Abstract
Background and Purpose
Many organs suffer from ischaemic injuries that reduce their ability to generate sufficient energy, which is required for functional maintenance and repair. Erythropoietin (EPO) ameliorates ischaemic injuries by pleiotropic effects. The aim of this study was to investigate the effect and mechanism of a small molecule EH-201, and found it as a potent EPO inducer and its effect in non-haematopoietic cells for therapeutic potential in ischemic disorders.
Experimental Approach
Mice kidney slices, primary hepatocytes, primary cardiomyocytes and C2C12 myoblasts were treated with EH-201. The effects of this treatment on EPO, Hb expression and mitochondrial biogenesis were analysed. In vivo, doxorubicin-induced cardiomyopathic mice were treated with EH-201. The mice were subjected to an endurance test, electrocardiography and echocardiography, and a histological examination of the isolated hearts was performed. EH-201 was also administered to cisplatin-induced nephropathic mice.
Key Results
In non-haematopoietic cells, EH-201 was potent at inducing EPO. EH-201 also stimulated mitochondrial biogenesis and enhanced the expression of Hb by a mechanism dependent on EPO-mediated signalling. In mechanistic studies, using EPO and EPO receptor-neutralizing antibodies, we confirmed that EH-201 enhances EPO-EPOR autocrine activity. EH-201 robustly increased the endurance performance activity of healthy and cardiomyopathic mice during hypoxic stress, enhanced myocardial mitochondrial biogenesis and Hb expression, and also improved cardiac function. EH-201 ameliorated anaemia and renal dysfunction in nephropathic mice.
Conclusions and Implications
The enhancement and recovery of cellular functions through the stimulation of mitochondrial activity and Hb production in non-haematopoietic cells by an inducer of endogenous EPO has potential as a therapeutic strategy for ischaemic diseases.
Keywords: erythropoietin, inducer, mitochondria, haemoglobin, non-haematopoietic cell, ischaemic disease, cardiomyopathy, anaemia, endurance performance
Introduction
Ischaemia causes oxygen deprivation, cell injury and related organ dysfunctions, such as heart failure, stroke, chronic obstructive pulmonary disease, ischaemic retinopathy, liver injury and acute renal failure (Wu et al., 2009; Perin et al., 2012). Mitochondrial dysfunction is a key factor in organ ischaemia injury; upon loss of oxygen, mitochondrial oxidative phosphorylation rapidly stops, with a resulting loss of the major source of ATP production for energy metabolism (Murphy and Steenbergen, 2007; Neubauer, 2007).
Erythropoietin (EPO) is essential for the regulation of the mass of erythrocytes in response to changes in tissue oxygenation during hypoxia and anaemia. The protective effects of EPO have been demonstrated in various tissues and experimental models of ischaemia-induced injury and have been attributed to its effect on non-haematopoietic metabolic adaptation, inhibition of apoptosis and stimulation of angiogenesis (Chatterjee, 2005). Recently, EPO has been reported to stimulate cardiac mitochondrial proliferation through the activation of mitochondrial biogenesis, which is mediated by PPAR co-activator 1-α (PGC-1α), a key regulator of cardiac bioenergetics (Carraway et al., 2010). Clinically, EPO reverses cardiac remodelling, improves cardiac function, and enhances the exercise tolerance and quality of life of patients by inducing protective effects beyond the correction of anaemia (Bergmann et al., 2011). These findings highlight the possibility that EPO-mediated protection may depend on its modulatory effects on intracellular energetics.
Hb is the main oxygen transporter in erythrocytes. Its main form, Hb-α, is a tetramer consisting of two α- and β-polypeptide chains, each carrying a haeme group. Recently, Hb was unexpectedly found to be expressed in many non-haematopoietic cells and it is possible that it facilitates tissue oxygen transport or increases cellular oxygenation and so provides an intrinsic protective mechanism against hypoxic/ischaemic injury (Liu et al., 1999; Newton et al., 2006; Nishi et al., 2008; Biagioli et al., 2009; Tezel et al., 2009; 2010).
Polygonum multiflorum Thunb. is a Chinese medicine used for the treatment of anaemia, liver diseases and other diseases commonly associated with aging (Yang et al., 2005; Huang et al., 2007). Using bioassay-based fractionation, we have identified a small molecule, 2,3,5,4′-tetrahydroxystilbene-2-o-β-d-glucoside (TSG or THSG) that, for the purpose of serial lead optimization by chemical modification, was termed EH-201. The protective effects of this compound have been reported in experimental models of cardiovascular diseases (W Zhang et al., 2009), cerebral ischaemia (Wang et al., 2009), Alzheimer's disease (Zhang et al., 2006) and inflammation (Wang et al., 2008), and have been attributed to its antioxidant and free radical-scavenging properties. However, the exact mechanism underlying its protective effects is not yet clear. In the present study, we identified a previously unexplored mechanism as we demonstrated that EH-201 is a potent inducer of endogenous EPO through the enhancement of EPO-EPO receptor (EPOR) autocrine activity. In addition, we showed that it enhanced mitochondrial function and Hb expression in non-haematopoietic cells. The therapeutic potential of EH-201 in animal models of ischaemic disorders was also investigated, as well as its underlying mechanisms of action.
Methods
The extraction, isolation and characterization of EH-201
EH-201 was extracted and isolated to 99.2% purity by bioassay-based fractionation from the dried, milled roots of P. multiflorum Thunb., and the compound was identified as EH-201 (TSG or THSG) (Figure 1A). Its chemical identity was confirmed by LC/MS/MS, UV, 1H-NMR and proton-decoupled 13C-NMR data (Supporting Information Figure S1 and Supporting Information Table S1).
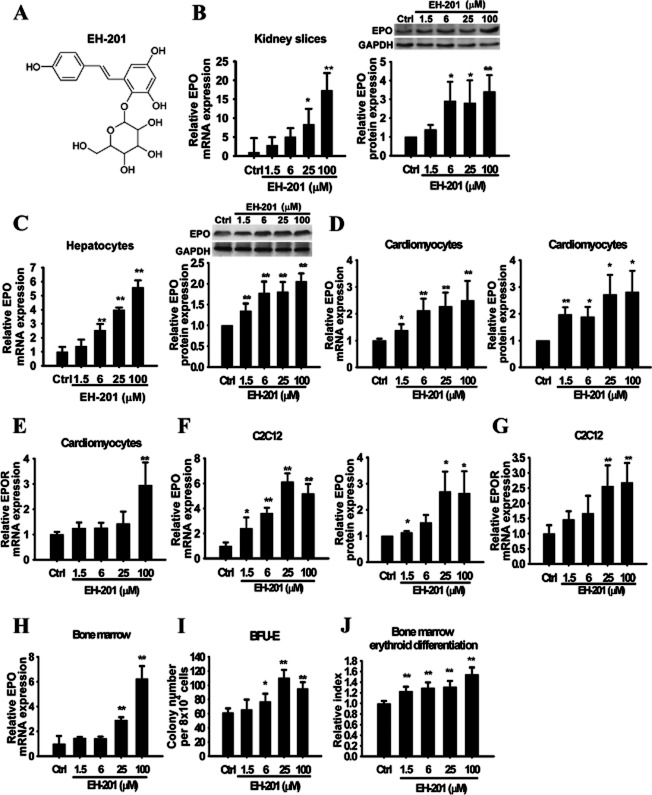
Figure 1.
EH-201 is a potent inducer of EPO expression. (A) The chemical structure of EH-201. (B, C) The EH–201-treated kidney slices and hepatocytes were analysed for EPO expression by Q-PCR and Western blotting. (D, E) Primary mice cardiomyocytes and (F, G) C2C12 myotubes were treated with EH-201, and the effects on EPO and EPOR expression were analysed by Q-PCR and Western blotting. (H) The bone marrow cells were incubated with EH-201 for 48 h, and the expression of EPO was detected by Q-PCR. (I) The bone marrow cells were incubated with EH-201, and the colonies were counted on day 9 for burst-forming units-erythroid (BFU-E). (J) The quantification of the differentiated erythroid progenitors was performed using a Hb colorimetric assay. The control represents vehicle treatment. The values are presented as the means ± SEM (n = 6 for each). *P < 0.01, *P < 0.05 versus control, Student's t-test.
