) were selected as our control group. The SSR results for both case and control groups are presented in Tables 1 and 2.
Table 2.
Normal sympathetic skin response data
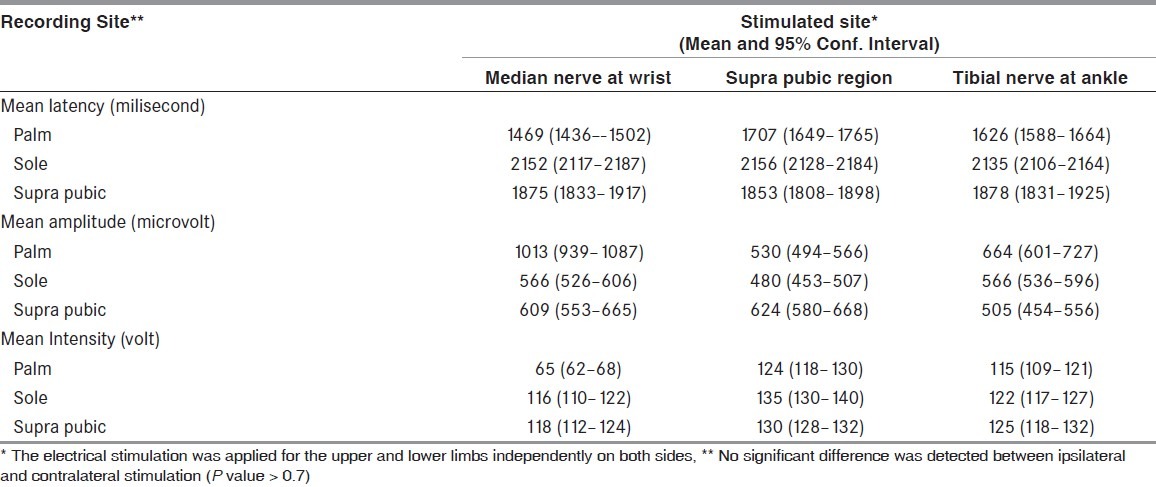
Control group: All the 30 healthy controls had palmar, plantar, and suprapubic areas SSR bilaterally. The SSR results in the palm’s larger amplitude and shorter latency than the sole. No significant difference was detected between ipsilateral and contralateral stimulation in SSR latencies and amplitudes from our controls (P value > 0.7).
Group B ASIA (sensory incomplete, but no motor function was preserved below the neurological level): Four patients with different neurological spinal cord injury were included in this group. No SSR response was obtained in the plantar and suprapubic areas in subjects from this group. Palmar SSR to median nerve stimuli was obtained in only one patient (NO 4 with thoracic injury) and the others had no palmar SSR in response to median nerve stimulation. There were no significant differences in intensity (85 V), latency (1620 ms) or amplitude between the palmar SSR obtained in-patient NO 4 and in the control group (P > 0.09).
Group C ASIA (Motor function was preserved below the neurological level with more than half-key muscles grade less than 3): Seven patients with different spinal neurological levels were included in this group. No patients from group C had palmar SSR to the tibial nerve or suprapubic area stimuli. Six patients from this group had palmar SSR in response to median nerve stimuli and there were no significant differences in latency (1473 ± 25 ms, P value > 0.8), but there were significant differences in amplitude (449 ± 131 μV, P value < 0.0007) and intensity (175 ± 46 V, P value < 0.001) between the palmar SSR obtained in group C and in the control group.
No patient had plantar SSR in response to suprapubic area stimuli in this group. One patient had plantar SSR after stimulation of the median nerve (PT NO: 11) and three had plantar SSR to the tibial nerve stimulation with a significant difference in amplitude with our controls (P value < 0.01).
Only patient no. 11 had suprapubic SSR to median N and suprapubic area stimulation with a significant difference in stimulus intensity with our control groups (P value < 0.05).
Group D ASIA (Motor function was preserved below the neurological level, and at least half key muscles below the neurological level had grade 3 or more): Five patients participated in this group. Except patient no. 12 with C4 level of lesion that had no palmar SSR to tibial nerve stimulation and no suprapubic SSR to suprapubic stimulation, all the other patients had palmar, plantar, and suprapubic areas SSR. Palmar SSR in response to median nerve stimuli in this group had no significant difference (P value > 0.07) in latency, amplitude and intensity with controls. However, there were significant differences (P < 0.01) in latency (2303 ± 46 ms), amplitude (381 ± 28 μV), and stimulus intensity (147 ± 8 V) from plantar SSR after tibial N stimulation compared with the normal controls. Stimulation and recording in the suprapubic region showed a significant decrease in the amplitude (287 ± 62 μV, P value < 0.001) and a significant higher stimulus intensities were required for the SSR responses (P value < 0.001).