Animals
Eight- to ten-week-old specific pathogen-free C57BL/6J male mice (20–25 g), obtained from the National Laboratory Animal Centre (Taiwan) were housed five to six per cage at a constant temperature of 22 ± 2°C. They were fed standard laboratory chow (PMI, Brentwood, MO, USA) and water ad libitum and kept under a 12 h dark/light cycle. The experimental protocol was approved by the Animal Research Committee of National Yang-Ming University (Guide for Animal Experiments, National Yang-Ming University). All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques, if available. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Cell culture and treatment
The C2C12 myoblast, HEK293 and TF-1 cells were purchased from Bioresources Collection and Research Centre (Hsinchu, Taiwan). The C2C12 myoblasts were differentiated to myotubes, as described previously (Zebedin et al., 2007), and were treated with drugs for 24 h. Ex vivo 250 μm-thick kidney slices were prepared from 8- to 10-week-old C57BL/6J mice, as previously described (Obatomi et al., 1998). The slices were treated with drugs in the gassed media (DMEM/F12 buffered with 15 mM HEPES and 20 mM sodium bicarbonate) in an atmospheric chamber at 37°C with 50% O2: 5% CO2: 45% N2 for 18 h. Mouse primary hepatocytes were isolated and purified from 8- to 10-week-old C57BL/6J mice, as previously described (Kreamer et al., 1986), and plated onto 1% gelatin-coated microplates in DMEM supplemented with 10% FBS (Gibco-BRL, Gaithersburg, MD, USA). After the hepatocytes had attached, fresh medium containing drugs was added for 24 h. Neonatal C57BL/6J mouse cardiomyocyte cultures were prepared from post-natal 1-day-old C57BL/6J mice obtained from the Animal Centre at the National Yang-Ming University as described previously (Song et al., 2000), and the isolated ventricular cells were resuspended in 10% FCS-containing M199 medium (Gibco). The cardiomyocytes were incubated in a humidified atmosphere at 37°C with 5% CO2 on plates pre-coated with 1% gelatin. The subconfluence of spontaneously beating cells was achieved after 48 h of culture, after which the cells were treated with various drugs for 24 h. The bone marrow progenitor cell cultures for the colony-forming assay and the Hb colorimetric assay (Worthington et al., 1987) were prepared as previously described. In the knockdown experiment, the C2C12 myotubes were transfected with scrambled or PGC–1α-specific siRNA (Supporting Information Table S2) using Lipofectamine 2000 reagent, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). These cultured cells were treated with rhEPO (recombinant human EPO, Roche, Manheim, Germany) or EH-201 or were co-incubated with EPO neutralizing antibody (R&D Systems Inc., Minneapolis, MN, USA) for the time periods indicated. Thereafter, the drug-treated cell and tissue lysates were collected and homogenized to determine the specific expression of mRNA and protein, as well as their mitochondrial activity.
Real-time PCR
The total RNA was extracted using the TRIzol reagent (Invitrogen) and was reverse transcribed by M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). The EPO, EPOR, PGC-1α, Hb-α, Hb-β and GAPDH mRNA expression was quantified by quantitative real-time PCR (Q-PCR) with an ABI 7500 sequence detector (Applied Biosystems, Foster City, CA, USA) using SYBR Green Master Mix® (ABI-7500). The relative mRNA expression levels were determined using the ΔΔCt method, with GAPDH as the endogenous control. The primers used are listed in Supporting Information Table S2.
Western blot
The total protein (50 μg) was separated by 12% SDS-PAGE, transferred onto PVDF membranes and probed with antibodies against EPO, PGC-1α, GAPDH, PCNA (from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), sirtuin (Sirt1; Millipore, Billerica, MA, USA), or hypoxia-inducible factor (Hif)-2α (Novus Biologicals, Littleton, CO, USA). Following incubation with the appropriate HRP-conjugated secondary antibody, the signals were visualized by luminal enhanced chemiluminescence detection, according to the manufacturer's protocol (Perkin-Elmer Life Sciences, Waltham, MA, USA).
Quantification of the mtDNA copy number
The total cellular DNA was purified using a conventional phenol-chloroform method, and the mitochondrial DNA (mtDNA) copy number was measured, as previously described (Lagouge et al., 2006).
The MitoTracker assay
The mitochondrial content was assessed by the MitoTracker microplate assay, as previously described (Chu et al., 2007). The treated cells were loaded with 0.1 μM green fluorescent MitoTracker-Green (MTG, Invitrogen) for 60 min at 37°C. The intracellular MTG content was measured by fluorescence photometry (Thermo Fisher Scientific Inc., Waltham, MA, USA). Subsequently, the fixed cells were labelled with H33342 to assess the cell density. The MTG/H33342 fluorescence ratios were calculated.
Measurement of citrate synthase activity
The citrate synthase activity was measured in tissue lysates, as described previously (Siu et al., 2003). The changes in absorbance at 412 nm were measured; and the activity was expressed as μmol·min−1·mg−1 protein.
TF-1 cell proliferation assay
The EPO-sensitive cell line TF-1 (Kitamura et al., 1989) was seeded in 96-well microplates at a 1 × 105·mL−1 cell density in RPMI 1640 medium with 2% FBS, and the cells were treated with rhEPO and EH-201 with or without EPOR neutralizing antibody (Santa Cruz Biotechnology, Inc.) for 48 h. The cell numbers were determined by a trypan blue dye exclusion assay.
Rotarod endurance assessment
Before being divided into treatment groups, 8- to 10-week-old C57Bl/6J male mice were trained on a rotarod apparatus (14 rpm) for a maximum of 10 min for each of three consecutive training sessions per day for 3 days; animals that did not master this task were excluded from the experiments. After the training, the qualified mice were randomly divided into EH-201-treating groups (10, 30 or 90 mg·kg−1 per day, n = 5 for each group) for 7 days. On the testing day, each mouse was subjected to three trials on the rotarod at 22 r.p.m. under a normoxic or hypoxic (8% O2) atmosphere. The endurance performance was measured over time until the mice suffered from exhaustion and fell off of the rotarod. The maximum trial length was 60 min and there was a 30-min rest period between each trial.
EPO elisa
Serum EPO concentrations were analysed using an elisa kit specific for mouse EPO (R&D Systems Inc.), according to the manufacturer's instructions.
Doxorubicin-induced cardiomyopathy
Cardiomyopathy was induced in 8- to 10-week-old C57Bl/6J male mice by a single i.p. injection of 15 mg·kg−1 doxorubicin (Dox)-HCl (Sigma-Aldrich, St. Louis, MO, USA), and the normal group was injected with saline (n = 6). Seven days after the injection, the presence of Dox-induced cardiomyopathy was confirmed with an electrocardiogram by observing a prolonged S–T interval. An average of 80% of injected mice were found to have cardiomyopathy (27/34); the unaffected mice were excluded from the EH-201 experiments. The cardiomyopathic mice were randomly divided into four cohorts comprising the control (Dox, n = 9) and three EH-201-treated groups (n = 6 for each group) for an additional 1 week. EH-201 was administered p.o. by mixing it into the feed. The Dox group was fed the normal diet and the EH-201-treated groups were fed the normal diet containing different doses of EH-201 (10, 30 or 90 mg·kg−1 per day). One week later, the mice were subjected to the rotarod endurance test, echocardiography and electrocardiogram. The mice were killed after an electrocardiogram and the isolated hearts were subjected to histological examination and Hb analysis.
Hb staining
The staining for Hb in the isolated myocardium tissue lysates was performed with tetramethylbenzidine (TMBZ, Sigma-Aldrich), following non-reducing SDS-PAGE, as previously described (Maitra et al., 2011). The photography and scanning of the gels was performed using a Typhoon Trio™ imager (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The TMBZ stain was removed from the gels by the addition of a 70 mM sodium sulfite solution. Thereafter, 30% isopropanol was used to replace the sodium sulfite, and then the gels were stained with Coomassie blue for analysis of the protein loading control.
Echocardiography and ECG
The mice from all treatment groups were anaesthetized with isoflurane (0.75–1.5% inhalation); and echocardiographic measurements were taken in M-mode in triplicate for each mouse using an ATL HDI 5000 ultrasound system (Philips Medical Systems, ATL Ultrasound, Bothell, WA, USA). The depth of anaesthesia was monitored by assessing the reaction to toe pinch and skin pinch tests and also by determining jaw tone. To assess the ECG parameters, three electrodes were utilized. The ECG tracings from lead I were recorded by means of an electrocardiograph connected to s.c. needle electrodes in the isoflurane-anaesthetized mouse. All probes were connected to an amplifier and digital converter for signal recording at the 100 mV range with low-pass 1 Hz and high-pass 1 kHz filters. An acquisition data system with LabVIEW software (National Instruments Corp., Austin, TX, USA) was used to record and analyse the ECG signals.
Cisplatin-induced nephropathy
Forty 8- to 10-week-old C57Bl/6J male mice were injected i.p. with three doses of cisplatin (Sigma-Aldrich), following the scheme of 7, 6 and 6 mg·kg−1 body weight, at 4 day intervals, and the normal group (n = 6) was injected with saline (Figure 6A). On day 13, serum samples were collected and assayed for blood urea nitrogen content (BUN). Mice with BUN values greater than 100 mg·100 mL−1 were chosen for the experiment. An average 70% of injected mice were successfully induced to have renal dysfunction (26/40); the unaffected mice were excluded from the EH–201-treating experiments. The mice were subsequently divided randomly into four cohorts comprising the control (Ctrl, n = 8) and three EH-201-treated groups (n = 6 for each group) for an additional 2 weeks. Blood samples from all the mice were collected every 5 days. The red blood cell (RBC) numbers were determined from the complete blood cell count using a Sysmex Kx-21 haematology analyser (Sysmex America Inc., Mundelein, IL, USA), and the serum BUN levels were determined through the urease GLDH method using a commercial kit (DiaSys Diagnostic System GmbH, Frankfurt, Germany).
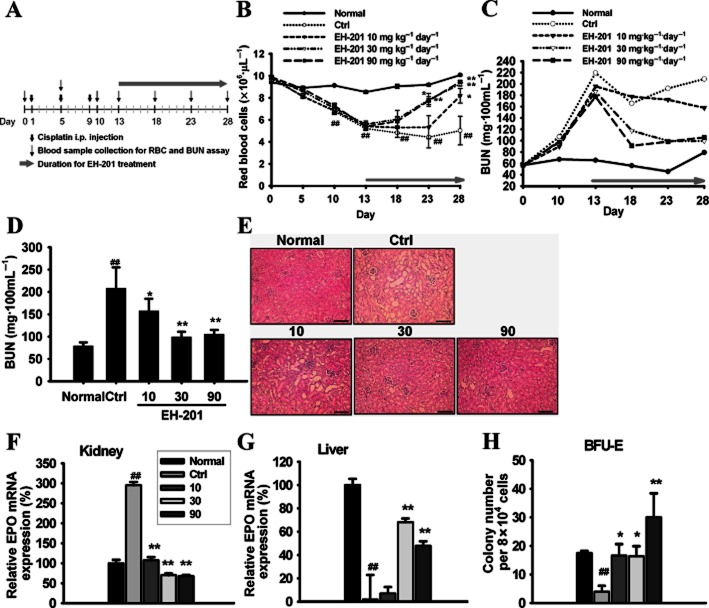
Figure 6.
EH-201 accelerates the recovery from anaemia and renal function in cisplatin-induced nephropathy in mice. (A) Schematic diagram protocol. (B) The time course kinetics of the RBC numbers in the peripheral blood. (C) The time course kinetics of the BUN values after cisplatin injection. (D) The functional recovery of the kidneys of mice treated with EH-201 on day 28. (E) The haematoxylin-eosin stain of kidney sections after EH-201 administration on day 28 (bars = 100 μm). (F, G) The EPO expression in the kidney and liver on day 28 was determined by Q-PCR. (H) The numbers of BFU-E colonies in the isolated bone marrow cells from the treated mice on day 28. The values are presented as the means ± SEM (n = 5–6 animals each group). ##P < 0.01 versus with normal group; **P < 0.01, *P < 0.05 versus control group by one-way anova with Tukey's post hoc test.
Statistics
All results are expressed as the mean ± SEM. The statistical analysis was performed using Student's t-test. One-way anova was used to examine the differences across the animal experimental groups. The post hoc differences between the means of the experimental groups were determined via Tukey's test. P < 0.05 was considered significant.
Results
EH-201 is a potent EPO inducer
To determine whether EH-201 has the ability to induce EPO expression, kidney slices and hepatocytes were treated with EH-201 ex vivo. EH-201 markedly enhanced EPO mRNA and protein expression in a concentration-dependent manner in the kidney slices and hepatocytes (Figure 1B and C). According to the gene expression pattern of EPO in human tissues in the publicly available database created by Su et al. (2004), the EPO transcript is expressed at a surprisingly high level in human cardiomyocytes. Therefore, we tested whether EH-201 also induces EPO expression in neonatal mice cardiomyocytes and C2C12 myocytes. We found that EH-201 concentration-dependently increased the expression of EPO and EPOR in the primary cardiomyocytes and C2C12 myocytes (Figure 1D–G). Because the bone marrow progenitor cells can express EPO, which then mediates haematopoiesis (Stopka et al., 1998; Sato et al., 2000), we cultured bone marrow cells with EH-201 to examine its effect on erythropoiesis. The expression of EPO mRNA was increased in the bone marrow cells exposed to EH-201 (Figure 1H). EH-201 significantly increased the number of BFU-E colonies (Figure 1I) and Hb expression in a concentration-dependent manner (Figure 1J). These data suggest that EH-201 is a potent inducer of EPO.
The induction of mitochondrial biogenesis by EH-201 is mediated by EPO
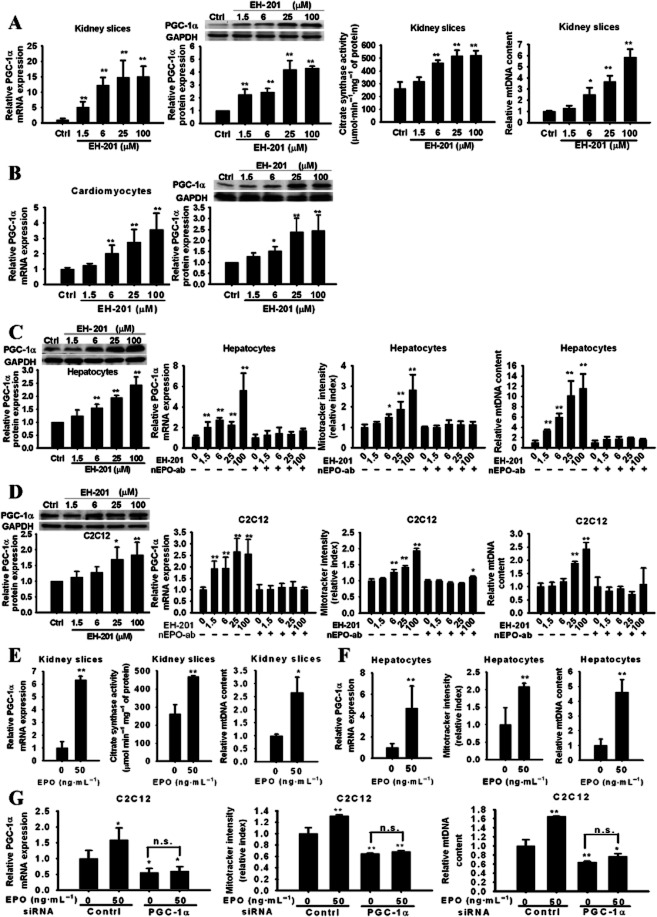
To determine whether EH-201 influences mitochondrial biogenesis, a series of experiments were performed to test the effects of the EPO inducer in non-haematopoietic cells. In the EH-201-treated kidney slices, the activity of the mitochondrial marker enzyme citrate synthase increased in a concentration-dependent manner, and a marked increase in the mitochondrial copy number and PGC-1α expression was also observed (Figure 2A). The stimulating effects of EH-201 on mitochondrial biogenesis were also observed with hepatocytes, cardiomyocytes and C2C12 myocytes (Figure 2B–D). However, treatment with a neutralizing-EPO antibody abolished these effects of EH-201 on mitochondrial biogenesis (Figure 2C and D), whereas EPO treatment increased PGC-1α expression and mitochondrial biogenesis (Figure 2E–G). Next, we examined whether these effects were mediated by a PGC–1α-dependent pathway using PGC–1α-specific siRNA-transfected C2C12 myocytes. The PGC–1α siRNA resulted in a 44% reduction in PGC-1α mRNA expression and prevented the stimulating effect of EPO on mitochondrial biogenesis (Figure 2G), which indicates that the activation of mitochondrial biogenesis by EPO is PGC–1α-dependent. Additionally, because the mammalian Sirt1 regulates mitochondrial function and biogenesis in the skeletal muscles and liver along with PGC-1α (Baur et al., 2006; Lagouge et al., 2006), we determined changes in the expression of Sirt1 and observed that EH-201 treatment increased its expression (Supporting Information Figure S2), which suggests that the induction of EPO-mediated mitochondrial activity by EH-201 might occur through the Sirt1/PGC-1α pathway. These data suggest that the stimulation of mitochondrial biogenesis in non-haematopoietic cells induced by EH-201 is mediated by an increase in EPO levels, which are dependent on an increase in PGC-1α.