Discussion
The sympathetic skin response is a sign of the sympathetic innervations of the skin sweat glands involving afferent pathway, brain stem nuclei, and sympathetic output.[11,16] It can be used to assess the spinal sympathetic nervous system and the respective peripheral sympathetic nerve fibers connecting the recorded skin areas.[13–15] This test has been utilized in a number of studies involving SCI; it was shown that SSR provides a good measure of spinal sympathetic pathways in SCI;[11,16] however, it is not the only means of assessing the integrity of sympathetic pathways following SCI due to some conflicting studies using this technique. In our study, it was used to control sympathetic pathways’ integrity in incomplete SCI patients with neurogenic bladder. Some of the previously tested areas are palmar, plantar, and perineal that are specifically assessed along with central sympathetic pathways of the upper thoracic segments for palmar SSR, and possibly all thoracic segments for plantar SSR.[14,15]
In our study, the difference in the stimulus intensity for the same site in the control group for recording of sympathetic skin response from the three sites (palm, sole, and suprapubic) may be due to different peripheral or central afferent sympathetic skin response pathways of these three sites.
The Illiohypogastric N provides sympathetic innervations to the bladder neck or internal urethral sphincter and the skin eccrine glands of the abdomen above the pubis.[1] Now as a new technique, suprapubic SSR can be used to detect autonomic system dysfunctions in incomplete SCI patients with urinary incontinence and peripheral injury to this nerve.
The results of this study showed significantly reduced SSR amplitudes (P value < 0.01) with more prominent reduction in the suprapubic area recording site (P value < 0.0004) in cases compared with the control group. In both control and patient groups, no significant reduction was found in amplitude over the 10 responses, indicating that habituation did not affect the results. We did not use automatic average due to possible phase cancellation and reduction of the amplitude. This significant amplitude reduction can be a clue to the involvement of the sympathetic nervous system or effect of injury to the sensory pathways of the sympathetic skin response.
SSR with significant prolonged latencies were recorded from palm and plantar areas in response to the suprapubic area and tibial N stimuli, respectively (P value < 0.02), showing some deterioration of sympathetic skin response pathways in our cases. In this study, a significantly higher intensity (P value < 0.01) was needed to elicit SSR in the cases compared with the control group; this might be due to some injury to sympathetic afferent fibers or skin hypertrophic changes in these patients.
Additionally, there were no correlation between duration from injury and SSR results in this study (P value > 0.05), which may be the result of static changes after spinal shock level of spinal cord injury.
There were no significant differences in latency, amplitude, and intensity of SSR responses (from those were obtainable) between patients’ upper T6 level of lesion with the patients’ lower T6 level of lesion (P value > 0.07) that may be due to incomplete SCI. All the patients with thoracic lesion had palmar SSR in response to median N stimuli but in four patients with cervical lesion (three ASIA grade B, one ASIA grade C) no palmar SSR was seen. These findings showed that the presence or absence of SSRs might be explained by connections with the supraspinal connection that may be deteriorated in these patients.
According to the absence or presence of SSR in all recording sites, in-group D ASIA patients had closer to normal response than other groups but they revealed a significant reduction in amplitude in the suprapubic area that may be a clue of the bladder autonomic nervous system involvement in this group. Absent SSR can occur due to inadequate stimulation and habituation; to resolve these problems, we administered ten stimuli at two times the motor threshold with random intervals of more than 60 s to avoid habituation for each stimulation site.
In our study, the patients with lesion under C7 and patients with ASIA D scores had better outcomes in bladder management and prineal hygiene (independent intermittent catheterization by straight catheters). This better outcome is more likely due to the spinal cord lesion level and their ASIA impairment score (AIS). In addition, patients with upper cervical lesion and ASIA B score had poorer outcome in bladder management (some to total assist for inserting transurethral indwelling catheter, or applying an external catheter to penis), which might be results of poor hand control and impaired fine motor coordination in this patient.
Previous studies have shown that SSR recordings above the level of the lesion by electrical stimulation of the urethral afferents can objectively assess the desire to void and contribute to the evaluation of the afferent nerve pathways of the lower urinary tract.[17] Rodic et al. have demonstrated that recording of the perineal sympathetic skin response as well as that of the hand and foot represents a sensitive diagnostic value for evaluating neurogenic bladder neck incompetence in spinal cord injured patients.[18] Reitz et al. have shown that recording of SSR below a complete SCI lesion after pudendal nerve stimulation is a new way to evaluate the integrity of sympathetic pathways in these patients that may help to identify spinal interactions between the sacral somatic afferents and the sympathetic outflow in the spinal cord injury.[19] However, in those previous studies, to add to the therapeutic or diagnostic value, even perineal SSR and pudendal nerve stimulation might pose social issues for some cases but not for suprapubic SSR.
However, for clinical management urodynamic testing, for instance, is of much more interest, but the results of our study have shown abnormal SSR in incomplete SCI patients with urinary incontinence as a possible sign of the clinical involvement of the autonomic nervous system. To better assess the value of this test in clinical evaluation of patients with urinary incontinence due to SCI, we suggest another study to evaluate the SSR in incomplete SCI patients but without incontinence of urine for comparison with the current study.
Conclusion
The results of our study have shown abolished or impaired sympathetic skin response in incomplete SCI patients with urinary incontinence as a sign of the clinical involvement of the autonomic nervous system. In addition, we have described recording abnormal SSR from the suprapubic area as another way to show bladder sympathetic system involvement.