Figure 2.
The induction of mitochondrial biogenesis by EH-201 is mediated by EPO. (A, B) EH–201-treated kidney slices and primary cardiomyocytes and (C, D) EH–201-treated hepatocytes and C2C12 myotubes with or without the neutralizing EPO antibody (nEPO-ab, 1 μg·mL−1) were analysed for PGC-1α expression by QPCR (n = 6) and Western blotting (n = 4), citrate synthase activity (n = 3), and mtDNA copy number (n = 6) and via the MitoTracker assay (n = 6). The control represents vehicle treatment. (E, F and G) rhEPO was given to kidney slices, hepatocytes and C2C12 myotubes. The mitochondrial activity was determined by PGC-1α Q-PCR (n = 6), citrate synthase activity (n = 3), mtDNA copy number (n = 6), and MitoTracker assays (n = 6). The control represents vehicle treatment. PGC-1α siRNA-transfected C2C12 myotubes were treated with rhEPO (n = 6). The control represents the scrambled siRNA treatment. The values are presented as the means ± SEM. **P < 0.01, *P < 0.05 versus untreated control, n.s., not significant, Student's t-test.
The induction of haemoglobin expression in non-haematopoietic cells by EH-201 is mediated by EPO
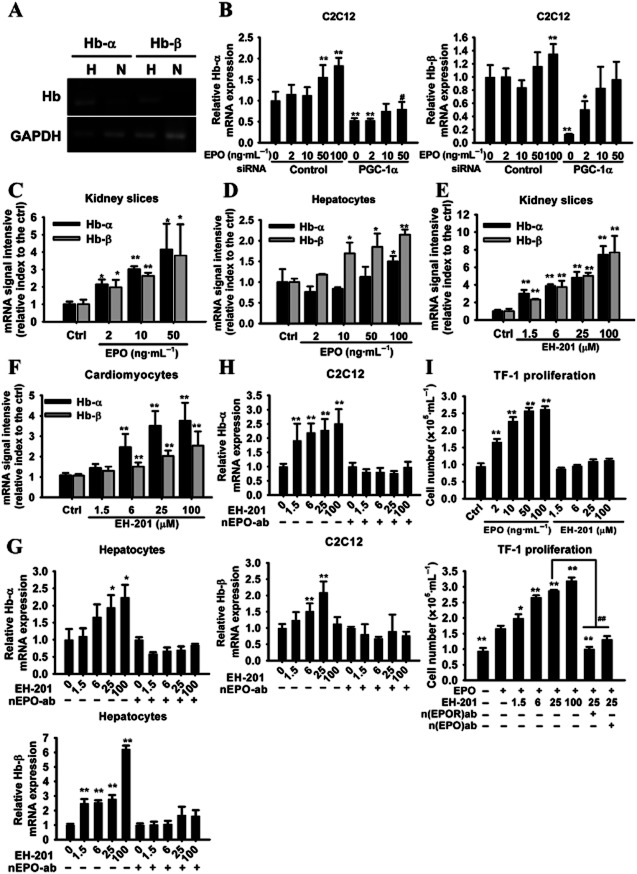
We further determined whether the expression of Hb was regulated by hypoxia-inducible EPO signalling in non-haematopoietic cells. In vitro experiments were performed in which C2C12 cells were incubated in the absence and presence of hypoxic conditions. The exposure of the C2C12 myoblasts to hypoxia resulted in a noticeable increase in the expression of Hb-α and Hb-β (Figure 3A). In the EPO-treated C2C12 myocytes, the expression of Hb-α was more affected by the treatment than that of Hb-β (Figure 3B). In addition, the expression of both Hb-α and Hb-β was increased in a concentration-dependent manner by EPO in the kidney slices, whereas only the expression of Hb-β was enhanced by EPO in the hepatocytes (Figure 3C and D). In the context of EH-201, the expression of Hb subunits was significantly increased in EH–201-treated non-haematopoietic cells (Figure 3E–H), and this increase was abolished by concomitant treatment with a neutralizing-EPO antibody (Figure 3G and H). Studies were also conducted to determine the role of PGC-1α in EPO signalling of the induction of Hb expression, by using PGC-1α siRNA. Interestingly, reducing the expression of PGC-1α in C2C12 myocytes led to a decrease in the expression of both Hb-α and Hb-β mRNA and also attenuated the stimulating effect of EPO on Hb-α (Figure 3B), which indicates that the regulation of Hb expression in non-haematopoietic cells mediated by EPO occurs through both PGC-1α-dependent and -independent pathways. These results suggest that EPO-mediated signalling is required for the enhanced expression of Hb induced by EH-201 in non-haematopoietic cells.
Figure 3.
The induction of Hb expression in non-haematopoietic cells by EH-201 is mediated by EPO. (A) Cultured C2C12 myotubes under normoxia or hypoxia (5% O2) for 24 h were analysed for the expression of Hb-α and Hb-β by RT-PCR, followed by 1.5% agarose gel electrophoresis. (B) The rhEPO-treated C2C12 myotubes with or without PGC-1α siRNA transfection were analysed for Hb expression by Q-PCR (n = 6). The control represents the scrambled siRNA treatment. (C, D) The rhEPO-treated kidney slices and hepatocytes were analysed for Hb expression by Q-PCR (n = 6). The control represents vehicle treatment. (E, F) EH-201-treated kidney slices and primary mice cardiomyocytes and (G, H) EH-201-treated hepatocytes and C2C12 myotubes with or without the neutralizing EPO antibody (nEPO-ab, 1 μg·mL−1) were analysed for Hb expression by Q-PCR (n = 6). The values are presented as the means ± SEM. **P < 0.01, *P < 0.05 versus untreated control, #P < 0.05 versus rhEPO-treated control (50 ng·mL−1). (I) The effects of rhEPO and EH-201 on the proliferation of TF-1 cells were determined by a trypan blue dye exclusion assay (upper, n = 6). The rhEPO (2 ng·mL−1) and EH-201 co-treated TF-1 cells were incubated with or without the neutralizing antibody (nEPOR-ab, 0.5 μg·mL−1; nEPO-ab, 1 μg·mL−1) for a 48 h proliferation assay (lower, n = 6). The values represent means ± SEM. **P < 0.01, *P < 0.05 versus control (upper) or rhEPO alone (lower), ##P < 0.01 versus rhEPO+ EH-201 25 μM, Student's t-test.
EH-201 enhances the binding of EPO to EPOR by a mechanism that does not involve Hif-α
To examine the mechanism behind EH-201's activity, we carried out computational docking studies (Bikadi and Hazai, 2009) to predict the binding of EH-201 to EPOR. We found that EH-201 binds preferentially to the EPO-bound EPOR complex (EPO/EPOR) rather than the EPO-free naïve EPOR (estimated total intermolecular energy −7.48 and −6.30 kcal·mol−1, respectively). Autodock identified more than two preformed binding sites in the EPO/EPOR complex for EH-201 with negative favourable binding free energy, and the predicted interaction residues on EPOR (Met150, Thr151, Supporting Information Figure S3) involve the hotspot residues located in loop 5 (Livnah et al., 1996). Because autocrine activity of EPO also plays an important role in EPOR activation and the regulation of EPO production (Villeval et al., 1994), we tested the hypothesis that EH-201 may act as binding enhancer of EPO to EPOR, thus enhancing the activation of EPOR. A TF-1 cell (EPOR positive) proliferation assay was performed to assess the biological activity of EPO. Interestingly, rhEPO induced the proliferation of TF-1 cells concentration-dependently, whereas, in the absence of rhEPO, EH-201 alone was unable to induce cell proliferation (Figure 3I). In the presence of even very low concentrations of rhEPO, for example 2 ng·mL−1, EH-201 significantly enhanced TF-1 cell proliferation in a concentration-dependent manner. The addition of neutralizing EPO or neutralizing EPOR antibodies significantly reduced TF-1 cell proliferation (Figure 3I). These data indicate that EPO is required for the activity of EH-201 and that the EPO/EPOR complex is the target of EH-201, which serves as an enhancer of EPO and EPOR binding. We also investigated whether EH–201-induced expression of EPO involves the activation of the Hif, as the expression of EPO is regulated by Hif. As shown in Supporting Information Figure S4A, using a hypoxia response element-driven luciferase reporter to assess the activation of Hif-1α, we found that EH-201 treatment did not stimulate the promoter activity. Furthermore, Hif–1α-targeted VEGF expression was up-regulated during hypoxia, whereas EH-201 did not alter the expression of VEGF (Supporting Information Figure S4B). EH-201 treatment also did not stabilize the Hif-2α protein levels (Supporting Information Figure S4C). These findings indicate that the induction of EPO by EH-201 is not due to the activation of Hif-1α or Hif-2α.