Acknowledgments
The authors would like to thank Dr. Nasrin Shokrpour at Center for Development of Clinical Research of Nemazee Hospital (Shiraz University of Medical Sciences, Shiraz Iran) for editorial assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Bryce TN, Ragnarsson KT, Stein AB, Biering-Sorensen F. Spinal cord injury. In: Braddom RL, Chan L, Harrast MA, Kowalske KJ, Matthews DJ, Ragnarsson KJ, et al., editors. Physical Medicine and Rehabilitation. 4th ed. Philadelphia: Elsevier; 2011. pp. 1293–347. [Google Scholar]
- 2.Shahani BT, Halperin JJ, Boulu P, Cohen J. Sympathetic skin response--a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 1984;47:536–42. doi: 10.1136/jnnp.47.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knezevic W, Bajada S. Peripheral autonomic surface potential: A quantitative technique for recording autonomic neural function in man. Clin Exp Neurol. 1985;21:201–10. [PubMed] [Google Scholar]
- 4.Elie B, Guiheneuc P. Sympathetic skin response: Normal results in different experimental conditions. Electroencephalogr Clin Neurophysiol. 1990;76:258–67. doi: 10.1016/0013-4694(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 5.Sato A, Schmidt RF. Somatosympathetic reflexes: Afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973;53:916–47. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- 6.Ertekin C, Ertekin N, Mutlu S, Almis S, Akçam A. Skin potentials (SP) recorded from the extremities and genital regions in normal and impotent subjects. Acta Neurol Scand. 1987;76:28–36. doi: 10.1111/j.1600-0404.1987.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 7.Ogura T, Kubo T, Lee K, Katayama Y, Kira Y, Aramaki S. Sympathetic skin response in patients with spinal cord injury. J Orthop Surg (Hong Kong) 2004;12:35–9. doi: 10.1177/230949900401200108. [DOI] [PubMed] [Google Scholar]
- 8.Jazayeri M, Ghavanini MR, Rahimi HR, Raissi GR. A study of the sympathetic skin response and sensory nerve action potential after median and ulnar nerve repair. Electromyogr Clin Neurophysiol. 2003;43:277–9. [PubMed] [Google Scholar]
- 9.Jost WH, Derouet H, Osterhage J, Schimrigk K, Ziegler M. Electrophysiologic diagnosis in erectile dysfunction. Urologe A. 1996;35:120–6. [PubMed] [Google Scholar]
- 10.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: Basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–70. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 11.Cariga P, Catley M, Mathias CJ, Savic G, Frankel HL, Ellaway PH. Organisation of the sympathetic skin response in spinal cord injury. J Neurol Neurosurg Psychiatry. 2002;72:356–60. doi: 10.1136/jnnp.72.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashraf A, Mohammadi A, Roshanzamir S, Ayaz M, Tolide-Ie H, Ghasempoor MZ. Sympathetic skin response in electrical burn injury. Burns. 2012;38:232–5. doi: 10.1016/j.burns.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Kucera P, Goldenberg Z, Kurca E. Sympathetic skin response: Review of the method and its clinical use. Bratisl Lek Listy. 2004;105:108–16. [PubMed] [Google Scholar]
- 14.Baba M, Watahiki Y, Matsunaga M, Takebe K. Sympathetic skin response in healthy man. Electromyogr Clin Neurophysiol. 1988;28:277–83. [PubMed] [Google Scholar]
- 15.Uncini A, Pullman SL, Lovelace RE, Gambi D. The sympathetic skin response: Normal values, elucidation of afferent components and application limits. J Neurol Sci. 1988;87:299–306. doi: 10.1016/0022-510x(88)90254-7. [DOI] [PubMed] [Google Scholar]
- 16.Curt A, Weinhardt C, Dietz V. Significance of sympathetic skin response in the assessment of autonomic failure in patients with spinal cord injury. J Auton Nerv Syst. 1996;61:175–80. doi: 10.1016/s0165-1838(96)00080-x. [DOI] [PubMed] [Google Scholar]
- 17.Schmid DM, Reitz A, Curt A, Hauri D, Schurch B. Urethral evoked sympathetic skin responses and viscerosensory evoked potentials as diagnostic tools to evaluate urogenital autonomic afferent innervation in spinal cord injured patients. J Urol. 2004;171:1156–60. doi: 10.1097/01.ju.0000111809.81966.8b. [DOI] [PubMed] [Google Scholar]
- 18.Rodic B, Curt A, Dietz V, Schurch B. Bladder neck incompetence in patients with spinal cord injury: Significance of sympathetic skin response. J Urol. 2000;163:1223–7. [PubMed] [Google Scholar]
- 19.Reitz A, Schmid DM, Curt A, Knapp PA, Schurch B. Sympathetic sudomotor skin activity in human after complete spinal cord injury. Auton Neurosci. 2002;102:78–84. doi: 10.1016/s1566-0702(02)00207-2. [DOI] [PubMed] [Google Scholar]