EH-201 administration enhances the endurance performance of mice
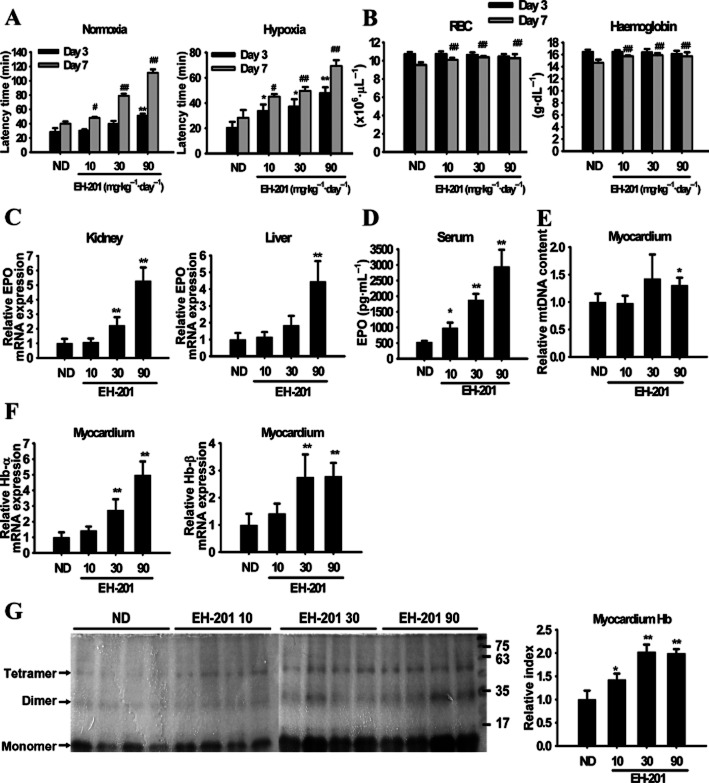
Given the ability of EH-201 to induce EPO, we tested whether EH-201 also enhances the endurance performance in mice undergoing hypoxic stress. Notably, the administration of EH-201 for 3 days increased the run time to exhaustion under both normoxic and hypoxic conditions in a dose-dependent manner (Figure 4A), with a further enhancement at 7 days. However, there was only a slight increase in RBC counts and Hb content in the peripheral blood (Figure 4B), which indicates that EH-201 increased the RBC numbers by inducing an increase in the endogenous EPO levels (Figure 4D), as confirmed by the increase in the production of renal and hepatic EPO induced by EH-201 (Figure 4C). The expression of Hb-α and Hb-β in the myocardium of the EH–201-treated mice was significantly increased (Figure 4F); this was confirmed by the increase in Hb protein expression observed with TMBZ staining (Figure 4G). High doses of EH-201 also induced cardiac mitochondrial biogenesis (Figure 4E). Furthermore, EH-201 treatment resulted in a significantly increased expression of PGC-1α and mitochondria content and activity in the liver and skeletal muscles (Supporting Information Figure S5A and B). These results suggest that EH-201 treatment dramatically enhances the endurance performance and hypoxic tolerance of the mice by increasing the endogenous expression of EPO and stimulating mitochondrial biogenesis and Hb expression in non-haematopoietic tissues.
Figure 4.
EH-201 increases endurance performance and activation of mitochondrial activity and Hb expression in mice. (A) The endurance of normal mice was measured with the rotarod exercise under normoxic or hypoxic (8% O2) conditions (ND: normal diet). (B) The effect of EH-201 on plasma RBC numbers and Hb levels. (C, D) EPO mRNA expression in the kidney and liver of mice was measured by Q-PCR after 3 days of EH-201 administration. The serum levels of EPO were determined by elisa. (E, F) Isolated myocardium tissues after 3 days of EH-201 administration were analysed for Hb expression by Q-PCR, and the mitochondrial biogenesis was determined by mtDNA copy number. (G) The effects of EH-201 treatment on ventricular Hb expression were quantified by TMBZ staining in SDS-PAGE (left). The quantification of Hb expression (tetramer and dimer, right). The values are presented as the means ± SEM (n = 5 animals each group). **P < 0.01, *P < 0.05 versus the ND group; ##P < 0.01, #P < 0.05 versus the day 7 ND group by one-way anova with Tukey's post hoc test.
Therapeutic effect of EH-201 on established doxorubicin-induced cardiomyopathy
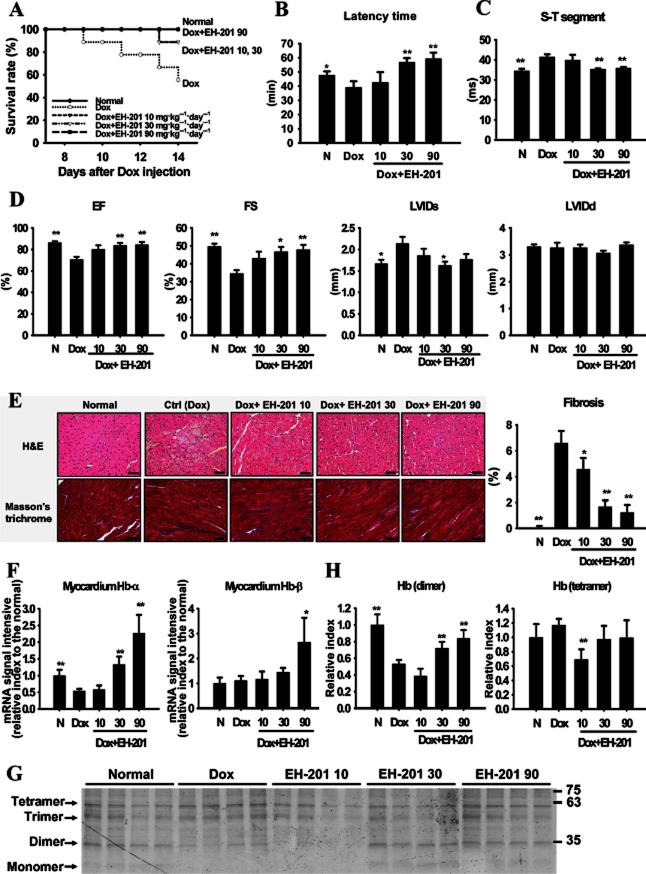
To assess the therapeutic potential of EH-201 in myocardial ischaemia, a Dox-induced cardiomyopathy model was used (Calvo-Romero et al., 2001; Ou et al., 2010). One week after Dox injection, the cardiomyopathic mice, as identified by ECG measurements (Farraj et al., 2011), were started on EH-201 treatment for 7 days to examine EH-201's therapeutic effects. We observed that the survival rates of the EH–201-treated groups were improved, and the high-dose group remained alive until the end of the study period (Figure 5A). Following the hypoxic rotarod endurance tests, we found that, although none of the groups recovered from the initial changes in body weight (Supporting Information Figure S6A), the endurance performance activity of the EH–201-treated groups were robustly increased (especially for the 30 and 90 mg·kg−1 doses), whereas that of the Dox group was significantly reduced (Figure 5B). Myocardium injury was measured by ECG up to 2 weeks following the injection of Dox, and these ECG parameters were significantly abnormal, which reflected the extensive cardiac damage caused by Dox (Figure 5C). Seven days after the administration of EH-201, these ECG signs were significantly recovered in the mice treated with EH-201 (30 and 90 mg·kg−1), which indicates an improvement in cardiac activity (Figure 5C and Supporting Information Figure S6B). Echocardiography performed 2 weeks after Dox administration demonstrated that mice receiving Dox alone had a significant deterioration in cardiac function, as characterized by decreased ejection fractions and fractional shortening. By comparison, mice treted with EH-201 (30 and 90 mg·kg−1) had significantly greater ejection fractions and fractional shortening (Figure 5D). However, there were no significant differences in the left ventricular diameters at the systole and diastole between the groups. These results indicate that treatment with EH-201 significantly attenuated the Dox-induced impairment of cardiac function. In addition, the Dox-damaged hearts presented with cytoplasmic vacuolization, myofibrillar loss and developed myocardial fibrosis and these effects were ameliorated by EH-201 treatment (Figure 5E). The image quantification results indicated that Dox increased the area of fibrosis in the ventricular endomysium, compared with normal mice (normal, 1.71 ± 0.18% vs. Dox, 8.31% ± 0.94%, Figure 5E), whereas fibrosis was almost absent in the mice treated with medium to high doses of EH-201. We also observed an up-regulation of Hb expression in the isolated myocardium of the EH–201-treated mice (Figure 5F, 30 and 90 mg·kg−1) and found an increase in Hb dimers (Figure 5G and H). Taken together, these data suggest that EH-201 has therapeutic potential as it improved the cardiac function and ischaemic tolerance of Dox-induced cardiomyopathic mice.
Figure 5.
EH-201 has therapeutic effects on cardiac dysfunction in doxorubicin (Dox)-induced cardiomyopathy in mice. (A) The survival rate was analysed using the Kaplan–Meier method (detailed treatment protocol in Methods). The normal (N) group represents saline injection. (B) The effect of EH-201 treatment on mice performing the hypoxic rotarod endurance test 2 weeks after Dox injection. (C, D) The effect of EH-201 on cardiac abnormality and functionality was characterized by ECG and echocardiography. EF, ejection fraction; FS, fractional shortening; LVIDs/d, left ventricular internal diameter at systole/diastole. (E) Representative photomicrographs of left ventricular sections of mouse hearts stained with haematoxylin-eosin and Masson's trichrome (left, bars = 10 μm). The blue staining indicates fibrosis, and quantification of the interstitial fibrosis was performed (right). (F) Isolated myocardium tissues after 2 weeks of Dox were analysed for Hb expression by Q-PCR and (G) a TMBZ stain of each myocardium lysate of the treatment groups in SDS-PAGE was performed, with (H) quantitative values. The values are presented as the means ± SEM (n = 5–6 animals each group). **P < 0.01, *P < 0.05 versus Dox group by one-way anova with Tukey's post hoc test.
EH-201 ameliorates anaemia and renal function in cisplatin-induced nephropathy
We used an established cisplatin-induced nephropathy mouse model to examine the effect of EH-201-induced EPO production on the anaemia accompanying the impaired renal function resulting from the use of a nephrotoxic agent (Figure 6A). We observed significant anaemia from day 10 and impaired renal function from day 13 after the first injection of cisplatin (Figure 6B and C). Notably, the administration of 30 and 90 mg·kg−1 of EH-201 for 2 weeks (on day 28, Figure 6B) led to an almost complete recovery of anaemia. Moreover, the BUN levels of the EH-201 30 and 90 mg·kg−1 treatment groups were also significantly recovered (Figure 6D). The histochemical examination revealed renal tubuloepithelial necrosis, vacuolation and desquamation from day 13; however, treatment with EH-201 significantly attenuated this renal damage (Figure 6E). In addition, we observed a significant increase in EPO in the kidneys of the anaemic mice, and EH-201 treatment did not lead to any further increases (Figure 6F), a finding which may be due to the compensatory effect of the remaining functional kidney cells and the recovery of renal function generated by EH-201 relieving the hypoxic stress on the kidney. The EH-201 30 and 90 mg·kg−1 treatments induced significant recovery of the hepatic EPO expression (Figure 6G). Furthermore, EH-201 administration also activated the erythroid progenitor cells in bone marrow (Figure 6H). Collectively, these findings suggest that EH-201 improved the recovery from cisplatin-induced anaemia and renal dysfunction by inducing the production of EPO.
Discussion and conclusions
EPO has been widely used for the treatment of anaemia in chronic kidney disease and that induced by chemotherapy in cancer patients. However, these therapies involve the administration of large doses of rhEPO to achieve circulating levels of EPO that are hundreds of times higher than normal (Fishbane and Besarab, 2007). In chronic heart failure, treatment with EPO was reported to have a beneficial effect on heart failure after hospitalization (van der Meer et al., 2009). However, EPO injections also result in increased haematocrit levels, which are associated with an increased blood viscosity. Therefore, an increased risk of thromboembolic events may limit its clinical use (Timmer et al., 2009; Skali et al., 2011). In comparison with EH-201, a small molecule, the size of the protein molecule EPO may reduce its penetration or delivery to the multilayer cardiomyocytes. In contrast to the supraphysiological levels of circulating EPO required to produce an effect, we found that EH-201 is a potent inducer of endogenous EPO and stimulates EPO-mediated mitochondrial biogenesis in non-haematopoietic cells. The compound can be administered orally and revives RBC numbers under pathological conditions by raising the endogenous levels of EPO to within a normal physiological range. Hypoxia is a major consequence of ischaemia, and EH-201 administration robustly increased exercise endurance in healthy and cardiomyopathic mice under hypoxic stress. In addition, EH-201 acted as an EPO/EPOR enhancer, which may help to increase EPOR sensitivity. Low-dose EPO treatment has been shown to be beneficial in animal models of myocardial infarction (Ben-Dor et al., 2007) and when applied clinically (Minamino et al., 2012). Additionally, the endogenous EPO/EPOR system in non-haematopoietic cells has been found to exert cardioprotective effects (Asaumi et al., 2007). Thus, an easily accessible oral therapy that uses a small molecule to raise the endogenous levels of EPO and enhance its autocrine activity may have benefits in ameliorating tissue ischaemia.
Unexpectedly, Hb has been found to be expressed in non-haematopoietic cells, where it may facilitate tissue oxygen transport or increase cellular oxygenation, thus linking Hb expression to mitochondrial function (Biagioli et al., 2009). The myocardium has a much higher Hb content than skeletal muscles, and also contains more Hb than myoglobin (Kranen et al., 1999), which indicates that Hb plays an important role in cardiomyocyte oxygenation. The dimeric form of Hb has been reported to possess a higher affinity for oxygen than the conventional tetrameric form (Marden et al., 1995). We found that EH-201 induced the expression of Hb-α, Hb-β, and dimeric Hb in non-haematopoietic cells and tissues with high oxygen demand such as myocytes, kidney slices, hepatocytes and myocardium. However, the expression of each form of Hb varied between these non-haematopoietic cells, probably as a result of the tissue-restricted transcription factors regulating the α- and β-globin genes (Blobel, 2000; Voon and Vadolas, 2008). The EPO-mediated increase in mitochondrial biogenesis and Hb expression induced by EH-201 in non-haematopoietic cells may provide a means of facilitating oxygen extraction from the capillaries, as well as improving the inter- and intracellular oxygen transport (Hb) that is associated with mitochondrial biogenesis and is needed to improve tissue oxygenation and energy production.
The preventative and protective effects of EH-201 acting as a reactive oxygen species scavenger in cerebral ischaemia and Dox-induced cardiotoxicity in mice were explored in previous studies (Wang et al., 2009; SH Zhang et al., 2009). In contrast, we adopted a therapeutic protocol to investigate whether EH-201 is able to generate recovery from cardiac and renal damage in established models of ischaemic damage. Our results suggest that EH-201 does indeed have therapeutic potential. However, the present findings are not consistent with previous results suggesting that exogenous EPO improves cardiac function in rats with myocardial infarction through a pro-angiogenic effect produced by stimulating VEGF production in cardiomyocytes (Westenbrink et al., 2010). We found that EH-201 did not increase VEGF levels. These differences may be due to the models used, as the cardioprotective effects of EPO on Dox-induced cardiomyopathy may occur without affecting coronary angiogenesis (Li et al., 2006). Therefore, EH-201 may target the cardiomyocytes to induce the production of endogenous EPO and ameliorate cardiac function. Although the development of a propyl hydroxylase inhibitor or Hif stabilizer has the potential to induce endogenous EPO (Hsieh et al., 2007), Hif-α transcription factors regulate a multitude of biological processes, and intermittent Hif-α activation over prolonged periods of time may lead to profound changes in cellular metabolism, growth and differentiation that will inevitably result in undesireable side effects (Nangaku et al., 2008). Additionally, the long-term inactivation of prolyl hydroxylase may contribute to the pathogenesis of ischaemic cardiomyopathy (Moslehi et al., 2010). In contrast, EH-201 can act as an enhancer of EPO and EPOR binding, by a mechanism that does not involve Hif-α activation, thus increasing EPOR sensitivity and potentially overcoming the problems associated with EPO resistance or insensitivity. Moreover, in contrast to a Hif stabilizer that stimulates high levels of erythropoiesis, EH–201 activated mitochondrial function and Hb expression in non-haematopoietic cells and enhanced RBC numbers to within a normal range. This suggests that EH-201, with its novel mechanism of action, might be a safer inducer of endogenous EPO than previous established compounds. There are some precedents for positive allosteric modulators (PAMs) in the regulation of catalytic receptors, such as the small-molecule non-peptidyl fungal metabolite, L-783,281, which has been shown to be an allosteric activator of trkA and insulin receptors (Zhang et al., 1999; Wilkie et al., 2001). The staurosporine-like compound L-753,000 potentiates the neurotrophic effects of neurotrophin 3 by acting selectively at the trkA receptor (Pollack et al., 1999). Although there is some evidence that EH-201 might be acting as a PAM of EPOR function, further characterization remains to be achieved. Our mechanistic studies indicate that EH-201 serves as an enhancer of EPO and EPOR binding but this requires further confirmation through chemical and structural biology studies.
In addition, according to pharmacokinetics studies performed in vivo (Lv et al., 2011; Zhao et al., 2013), this compound is absorbed rapidly, distributed abundantly in the heart, kidney, liver and lung, and then excreted quickly after oral administration. The favourable pharmacokinetic profiles of this compound probably help to provide its promising in vivo efficacy in the present study.
In conclusion, based on our findings, EH-201 is a novel inducer of endogenous EPO expression in the heart, kidney and liver and induces mitochondrial biogenesis and Hb expression through EPO-mediated activation in non-haematopoietic cells and is also able to enhance and preserve efficient cellular oxygenation and mitochondrial energetics. The activation of mitochondrial function and Hb expression in non-haematopoietic cells by this endogenous EPO inducer is a potential therapeutic strategy for ischaemic diseases.
Acknowledgments
We thank the Taiwan Mouse Clinic, which is funded by the National Research Program for Biopharmaceuticals (NRPB) at the National Science Council (NSC) of Taiwan for technical support in echocardiography experiment. We are thankful for the Grant-in-Aid (NSC-100-2320-B-010-032) from the National Science Council, and the grant from the Ministry of Education, Aim for the Top University Plan, Republic of China (Taiwan).
Glossary
- BUN
blood urea nitrogen
- Dox
doxorubicin
- EH-201
2,3,5,4′-tetrahydroxystilbene-2-o-β-d-glucoside
- EPO
erythropoietin
- EPOR
EPO receptor
- Hif
hypoxia-inducible factor
- mtDNA
mitochondrial DNA
- PGC-1α
PPAR co-activator 1α
- Sirt1
sirtuin
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 EH-201 characterization. (A) HPLC profile of EH-201. Mightysil RP-18 column (4.6 × 250 mm i.d., 5 μm) was used at flow rate of 0.8 mL·min-1 with MeOH/H2O (20/80, v/v) gradient to 100% MeOH in 60 min in the detection wavelength of 280 and 300 nm. (B) Positive ion mode LC-APCI/MS/MS of EH-201.
Figure S2 EH-201 induces Sirt1 expression. Sirt1 protein expression in the lysates of the EH-201-treated kidney slices and hepatocytes were analysed by Western blotting (n = 4). The control represents vehicle treatment. The values are presented as the means ± SEM. **P < 0.01, *P < 0.05 compared with control.
Figure S3 Ribbon diagrams of the computational docking results for EH-201 on EPO/EPOR complex. Docking calculations were carried out using DockingServer (Bikadi et al., 2009) on EPO complexed with extracellular domain of EPOR protein model (PDB entry code 1cn4). The carbon backbone (green colour) with balls and sticks indicated the ligand molecule EH-201, the helix (red colour, left) indicated the helix A of EPO, and the loop (gray colour, right) indicated the loop 5 of EPOR. The predictive interaction residues including PRO144, GLU147, PRO149, Met150, and THR151 are located in loop 5 of EPOR, which is important for EPO binding.
Figure S4 EH-201-induced EPO production does not involve Hif-α activation. (A) The hypoxia response element (HRE)-driven luciferase reporter (Luci) transfected HEK 293 cells were incubated with EH-201 under normoxia or hypoxia (5% O2, as the positive control) for 24 h. The plasmid for β-Galactosidase (β-Gal) was used as a transfection control, and the pGL3-v served as a vector control. Similar results were observed in three additional independent experiments. (B) The VEGF expression of the EH-201-treated hepatocytes were analysed by Q-PCR (n = 3). Hypoxia condition served as a positive control. (C) The Hif-2α protein expression levels in the nuclear lysates of the EH-201-treated kidney slices were analysed by Western blotting (H: 5% O2 hypoxia as a positive control). The control represents vehicle treatment. The values are presented as the means ± SEM. **P < 0.01, *P < 0.05 compared with normoxia, Student's t-test.
Figure S5 EH-201 increases mitochondrial function and biogenesis in the liver and skeletal muscle. (A, B) Isolated liver and skeletal muscle tissues after 14 days of EH-201 administration were analysed for the mitochondrial activity by PGC-1α Q-PCR, citrate synthase activity and mtDNA copy number. The values are presented as the means ± SEM (n = 5 animals each group). **P < 0.01, *P < 0.05 versus the ND group; ##P < 0.01, #P < 0.05 versus the day 7 ND group by one-way anova with Tukey's posthoc test.
Figure S6 EH-201 has therapeutic effects on cardiac dysfunction in doxorubicin (Dox)-induced cardiomyopathy in mice. (A) The effect of EH-201 on the body weight of mice two weeks after Dox injection. (B) The effect of EH-201 on cardiac function was characterized by ECG, heart rate presented as the beat per second (bps). The values are presented as the means ± SEM (n = 5–6 animals each group). **P < 0.01, *P < 0.05 versus Dox group by one-way anova with Tukey's posthoc test.
Table S1 Proton (500 MHz) and carbon (125 MHz) chemical shifts* of EH-201.
Table S2 Sequences of specific gene used for Q-PCR primers, siRNA, and HRE.
References
- Asaumi Y, Kagaya Y, Takeda M, Yamaguchi N, Tada H, Ito K, et al. Protective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload-induced left ventricular dysfunction in mice. Circulation. 2007;115:2022–2032. doi: 10.1161/CIRCULATIONAHA.106.659037. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dor I, Hardy B, Fuchs S, Kaganovsky E, Kadmon E, Sagie A, et al. Repeated low-dose of erythropoietin is associated with improved left ventricular function in rat acute myocardial infarction model. Cardiovasc Drugs Ther. 2007;21:339–346. doi: 10.1007/s10557-007-6049-8. [DOI] [PubMed] [Google Scholar]
- Bergmann MW, Haufe S, von Knobelsdorff-Brenkenhoff F, Mehling H, Wassmuth R, Munch I, et al. A pilot study of chronic, low-dose epoetin-{beta} following percutaneous coronary intervention suggests safety, feasibility, and efficacy in patients with symptomatic ischaemic heart failure. Eur J Heart Fail. 2011;13:560–568. doi: 10.1093/eurjhf/hfr002. [DOI] [PubMed] [Google Scholar]
- Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikadi Z, Hazai E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J Cheminform. 2009;1:1–15. doi: 10.1186/1758-2946-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel GA. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood. 2000;95:745–755. [PubMed] [Google Scholar]
- Calvo-Romero JM, Fernandez-Soria-Pantoja R, Arrebola-Garcia JD, Gil-Cubero M. Ischemic heart disease associated with vincristine and doxorubicin chemotherapy. Ann Pharmacother. 2001;35:1403–1405. doi: 10.1345/aph.10358. [DOI] [PubMed] [Google Scholar]
- Carraway MS, Suliman HB, Jones WS, Chen CW, Babiker A, Piantadosi CA. Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart. Circ Res. 2010;106:1722–1730. doi: 10.1161/CIRCRESAHA.109.214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK. Pleiotropic renal actions of erythropoietin. Lancet. 2005;365:1890–1892. doi: 10.1016/S0140-6736(05)66622-6. [DOI] [PubMed] [Google Scholar]
- Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 2007;113:659–673. doi: 10.1007/s00401-007-0199-4. [DOI] [PubMed] [Google Scholar]
- Farraj AK, Hazari MS, Cascio WE. The utility of the small rodent electrocardiogram in toxicology. Toxicol Sci. 2011;121:11–30. doi: 10.1093/toxsci/kfr021. [DOI] [PubMed] [Google Scholar]
- Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, et al. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Horng LY, Chen CF, Wu RT. Chinese herb Radix Polygoni Multiflori as a therapeutic drug for liver cirrhosis in mice. J Ethnopharmacol. 2007;114:199–206. doi: 10.1016/j.jep.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Kranen RW, van Kuppevelt TH, Goedhart HA, Veerkamp CH, Lambooy E, Veerkamp JH. Hemoglobin and myoglobin content in muscles of broiler chickens. Poult Sci. 1999;78:467–476. doi: 10.1093/ps/78.3.467. [DOI] [PubMed] [Google Scholar]
- Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, et al. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, et al. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science. 1996;273:464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- Lv G, Lou Z, Chen S, Gu H, Shan L. Pharmacokinetics and tissue distribution of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside from traditional Chinese medicine Polygonum multiflorum following oral administration to rats. J Ethnopharmacol. 2011;137:449–456. doi: 10.1016/j.jep.2011.05.049. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra D, Byun J, Andreana PR, Abdulhamid I, Diamond MP, Saed GM, et al. Reaction of hemoglobin with HOCl: mechanism of heme destruction and free iron release. Free Radic Biol Med. 2011;51:374–386. doi: 10.1016/j.freeradbiomed.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden MC, Griffon N, Poyart C. Oxygen delivery and autoxidation of hemoglobin. Transfus Clin Biol. 1995;2:473–480. doi: 10.1016/s1246-7820(05)80074-6. [DOI] [PubMed] [Google Scholar]
- van der Meer P, Groenveld HF, Januzzi JL, Jr, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309–1314. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- Minamino T, Toba K, Higo S, Nakatani D, Hikoso S, Umegaki M, et al. Design and rationale of low-dose erythropoietin in patients with ST-segment elevation myocardial infarction (EPO-AMI-II study): a randomized controlled clinical trial. Cardiovasc Drugs Ther. 2012;26:409–416. doi: 10.1007/s10557-012-6410-4. [DOI] [PubMed] [Google Scholar]
- Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, et al. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010;122:1004–1016. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008;110:e1–e7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart – an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, et al. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 2008;19:1500–1508. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obatomi DK, Brant S, Anthonypillai V, Early DA, Bach PH. Optimizing preincubation conditions for precision-cut rat kidney and liver tissue slices: effect of culture media and antioxidants. Toxicol In Vitro. 1998;12:725–737. doi: 10.1016/s0887-2333(98)00055-1. [DOI] [PubMed] [Google Scholar]
- Ou L, Li W, Liu Y, Zhang Y, Jie S, Kong D, et al. Animal models of cardiac disease and stem cell therapy. Open Cardiovasc Med J. 2010;4:231–239. doi: 10.2174/1874192401004010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S, Young L, Bilsland J, Wilkie N, Ellis S, Hefti F, et al. The staurosporine-like compound L-753,000 (NB-506) potentiates the neurotrophic effects of neurotrophin-3 by acting selectively at the TrkA receptor. Mol Pharmacol. 1999;56:185–195. doi: 10.1124/mol.56.1.185. [DOI] [PubMed] [Google Scholar]
- Sato T, Maekawa T, Watanabe S, Tsuji K, Nakahata T. Erythroid progenitors differentiate and mature in response to endogenous erythropoietin. J Clin Invest. 2000;106:263–270. doi: 10.1172/JCI9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol. 2003;94:555–560. doi: 10.1152/japplphysiol.00821.2002. [DOI] [PubMed] [Google Scholar]
- Skali H, Parving HH, Parfrey PS, Burdmann EA, Lewis EF, Ivanovich P, et al. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with Darbepoetin Alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation. 2011;124:2903–2908. doi: 10.1161/CIRCULATIONAHA.111.030411. [DOI] [PubMed] [Google Scholar]
- Song W, Lu X, Feng Q. Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res. 2000;45:595–602. doi: 10.1016/s0008-6363(99)00395-8. [DOI] [PubMed] [Google Scholar]
- Stopka T, Zivny JH, Stopkova P, Prchal JF, Prchal JT. Human hematopoietic progenitors express erythropoietin. Blood. 1998;91:3766–3772. [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel TH, Geng L, Lato EB, Schaal S, Liu Y, Dean D, et al. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1911–1919. doi: 10.1167/iovs.07-1372. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Kain AD, Powell DW, et al. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest Ophthalmol Vis Sci. 2010;51:907–919. doi: 10.1167/iovs.09-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer SA, De Boer K, Knaapen P, Gotte MJ, Van Rossum AC. The potential role of erythropoietin in chronic heart failure: from the correction of anemia to improved perfusion and reduced apoptosis? J Card Fail. 2009;15:353–361. doi: 10.1016/j.cardfail.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Villeval JL, Mitjavila MT, Dusanter-Fourt I, Wendling F, Mayeux P, Vainchenker W. Autocrine stimulation by erythropoietin (Epo) requires Epo secretion. Blood. 1994;84:2649–2662. [PubMed] [Google Scholar]
- Voon HP, Vadolas J. Controlling alpha-globin: a review of alpha-globin expression and its impact on beta-thalassemia. Haematologica. 2008;93:1868–1876. doi: 10.3324/haematol.13490. [DOI] [PubMed] [Google Scholar]
- Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, et al. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol. 2008;578:339–348. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Westenbrink BD, Ruifrok WP, Voors AA, Tilton RG, van Veldhuisen DJ, Schoemaker RG, et al. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovasc Res. 2010;87:30–39. doi: 10.1093/cvr/cvq041. [DOI] [PubMed] [Google Scholar]
- Wilkie N, Wingrove PB, Bilsland JG, Young L, Harper SJ, Hefti F, et al. The non-peptidyl fungal metabolite L-783,281 activates TRK neurotrophin receptors. J Neurochem. 2001;78:1135–1145. doi: 10.1046/j.1471-4159.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- Worthington RE, Bossie-Codreanu J, Van Zant G. Quantitation of erythroid differentiation in vitro using a sensitive colorimetric assay for hemoglobin. Exp Hematol. 1987;15:85–92. [PubMed] [Google Scholar]
- Wu J, Hecker JG, Chiamvimonvat N. Antioxidant enzyme gene transfer for ischemic diseases. Adv Drug Deliv Rev. 2009;61:351–363. doi: 10.1016/j.addr.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PY, Almofti MR, Lu L, Kang H, Zhang J, Li TJ, et al. Reduction of atherosclerosis in cholesterol-fed rabbits and decrease of expressions of intracellular adhesion molecule-1 and vascular endothelial growth factor in foam cells by a water-soluble fraction of Polygonum multiflorum. J Pharmacol Sci. 2005;99:294–300. doi: 10.1254/jphs.fp0050333. [DOI] [PubMed] [Google Scholar]
- Zebedin E, Mille M, Speiser M, Zarrabi T, Sandtner W, Latzenhofer B, et al. C2C12 skeletal muscle cells adopt cardiac-like sodium current properties in a cardiac cell environment. Am J Physiol Heart Circ Physiol. 2007;292:H439–H450. doi: 10.1152/ajpheart.00119.2006. [DOI] [PubMed] [Google Scholar]
- Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, Royo I, et al. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284:974–977. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing Y, Ye CF, Ai HX, Wei HF, Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer's disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res. 2006;173:246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Wang WQ, Wang JL. Protective effect of tetrahydroxystilbene glucoside on cardiotoxicity induced by doxorubicin in vitro and in vivo. Acta Pharmacol Sin. 2009;30:1479–1487. doi: 10.1038/aps.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xu XL, Wang YQ, Wang CH, Zhu WZ. Effects of 2,3,4′,5-tetrahydroxystilbene 2-O-beta-D-glucoside on vascular endothelial dysfunction in atherogenic-diet rats. Planta Med. 2009;75:1209–1214. doi: 10.1055/s-0029-1185540. [DOI] [PubMed] [Google Scholar]
- Zhao Y-Y, Zhang L, Feng Y-L, Chen D-Q, Xi Z-H, Du X, et al. Pharmacokinetics of 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside in rat using ultra performance LC-quadrupole TOF-MS. J Sep Sci. 2013;36:863–871. doi: 10.1002/jssc.201200668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.